Antidiabetic, Anticholesterol, and Antioxidant Activity of Gryllusbimaculatus Fermented by Bacillus and Lactobacillus Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Fermentation
2.3. Microbiological Analysis
2.4. pH Measurement
2.5. In Vitro Digestion
2.6. α-Glucosidase Inhibitory (AGI) Activity
2.7. α-Amylase Inhibitory (AAI) Activity
2.8. HMG-CoA Reductase Inhibitory Activity
2.9. DPPH Radical Scavenging Activity
2.10. The SOD-Like Activity
2.11. Reducing Power
2.12. Total Polyphenol Contents
2.13. Statistical Analysis
3. Results and Discussion
3.1. Microbiological and Physicochemical Change during Fermentation
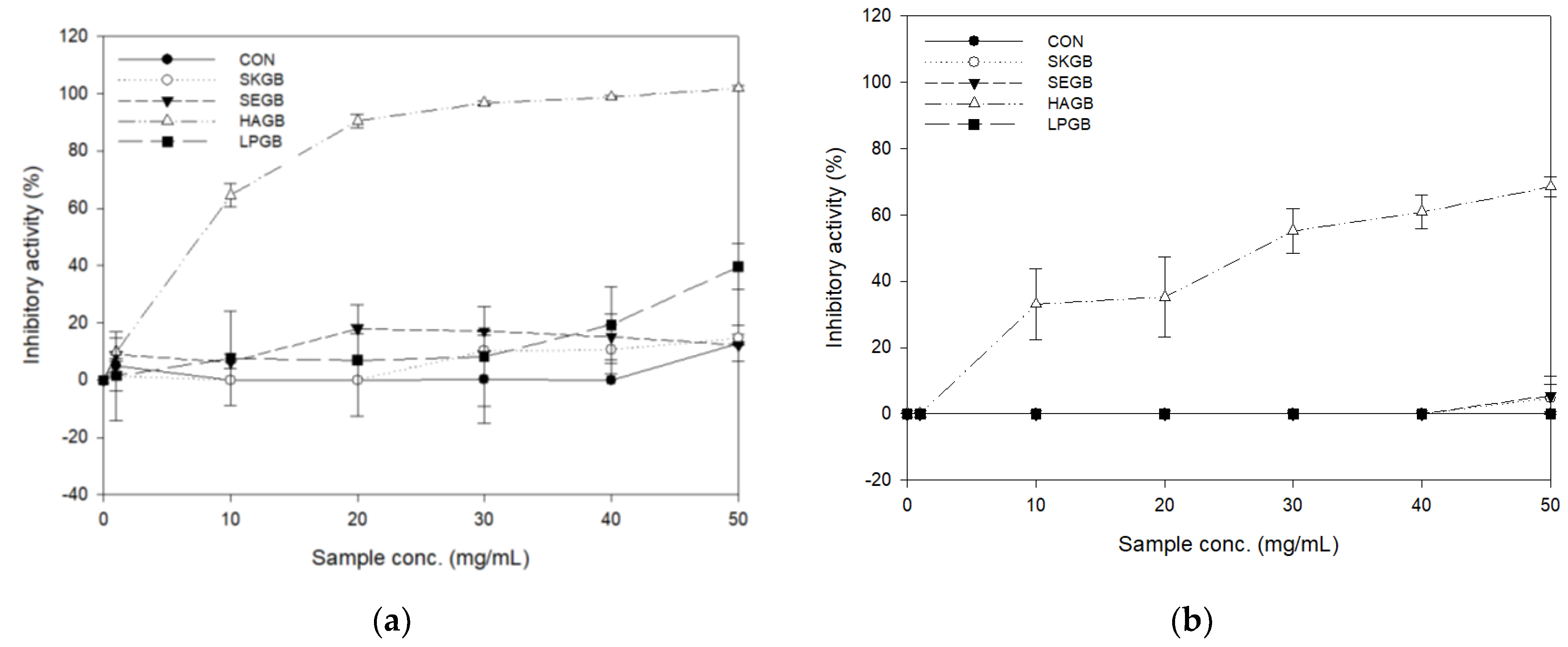
3.2. Inhibitory Activities of the Carbohydrate-Hydrolyzing Enzyme
3.3. Anticholesterol Activity
3.4. Antioxidant Activity and the TPC of Fermented GB
3.5. Antioxidant Activity and TPC After In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | MKHA15 1,2 | MKSK-J1 3,4 | |
|---|---|---|---|
| Acid and bile salt tolerance | Survival rate to pepsin (%) | 96.30 ± 0.10 5 | 91.85 ± 4.98 |
| Survival rate to bile salt (%) | 53.56 ± 5.86 | 90.31 ± 0.44 | |
| Safety test | γ-hemolysis | γ-hemolysis | |
| Enzymatic characteristics | Amylase | β-Glucuronidase negative | N.D.4 |
| Protease | Negative (qualitative) | 1.37 ± 0.63 (U/mL) | |
| Antioxidant activity | DPPH radical scavenging activity (%) | N.D.6 | 23.22 ± 0.21 (U/mL) |
| SOD-like activity (%) | 100.53 ± 6.91 | 7.69 ± 2.71 | |
| Reducing power (% ascorbic acid) | 56.62 ± 1.77 | 28.10 ± 2.04 | |
| Antidiabetic activity | α-Glucosidase inhibitory activity (%) | 98.76 ± 7.12 | 41.36 ± 0.63 |
| α-Amylase inhibitory activity (%) | 69.68 ± 2.38 | None | |
| Anticholesterol activity | HMG-CoA reductase inhibitory activity (%) | 65.81 ± 6.50 | −6.04 ± 7.70 |
References
- Zhao, H.L.; Cho, J.H.; Chung, C.H. Characteristics of seasoning pastes fermented by Aspergillus oryzae and Bacillus sutilis using edible insects. Korean J. Food Sci. Technol. 2018, 50, 297–307. [Google Scholar]
- Dobermann, D.; Field, L.N.; Michaelson, L.V. Impact of heat processing on the nutritional content of Gryllus bimaculatus (black cricket). Nutr. Bull. 2019, 44, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.Y.; Jang, S.H.; Ahn, H.Y.; Cho, H.D.; Seo, K.I.; Cho, Y.S. Optimization of fermentation conditions Protaetia brevitarsis seulensis larvae using Bacillus subtilis. Korean J. Food Preserv. 2019, 26, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Zielinska, E.; Baraniak, B.; Karas, M.; Rybczynska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, K.B.; Choi, S.K. Quality Characteristics of Two-spotted Cricket (Gryllus bimaculatus) Brown Stock by High Pressure Cooking. Culi. Sci. Hos. Res. 2017, 23, 163–174. [Google Scholar]
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016, 1, 3–8. [Google Scholar] [CrossRef]
- Taslimi, P.; Koksal, E.; Goren, A.C.; Bursal, E.; Aras, A.; Kilic, O.; Alwasel, S.; Gulcin, I. Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Akpan, J.L.; Uwaezuoke, N.J.; Nwobodo, N.N.; Ezeokpo, B.C.; Erhiano, E.; Araromi, E.J.; Ude, U.N.; Abdul, W.M.; Sulaiman, S.A. Effects of Honey on Postprandial Hyperlipidemia and Oxidative Stress in Wistar Rats: Role of HMG-CoA Reductase Inhibition and Antioxidant Effect. Niger. J. Physiol. Sci. 2018, 33, 129–138. [Google Scholar]
- Beatrice, A.G.; Marcella, A.E. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar]
- Wu, H.; Xu, B. Inhibitory effects of onion against α-glucosidase activity and its correlation with phenolic antioxidants. Int. J. Food Prop. 2014, 17, 599–609. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Smid, E.J.; Kleerebezem, M. Production of Aroma Compounds in Lactic Fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Frederic, L.; Jurgen, V.; Luc, D.V. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar]
- Omafuvbe, B.O.; Abiose, S.H.; Shonukan, O.O. Fermentation of soybean (Glycine max) for soy-daddawa production by starter cultures of Bacillus. Food Microbiol. 2002, 19, 561–566. [Google Scholar] [CrossRef]
- Kim, M.S.; Seong, H.Y.; Jang, H.A.; Jeong, Y.H.; Choi, J.A. Novel Lactobacillus plantarum MKHA15 and Uses thereof. Korea Granted Patent. Invertor: Industry-Academic Cooperation Foundation of Dankook University Cheonan Campus. Application number Korea 10-2019-0112122, 10 September 2019. [Google Scholar]
- Lee, S.K.; Lee, J.J.; Jin, Y.I.; Jeong, J.C.; Chang, Y.H.; Lee, Y.S.; Jeong, Y.H.; Kim, M.S. Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT-Food Sci. Technol. 2017, 79, 518–524. [Google Scholar] [CrossRef]
- Li, E.; Yang, H.; Zou, Y.; Wang, H.; Hu, T.; Li, Q.; Liao, S. In-Vitro digestion by simulated gastrointestinal juices of Lactobacillus rhamnosus cultured with mulberry oligosaccharides and subsequent fermentation with human fecal inocula. LWT-Food Sci. Technol. 2019, 101, 61–68. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Dilna, S.V.; Surya, H.; Aswathy, R.G.; Varsha, K.K.; Sakthikumar, D.N.; Pandey, A.; Nampoothiri, K.M. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT-Food Sci. Technol. 2015, 64, 1179–1186. [Google Scholar] [CrossRef]
- Lee, H.J.; Do, J.R.; Jung, S.K.; Kim, H.K. Physiological Properties of Sarcodon aspratus Extracts by Ethanol Concentration. J. Korean Soc. Food Sci. Nutr. 2014, 43, 656–660. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yen, C.L. Antioxidative Ability of Lactic Acid Bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef]
- Obanda, M.; Owuor, P.O. Flavanol Composition and Caffeine Content of Green Leaf as Quality Potential Indicators of Kenyan Black Teas. J. Sci. Food Agric. 1997, 74, 209–215. [Google Scholar] [CrossRef]
- An, B.; Sam, C.; Dries, V.; Ruben, S.; Christel, V.; Mik, V.D.B.; Bart, L.; Leen, V.C. Comparison of Six Commercial Meat Starter Cultures for the Fermentation of Yellow Mealworm (Tenebrio molitor) Paste. Microorganisms 2019, 7, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.J.; Lee, J.Y.; Kim, J.H. Microbial and Physiochemical Properties of Cheonggukjang Fermented Using Bacillus strains with Antibacterial or Antifungal Activities. Food Sci. Biotechnol. 2014, 23, 1525–1532. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shim, J.M.; Liu, X.; Yao, Z.; Lee, K.W.; Cho, K.M.; Kim, G.M.; Shin, J.H.; Kim, J.S.; Kim, J.H. Inhibition of Bacillus cereus in Cheonggukjang Fermentation with Bacillus Starters with Antimicrobial Activities. J. Korean Soc. Food Sci. Nutr. 2016, 45, 736–745. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Jang, H.A.; Kim, M.S. Characteristics of Vegetable Juice Fermented with Lactobacillus plantarum MKHA15 and Leuconostoc mesenteroides MKSR. J. Korean Diet. Assoc. 2019, 25, 281–294. [Google Scholar]
- Nurhayati, R.; Miftakhussolikhah; Frediansyah, A.; Rachmah, D.L. Lactic acid bacteria producing inhibitor of alpha glucosidase isolated from Ganyong (Canna Edulis) and Kimpul (Xanthosoma sagittifolium). Earth Environ. Sci. 2017, 101, 1–5. [Google Scholar] [CrossRef]
- Ramchandran, L.; Shah, N.P. Effect of exopolysaccharides and inulin on the proteolytic, angiotensin-I-converting enzyme- and α-glucosidase-inhibitory activities as well as on textural and rheological properties of low-fat yogurt during refrigerated storage. Dairy Sci. Technol. 2009, 89, 583–600. [Google Scholar] [CrossRef]
- Son, K.H.; Lee, J.Y.; Lee, J.S.; Kang, S.S.; Sohn, H.Y.; Kwon, C.S. Screening of Phenolic Compounds with Inhibitory Activities against HMG-CoA Reductase. J. Life Sci. 2017, 27, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Kim, H.J.; Im, N.K.; Lee, E.J.; Lee, S.P.; Lee, I.S. Antithrombotic and Cholesterol Reduction Effects of Defatted Soybean Grits Fermented by Bacillus subtilis NUC1. Korean J. Food Sci. Technol. 2009, 41, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Tomaro-Duchesneau, C.; Jones, M.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol Assimilation by Lactobacillus Probiotic Bacteria: An In Vitro Investigation. BioMed Res. Int. 2014, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Oh, N.S.; Kim, K.M.; Oh, S.N.; Kim, Y.H. Enhanced Production of Galactooligosaccharides Enriched Skim Milk and Applied to Potentially Synbiotic Fermented Milk with Lactobacillus rhamnosus 4B15. Food Sci. Anim. Resour. 2019, 39, 725–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Kim, J.H.; Lee, S.H.; Cho, E.J.; Kim, H.Y. Determination of Radical Scavenging Activity of Aster yomena (Kitam.) Honda. J. Korean Acad. Industr. Coop. Soc. 2018, 19, 402–407. [Google Scholar]
- Ferreira, I.C.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Kim, S.J.; Han, D.S.; Moon, K.D.; Rhee, J.S. Measurement of Superoxide Dismutase-like Activity of Natural Antioxidants. Biosci. Biotechnol. Biochem. 1995, 59, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Brioukhanov, A.L.; Thauer, R.K.; Netrusov, A.I. Catalase and superoxide dismutase in the cells of strictly anaerobic microorganisms. Microbiology 2002, 71, 281–285. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and Volatilome characterization. Food Chem. 2020, 333, 1–13. [Google Scholar] [CrossRef]
- Mattia, C.D.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant Activities In Vitro of Water and Liposoluble Extracts Obtained by Different Species of Edible Insects and Invertebrates. Front. Nutr. 2019, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Choi, Y.R.; Park, P.J.; Choi, J.H.; Moon, S.H. Purification and characterization of antioxidative peptides from enzymatic hydrolysate of Cod Teiset protein. J. Korean Fish. Soc. 2000, 33, 198–204. [Google Scholar]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; Kadouh, H.; Zhou, K. Phenolic compounds and antioxidant properties of gooseberry as affected by In Vitro digestion. LWT-Food Sci. Technol. 2013, 51, 417–422. [Google Scholar] [CrossRef]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The effect of In Vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT-Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
| Viable Cell Counts (log CFU/mL) | ||
| 0 h 1 | 24 h | |
| CON | 0.00 ± 0.00 b | 8.53 ± 0.19 a |
| SKGB | 5.40 ± 0.14 b | 8.88 ± 0.17 a |
| SEGB | 6.11 ± 0.00 b | 8.72 ± 0.17 a |
| HAGB | 8.22 ± 0.13 b | 9.52 ± 0.17 a |
| LPGB | 7.85 ± 0.00 b | 9.25 ± 0.03 a |
| pH | ||
| 0 h | 24 h | |
| CON | 7.08 a | 6.32 ± 0.21 b |
| SKGB | 7.05 a | 6.27 ± 0.01 b |
| SEGB | 6.72 a | 5.72 ± 0.00 b |
| HAGB | 6.67 a | 3.97 ± 0.02 b |
| LPGB | 6.37 a | 4.27 ± 0.00 b |
| HMG-CoA Reductase Inhibitory Activity (%) | |
|---|---|
| CON | 2.91 ± 6.02 d |
| SKGB | 45.50 ± 5.99 c |
| SEGB | 37.90 ± 2.88 c |
| HAGB | 120.89 ± 0.42 a |
| LPGB | 103.24 ± 9.11 b |
| Atorvastatin (100 mM) | 111.24 ± 8.60 |
| DPPH Radical Scavenging Activity (EC50 mg/mL) | SOD-Like Activity (IC50 mg/mL) | Reducing Power (EC50 mg/mL) | TPC (mg GAE/g) | |
|---|---|---|---|---|
| CON | 19.60 ± 3.94 a | 67.34 ± 8.22 a | 2.06 ± 0.30 a | 91.30 ± 24.18 c |
| SKGB | 8.21 ± 0.24 c | 49.79 ± 6.85 a,b | 0.29 ± 0.02 c | 367.50 ± 3.13 a,b |
| SEGB | 8.88 ± 1.80 c | 46.41 ± 2.64 b,c | 0.88 ± 0.03 b | 393.21 ± 30.81 a |
| HAGB | 17.05 ± 1.10 a,b | 22.37 ± 0.54 d | 0.69 ± 0.04 b | 329.40 ± 7.64 b |
| LPGB | 12.71 ± 0.82 b,c | 30.51 ± 3.06 c,d | 0.94 ± 0.00 b | 409.00 ± 16.00 a |
| DPPH Radical Scavenging Activity (%) | SOD-Like Activity (%) | Reducing Power (% Ascorbic Acid) | TPC (mg GAE/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| CON | 28.88 ± 4.73 A,a | 13.58 ± 3.53 A,b | 9.61 ± 1.94 A,b | 13.84 ± 2.40 A,c | 39.15 ± 2.78 B,b | 60.33 ± 0.71 A,b | 9.13 ± 2.42 A,a | 10.54 ± 0.86 A,b |
| SKGB | 28.55 ± 4.20 A,a | 17.77 ± 0.33 A,b | 17.13 ± 3.34 A,a | 15.34 ± 1.40 A,b,c | 46.47 ± 0.68 B,a | 86.06 ± 0.91 A,a | 1.28 ± 0.03 B,c | 12.48 ± 0.00 A,a |
| SEGB | 1.92 ± 0.87 B,c | 30.49 ± 1.15 A,a | 9.75 ± 3.63 A,b | 7.00 ± 1.79 A,d | 37.71 ± 0.07 A,b | 37.36 ± 0.04 B,d | 5.51 ± 0.24 A,b | 2.38 ± 0.27 B,d |
| HAGB | 12.42 ± 1.32 A,b | 5.62 ± 1.39 B,c | 9.60 ± 2.15 B,b | 21.18 ± 2.47 A,a | 29.28 ± 0.08 B,c | 43.08 ± 0.19 A,c | 0.64 ± 0.02 B,c | 7.99 ± 0.79 A,c |
| LPGB | 2.36 ± 1.76 B,c | 15.97 ± 0.78 A,b | 10.70 ± 2.11 B,b | 19.11 ± 0.50 A,a,b | 23.65 ± 0.56 A,c | 24.73 ± 0.17 A,e | 4.73 ± 0.04 A,b | 1.10 ± 0.07 B,d |
| Ascorbic acid (1 mg/mL) | 97.41 ± 1.94 | 72.15 ± 4.98 | 100 | N.D.4) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Kim, M. Antidiabetic, Anticholesterol, and Antioxidant Activity of Gryllusbimaculatus Fermented by Bacillus and Lactobacillus Strains. Appl. Sci. 2021, 11, 2090. https://doi.org/10.3390/app11052090
Jang H, Kim M. Antidiabetic, Anticholesterol, and Antioxidant Activity of Gryllusbimaculatus Fermented by Bacillus and Lactobacillus Strains. Applied Sciences. 2021; 11(5):2090. https://doi.org/10.3390/app11052090
Chicago/Turabian StyleJang, Hyunah, and Misook Kim. 2021. "Antidiabetic, Anticholesterol, and Antioxidant Activity of Gryllusbimaculatus Fermented by Bacillus and Lactobacillus Strains" Applied Sciences 11, no. 5: 2090. https://doi.org/10.3390/app11052090






