Terahertz Raman Measurements Using a Spatial Heterodyne Raman Spectrometer
Abstract
:1. Introduction
2. Theory
2.1. Basic Theory
2.2. Calibration Theory
3. Materials and Methods
3.1. Breadboard
3.2. Calibration
4. Results and Discussion
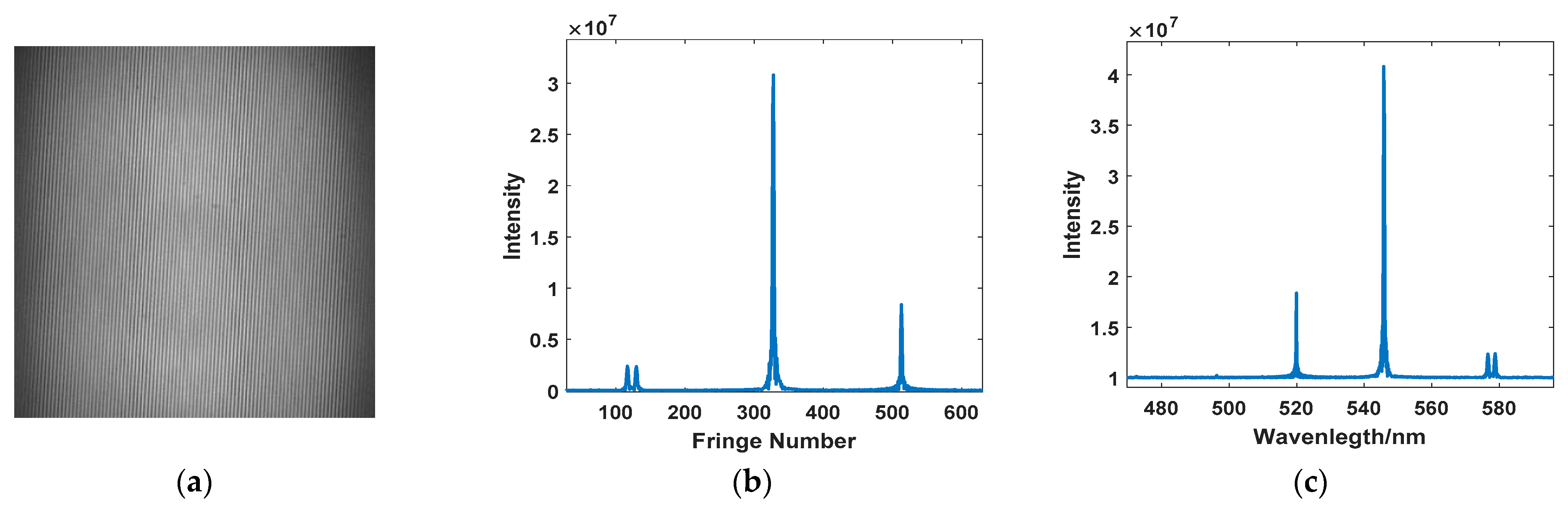
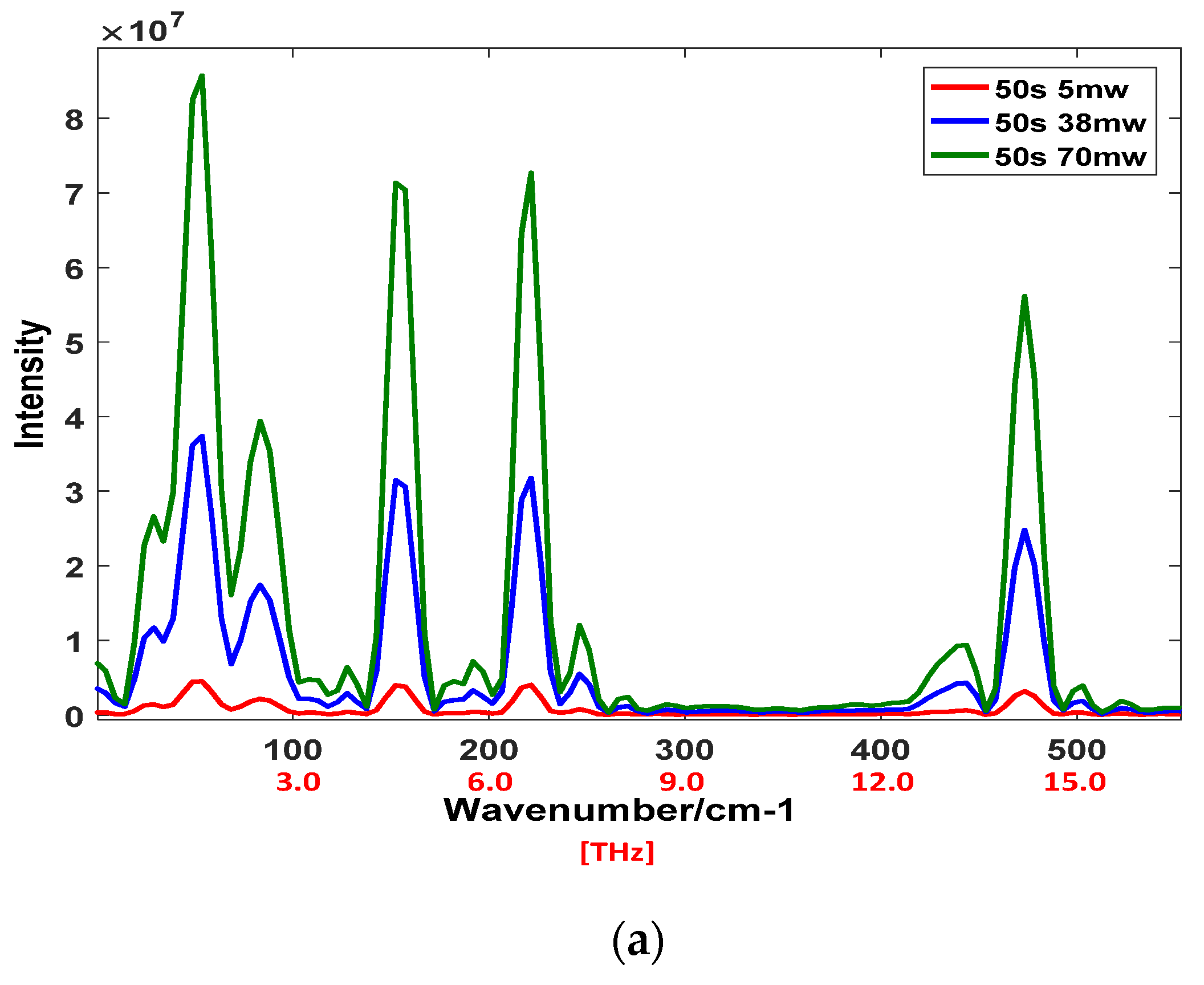
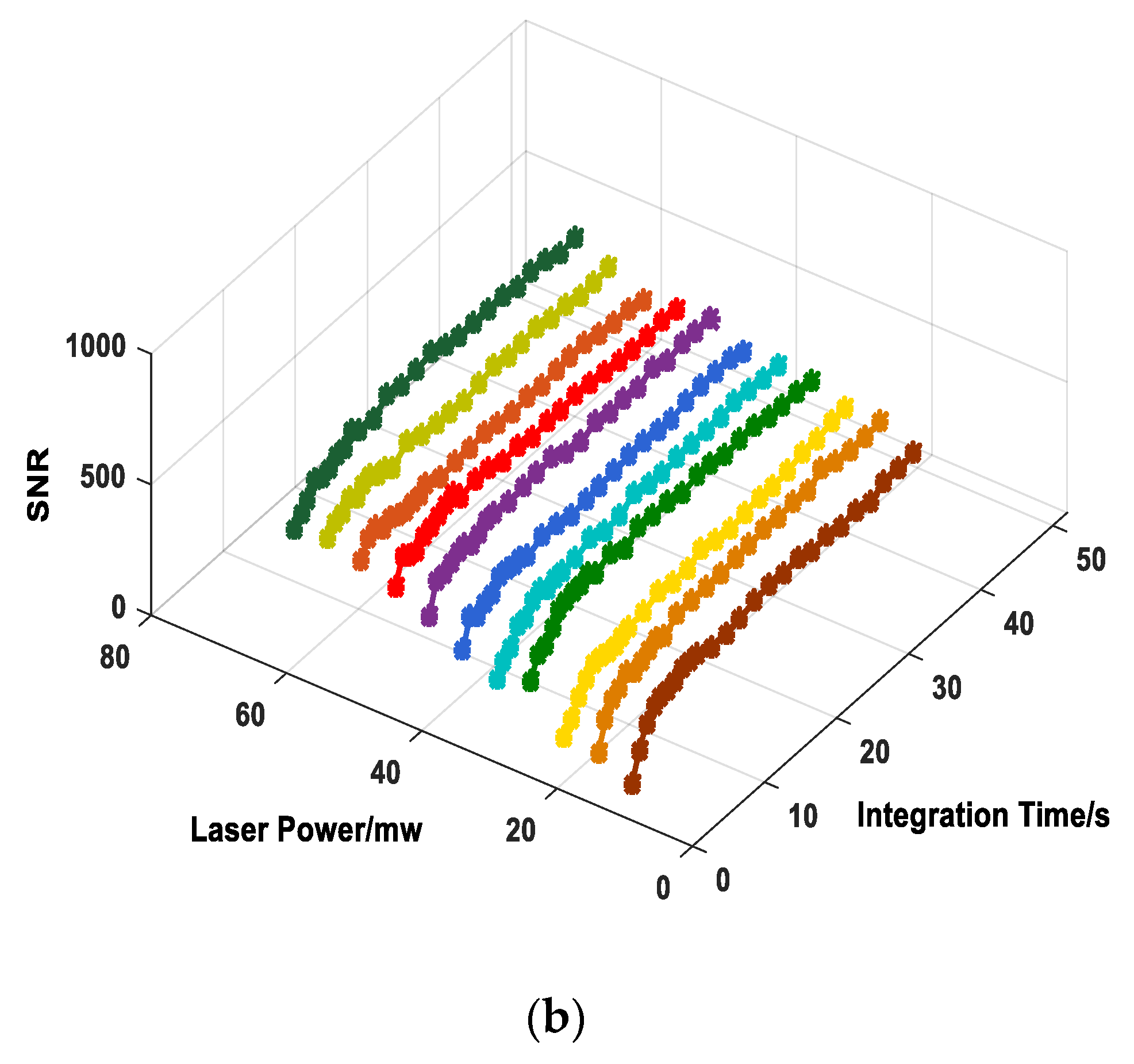
4.1. THz Raman Spectrum and SNR of Sulfur
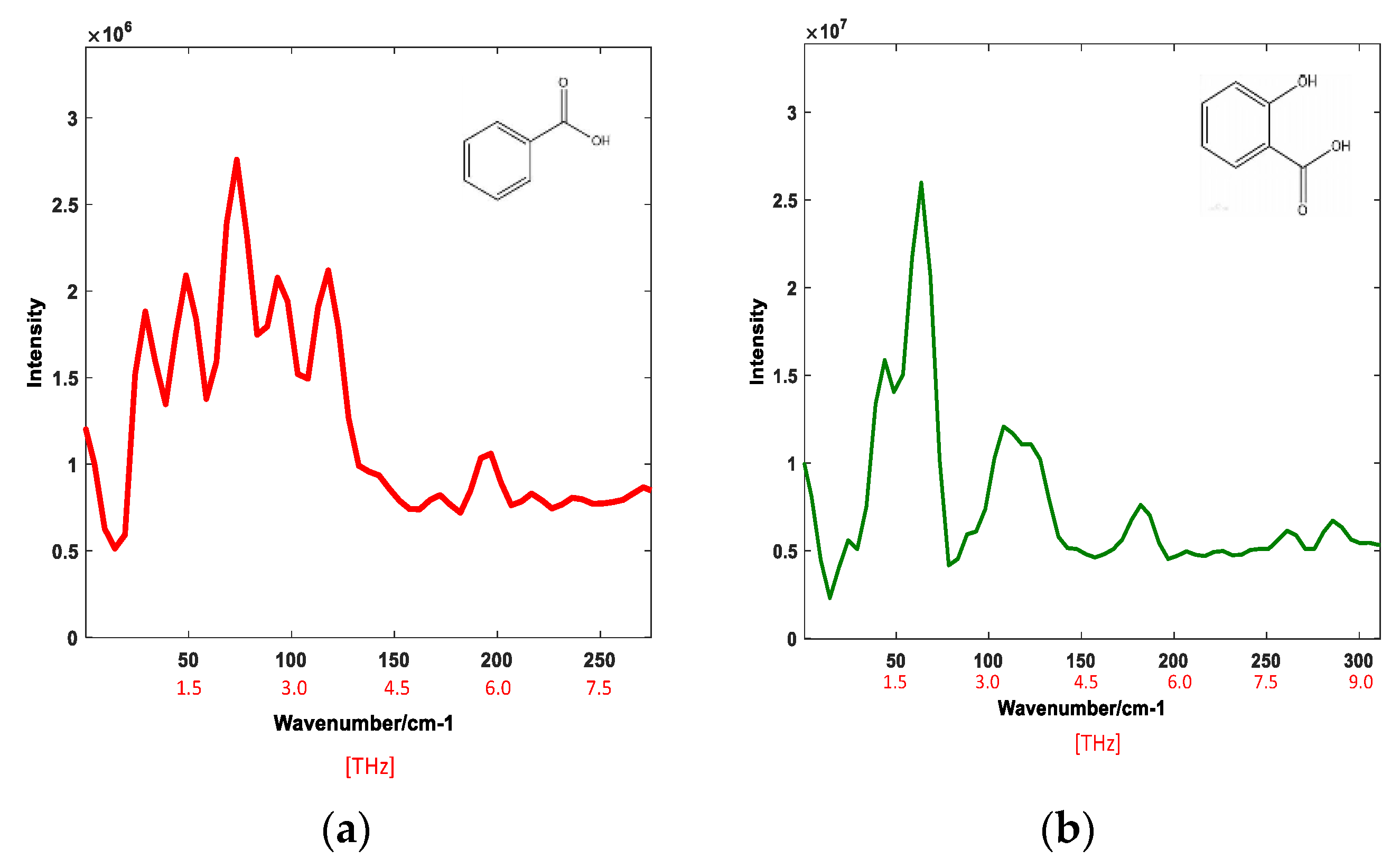
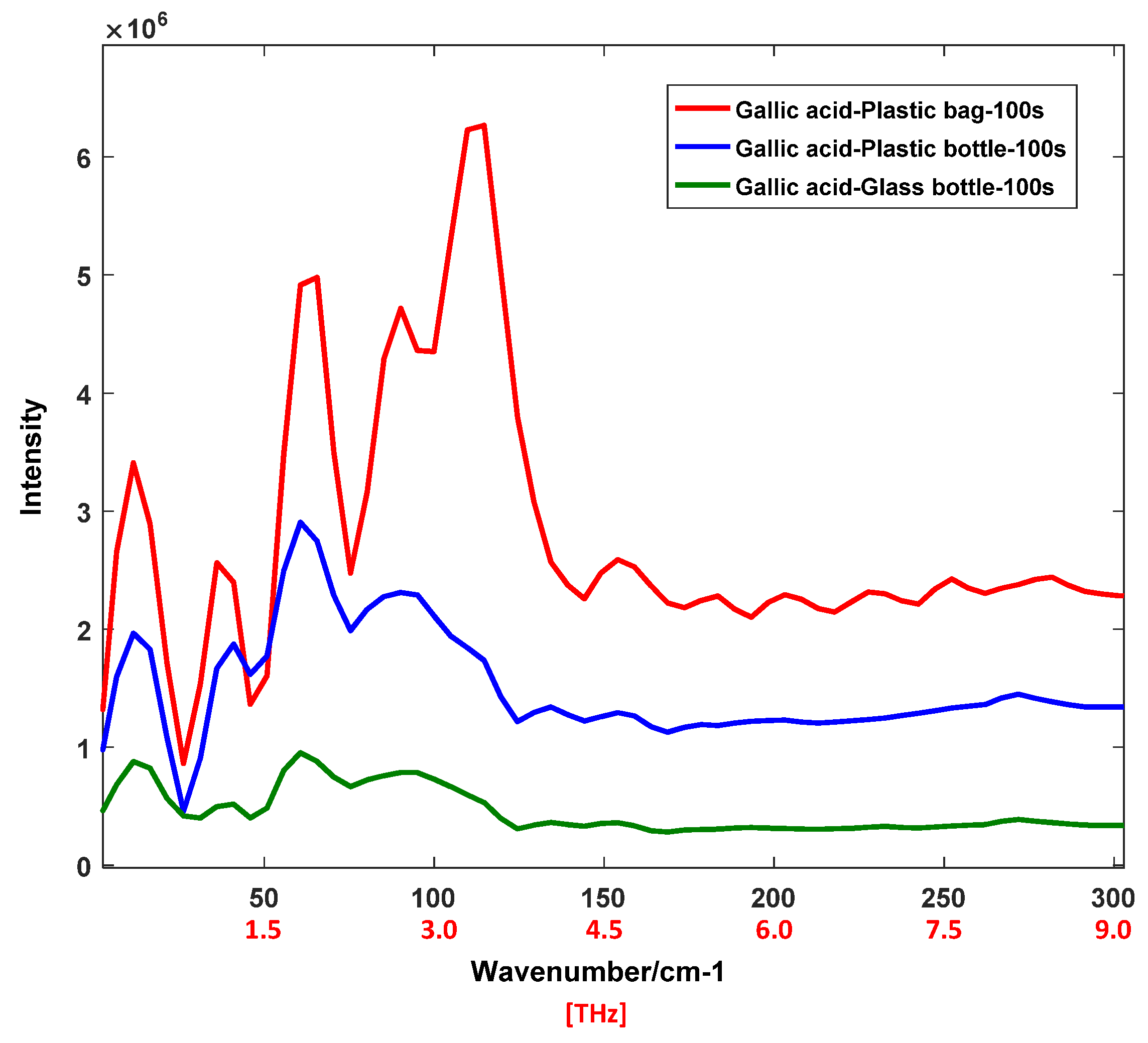
4.2. THz Raman Spectra of Organic Acids
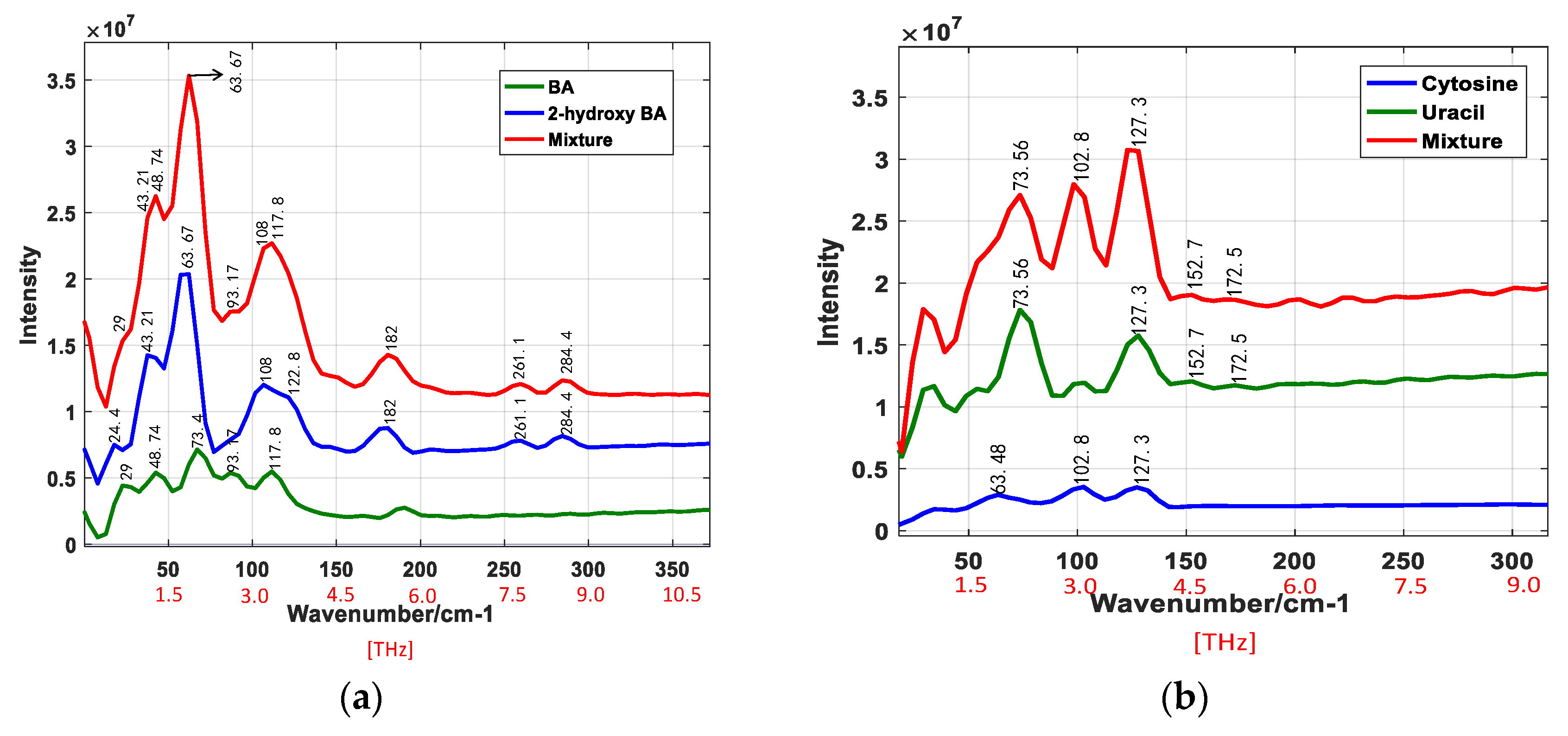
4.3. THz Raman Spectra of Nucleobases
4.4. THz Raman Spectra of Aqueous Solutions
4.5. THz Raman Spectra of Nucleobases
4.5.1. Spectra of Solid Mixtures
4.5.2. Spectra of Liquid Mixtures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Grechko, M.; Hasegawa, T.; D’Angelo, F.; Ito, H.; Turchinovich, D.; Nagata, Y.; Bonn, M. Coupling between intra- and intermolecular motions in liquid water revealed by two-dimensional terahertz-infrared-visible spectroscopy. Nat. Commun. 2018, 9, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Damari, R.; Weinberg, O.; Krotkov, D.; Demina, N.; Akulov, K.; Golombek, A.; Schwartz, T.; Fleischer, S. Strong coupling of collective intermolecular vibrations in organic materials at THz frequencies. Nat. Commun. 2019, 10, 2403–2412. [Google Scholar] [CrossRef]
- Morimoto, T.; Nagai, M.; Minowa, Y.; Ashida, M.; Yokotani, Y.; Okuyama, Y.; Kani, Y. Microscopic ion migration in solid electrolytes revealed by THz time-domain spectroscopy. Nat. Commun. 2019, 10, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bandurin, D.A.; Svintsov, D.; Gayduchenko, I.; Xu, S.; Principi, A.; Moskotin, M.; Tretyakov, I.; Yagodkin, D.; Zhukov, S.; Taniguchi, T.; et al. Resonant THz detection using graphene plasmons. Nat. Commun. 2018, 9, 5392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wei, Y.; Zang, X.; Zhu, Y.; Zhuang, S. Correction: Corrigendum: Excitation of dark multipolar plasmonic resonances at THz frequencies. Sci. Rep. 2016, 6, 27324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huisman, T.J.; Mikhaylovskiy, R.V.; Telegin, A.; Sukhorukov, Y.P.; Granovsky, A.; Naumov, S.; Rasing, T.; Kimel, A.V. THz magneto-optics in the ferromagnetic semiconductor HgCdCr2Se4. Appl. Phys. Lett. 2015, 106, 132411. [Google Scholar] [CrossRef] [Green Version]
- Dixit, V.; Cho, B.K.; Obendorf, K.; Tewari, J. Identifications of household’s spores using mid infrared spectroscopy. J. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 123, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Terracciano, A.C.; Masuno, V.A.E. Accurate prediction of THz spectra of molecular crystals of fentanyl and its analogs. J. Sci. Rep. 2021, 11, 11. [Google Scholar] [CrossRef]
- Chen, W.; Peng, Y.; Jiang, X.; Zhao, J.; Zhalo, H.; Zhu, Y. Isomers Identification of 2-hydroxyglutarate acid disodium salt (2HG) by THz Time-domain Spectroscopy. J. Sci. Rep. 2017, 7, 12166. [Google Scholar] [CrossRef] [Green Version]
- Richter, H.; Buchbender, C.; Güsten, R.; Higgins, R.; Klein, B.; Stutzki, J.; Wiesemeyer, H.; Hübers, H.-W. Direct measurements of atomic oxygen in the mesosphere and lower thermosphere using THz heterodyne spectroscopy. Commun. Earth Environ. 2021, 2, 19. [Google Scholar] [CrossRef]
- Hsieh, Y.D.; Nakamura, S.; Abdelsalam, D.; Minamikawa, T.; Mizutani, Y.; Yamamoto, H.; Iwata, T.; Hindle, F.; Yasui, T. Dynamic THz spectroscopy of gas molecules mixed with unwanted aerosol under atmospheric pressure using fibre-based asynchronous-optical-sampling THz time-domain spectroscopy. Sci. Rep. 2016, 6, 28114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Xie, L.; Ye, Z.; Gao, W.; Yao, Y.; Chen, M.; Qin, J.; Ying, Y. Discrimination of Transgenic Rice containing the Cry1Ab Protein using THz Spectroscopy and Chemometrics. Sci. Rep. 2015, 5, 11115. [Google Scholar] [CrossRef]
- Shi, X.; Han, Z. Enhanced THz fingerprint detection with ultrahigh sensitivity using the cavitydefect modes. J. Sci. Rep. 2017, 7, 13147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, S.; Fukushi, Y.; Kubota, O.; Itsuji, T.; Ouchi, T.; Yamamoto, S. Brain tumor imaging of rat fresh tissue using THz spectroscopy. J. Rep. 2016, 6, 30124. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Huang, H.; Hao, S.; Qiu, K.-F.; Gao, H.; Gao, L.; Tang, W.; Zhang, Z.; Zheng, Z.I. Insights into the water status in hydrous minerals using THz time-domain spectroscopy. J. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Julien, C. A triple monochromator for low-frequency Raman spectroscopy. J. Opt. 1980, 11, 257–267. [Google Scholar] [CrossRef]
- Moser, C.; Havermeyer, F.; Champion, P.M.; Ziegler, L.D. Compact Low Frequency Raman Spectroscopy System. J. Am. Inst. Phys. 2010, 794–795. [Google Scholar] [CrossRef]
- Guan, X.; Jiang, M.; Yang, F.; Yu, Y.; Chen, J. Portable Low Frequency Raman Spectrometer Based on Fliter. In Proceedings of the 2019 IEEE 4th Optoelectronics Global Conference (OGC), IEEE, Shenzhen, China, 3–6 September 2019; pp. 126–130. [Google Scholar] [CrossRef]
- Bell, R.J. Introductory Fourier transform spectroscopy. J. Acad. Press 1972, 41, 149–151. [Google Scholar] [CrossRef]
- Roesler, F.L.; Harlander, J.M. Spatial heterodyne spectroscopy-Interferometric performance at any wavelength without scanning. J. Proc. SPIE-Int. Soc. Opt. Eng. 1990, 1318. [Google Scholar] [CrossRef]
- Nims, C.; Cron, B.; Wetherington, M.; Macalady, J.; Cosmidis, J. Low frequency Raman Spectroscopy for micron-scale and in vivo characterization of elemental sulfur in microbial samples. J. Sci. Rep. 2019, 9, 7971. [Google Scholar] [CrossRef] [Green Version]
- Korobkov, V.S.; Zharikov, N.K. The low-frequency raman spectra of benzoic acid and some of its derivatives. J. Appl. Spectrosc. 1973, 19, 1306–1310. [Google Scholar] [CrossRef]






| Components | Parameters | Performance Index |
|---|---|---|
| Laser | Wavelength | 532 nm, CW |
| Beam diameter | ~2.0 (1/e, mm) | |
| Beam divergence | <1.5 (full angle, mrad) | |
| Grating 1 | Groove density | 150 gr/mm |
| Littrow angle | 2.324° | |
| Grating 2 | Groove density | 150 gr/mm |
| Littrow angle | 2.324° | |
| Prisms | Apex angle | 2.665° |
| Beam splitter | Size | 50.8 × 50.8 × 50.8 mm3 |
| CCD | Pixel numbers | 1024 × 1024 |
| Sensor size | 13.3 × 13.3 nm2 | |
| Pixel size | 13 × 13 | |
| BPF | Design wavelength | 532 nm |
| Diffraction efficiency | 99.8% | |
| Transmittance | >94% | |
| 500 nm long-pass filter | Edge wavelength | 508–2150 nm |
| Transition width | 15 nm | |
| Blocking region | 200–485 nm | |
| 550 nm short-pass filter | Cut-off wavelength | 400–543 nm |
| Transition width | 17 nm | |
| Blocking region | 567–715 nm | |
| BNF | Design wavelength | 532 nm |
| Optical density | OD3 | |
| Transmittance | >92% | |
| Imaging optics | Diameter | 62 mm |
| Focal length | 105 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, X.; Galantu, J.; Chu, Q.; Chen, J.; Liu, Z.; Mi, X.; Yao, X.; Li, P. Terahertz Raman Measurements Using a Spatial Heterodyne Raman Spectrometer. Appl. Sci. 2021, 11, 8094. https://doi.org/10.3390/app11178094
Sun Y, Li X, Galantu J, Chu Q, Chen J, Liu Z, Mi X, Yao X, Li P. Terahertz Raman Measurements Using a Spatial Heterodyne Raman Spectrometer. Applied Sciences. 2021; 11(17):8094. https://doi.org/10.3390/app11178094
Chicago/Turabian StyleSun, Yuqi, Xiaotian Li, Jiri Galantu, Qihang Chu, Jun Chen, Zhongkai Liu, Xiaotao Mi, Xuefeng Yao, and Pan Li. 2021. "Terahertz Raman Measurements Using a Spatial Heterodyne Raman Spectrometer" Applied Sciences 11, no. 17: 8094. https://doi.org/10.3390/app11178094





