In-Situ Photo-Dissociation and Polymerization of Carbon Disulfide with Vacuum Ultraviolet Microplasma Flat Lamp for Organic Thin Films
Abstract
:1. Introduction
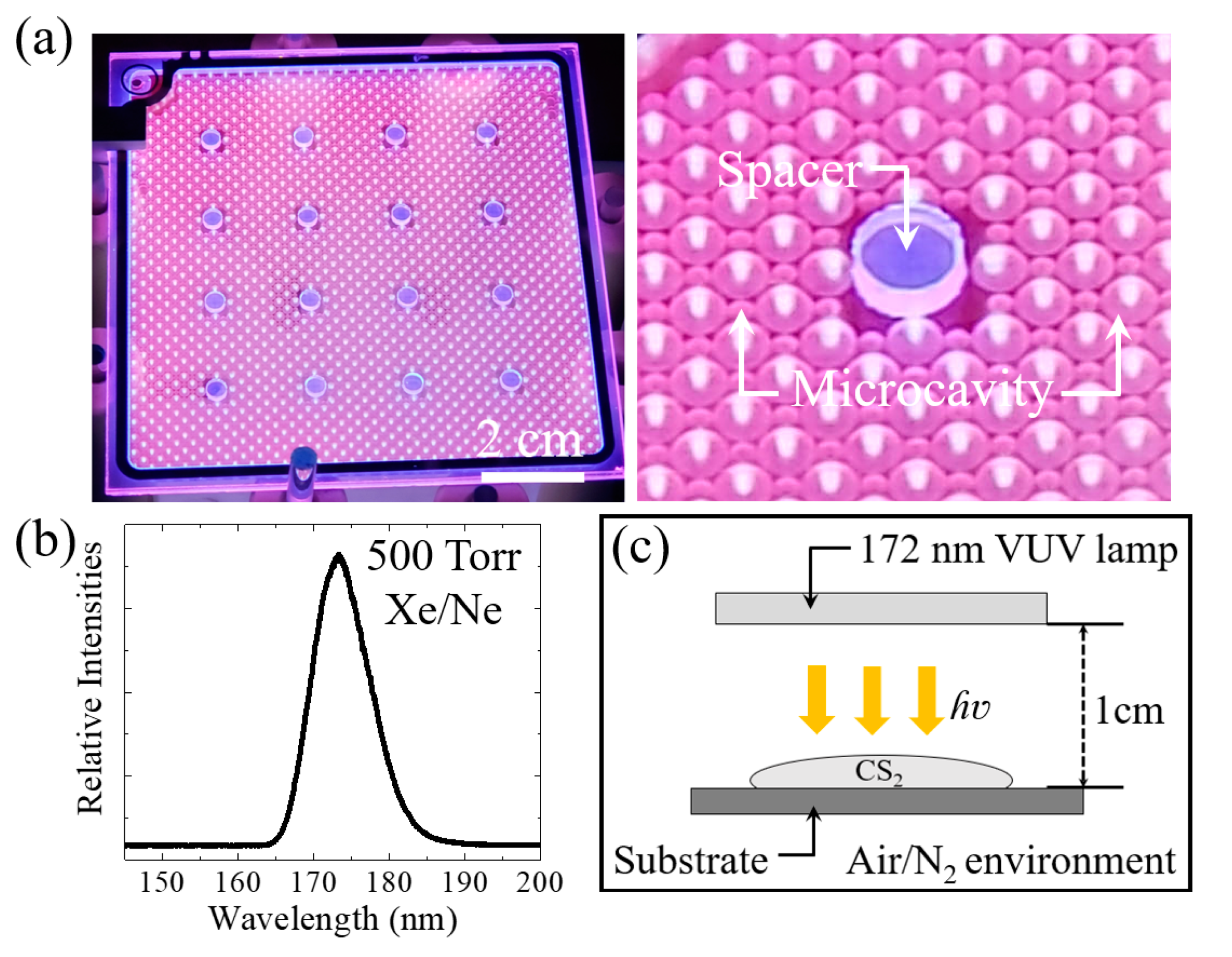
2. Experimental Method
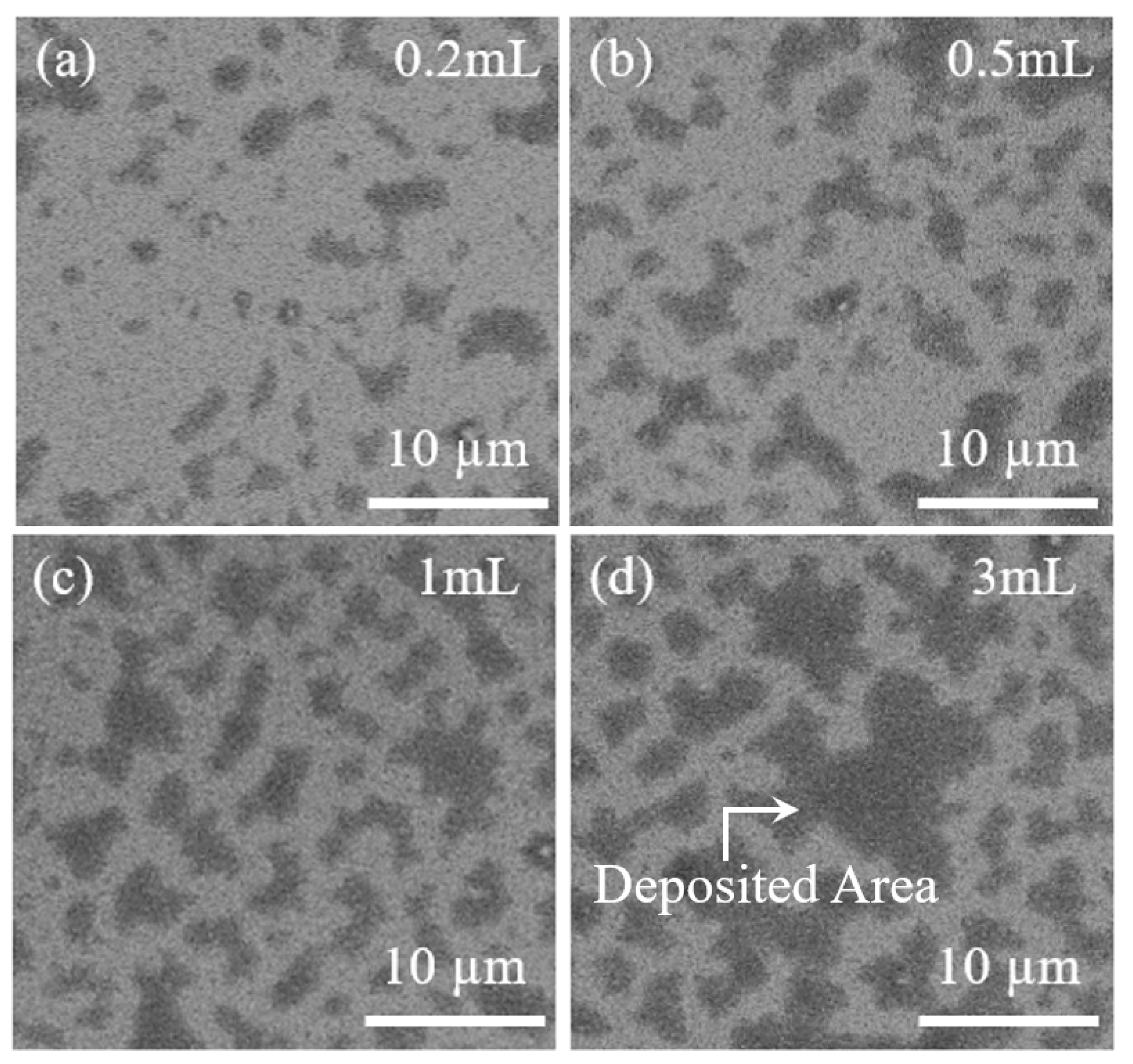
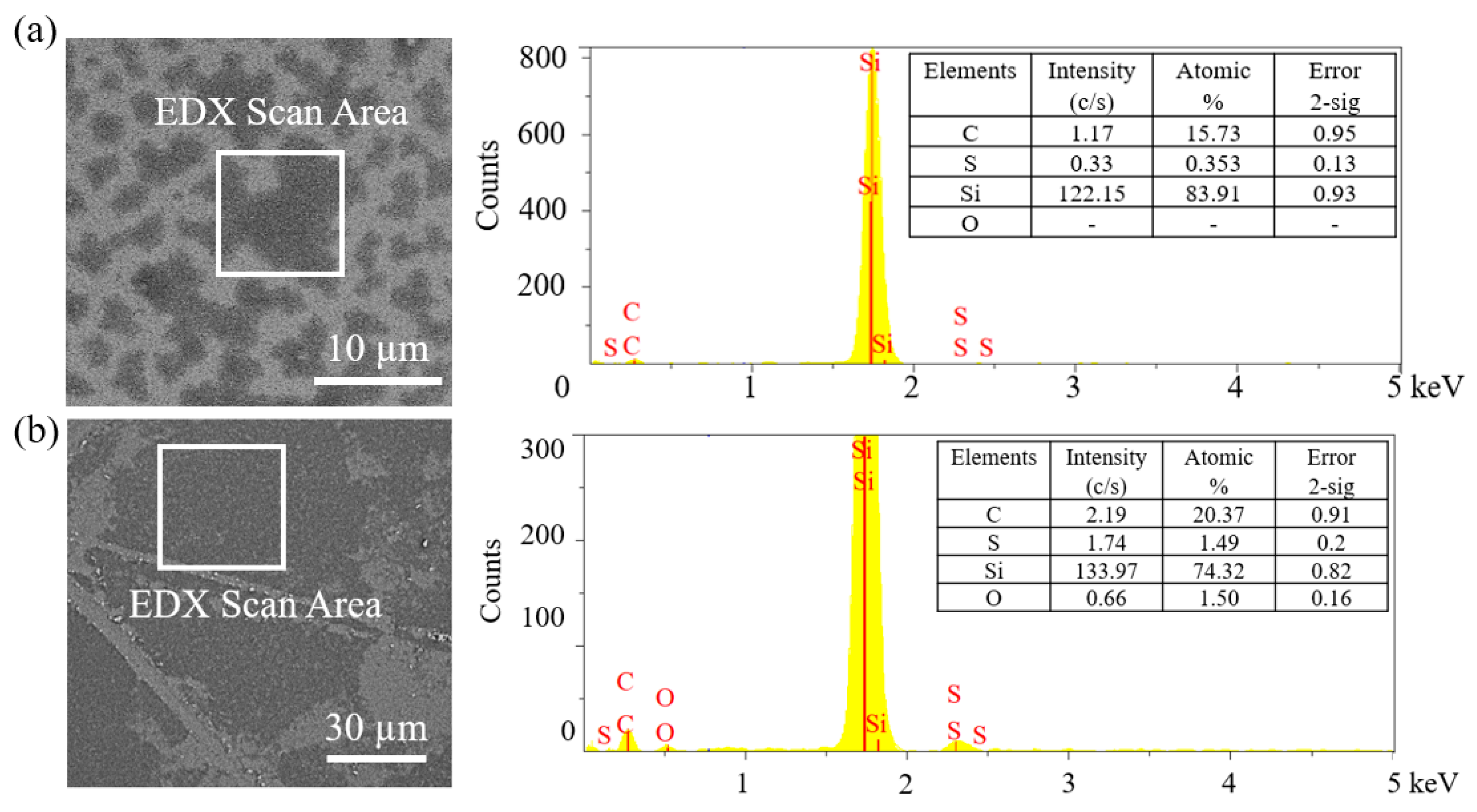
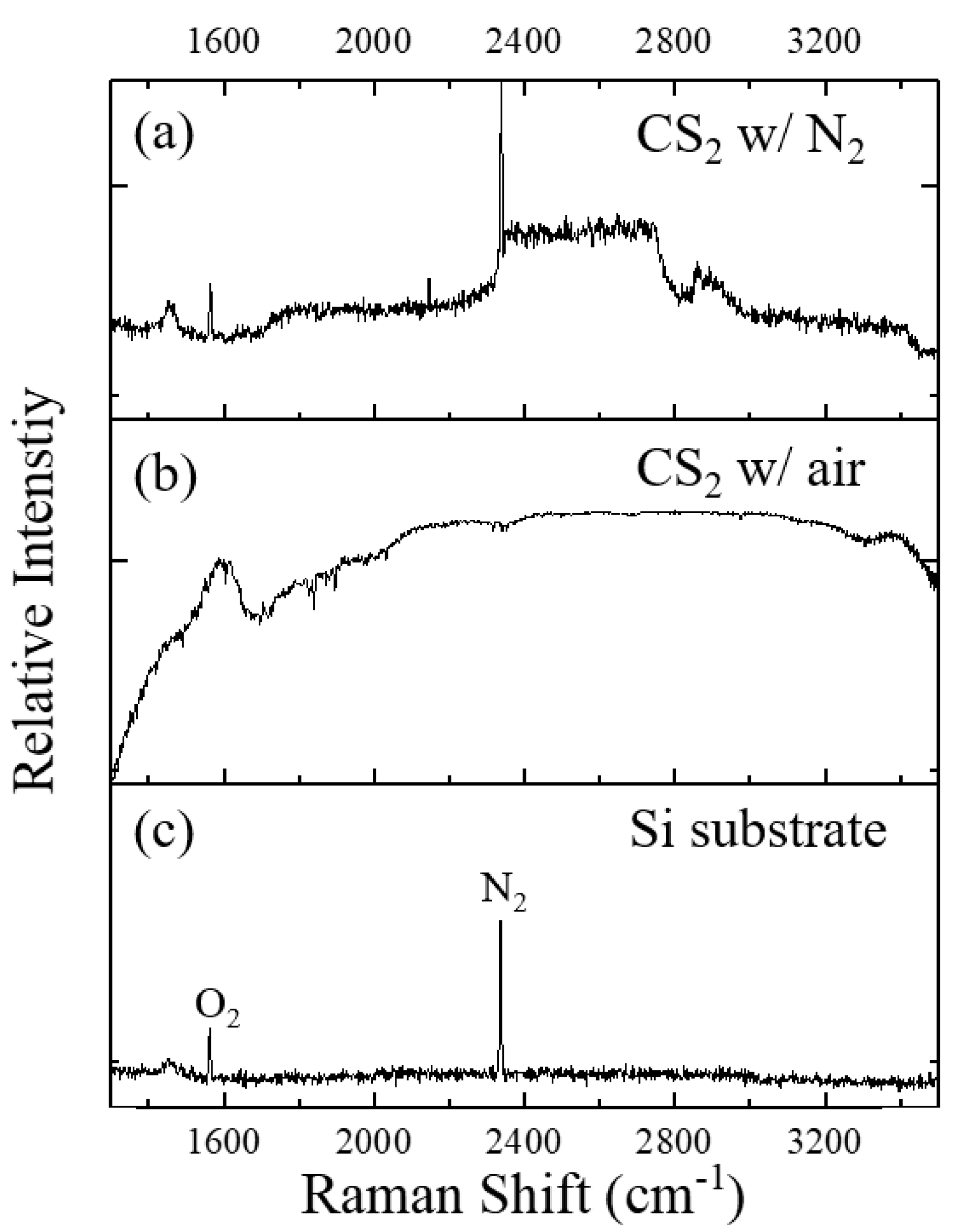
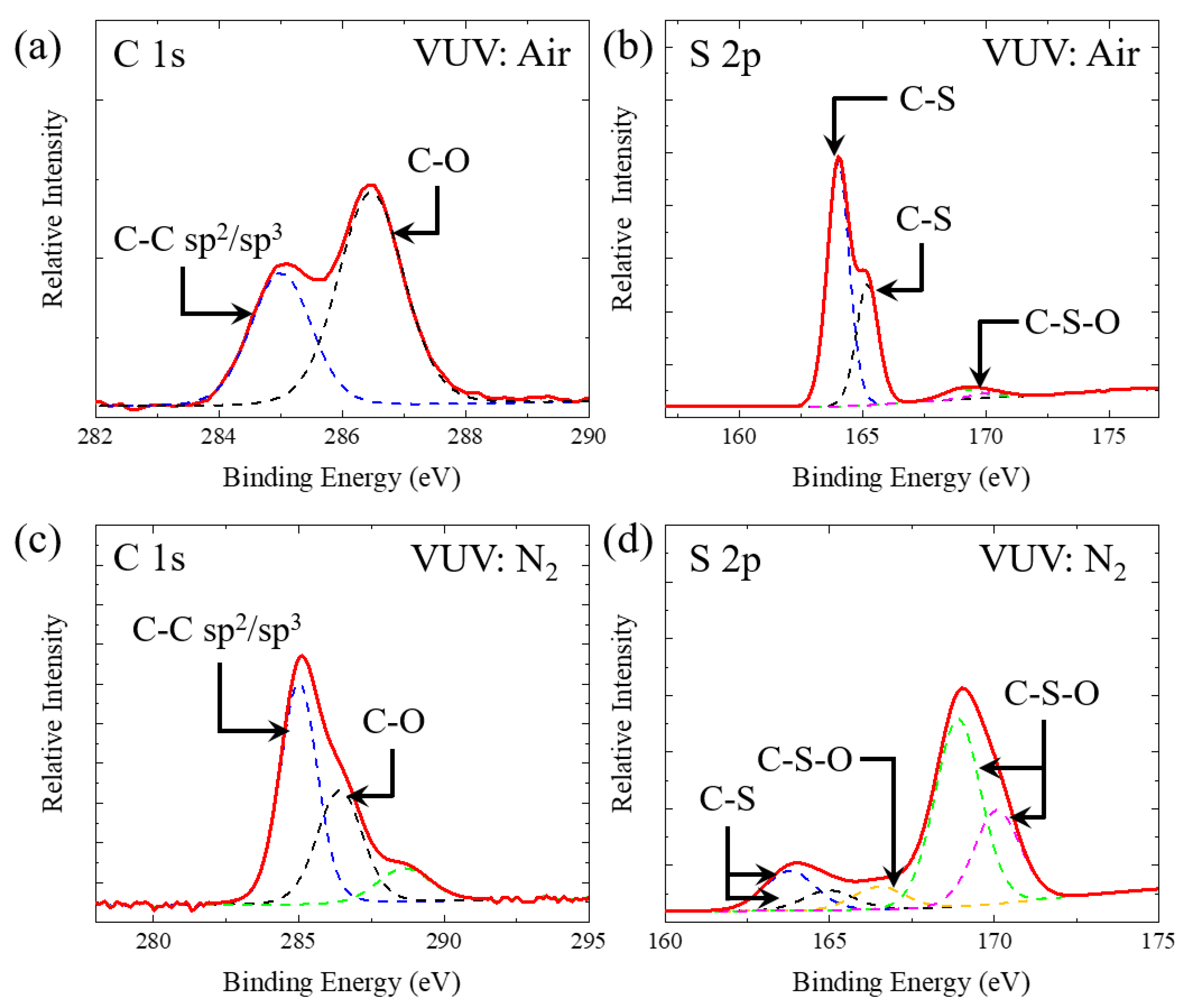
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of Plasma-Enhanced Chemical Vapor Deposition as a Method for Thin-Film Fabrication with Biological Applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef]
- Duty, C.; Jean, D.; Lackey, W. Laser chemical vapour deposition: Materials, modelling, and process control. Int. Mater. Rev. 2001, 46, 271–287. [Google Scholar] [CrossRef]
- Kow-Ming, C.; Wen-Chih, Y.; Chiu-Pao, T. Performance and reliability of low-temperature polysilicon TFT with a novel stack gate dielectric and stack optimization using PECVD nitrous oxide plasma. IEEE Trans. Electron Devices 2004, 51, 63–67. [Google Scholar]
- Fritz, A.K.; Olmer, L.J. Detecting and Preventing Gate Oxide Plasma Damage During PECVD Carbon Deposition Through Surface Photovoltage Measurements. IEEE Trans. Semicond. Manuf. 2017, 30, 426–433. [Google Scholar] [CrossRef]
- Zhiwei, X.; Christopher, B.; Maggie, L.; Chris, N. Plasma damage monitoring for PECVD deposition: A contact potential difference study and device yield analysis. In Proceedings of SPIE; Ajuria, S.A., Hossain, T.Z., Eds.; SPIE: Washington, DC, USA, 1998; Volume 3509. [Google Scholar]
- Park, S.-J.; Herring, C.M.; Mironov, A.E.; Cho, J.H.; Eden, J.G. 25 W of average power at 172 nm in the vacuum ultraviolet from flat, efficient lamps driven by interlaced arrays of microcavity plasmas. APL Photonics 2017, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, B.; Endo, T. Carbon dioxide and carbon disulfide as resources for functional polymers. Prog. Polym. Sci. 2005, 30, 183–215. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Nguyen, L.V.; Kirilenko, D.A.; Brunkov, P.N.; Rudenko, A.A.; Busleev, N.I.; Shakhmin, A.L.; Semencha, A.V.; Khmelnitsky, R.A.; Melnik, N.N.; et al. Large-Scale Laser Fabrication of Antifouling Silicon-Surface Nanosheet Arrays via Nanoplasmonic Ablative Self-Organization in Liquid CS2 Tracked by a Sulfur Dopant. ACS Appl. Nano Mater. 2018, 1, 2461–2468. [Google Scholar] [CrossRef]
- Kim, J.K.; Cho, S.I.; Kim, N.G.; Jhon, M.S.; Min, K.S.; Kim, C.K.; Yeom, G.Y. Study on the etching characteristics of amorphous carbon layer in oxygen plasma with carbonyl sulfide. J. Vac. Sci. Technol. A 2013, 31, 021301. [Google Scholar] [CrossRef] [Green Version]
- Colman, J.J.; Trogler, W.C. Photopolymerization of Carbon Disulfide Yields the High-Pressure-Phase (CS2)x. J. Am. Chem. Soc. 1995, 117, 11270–11277. [Google Scholar] [CrossRef]
- Heymann, D.; Cataldo, F.; Thiemens, M.H.; Fokkens, R.; Nibbering, N.M.M.; Vis, R.D. Formation of CmSn compounds by photopolymerization of CS2 in the atmosphere of Jupiter. Meteorit. Planet. Sci. 2000, 35, 355–361. [Google Scholar] [CrossRef]
- TAnderson, S.; Ma, J.H.; Park, S.; Eden, J.G. Multichannel Microchemical Reactor Comprising Replica-Molded Microplasma Devices: Chemiluminescence and Sulfur Deposition in Ar/CS2 Flows. IEEE Trans. Plasma Sci. 2008, 36, 1250–1251. [Google Scholar] [CrossRef]
- Horio, T.; Spesyvtsev, R.; Furumido, Y.; Suzuki, T. Real-time detection of S(1D2) photofragments produced from the 1B2(1Σu+) state of CS2 by vacuum ultraviolet photoelectron imaging using 133 nm probe pulses. J. Chem. Phys. 2017, 147, 013932. [Google Scholar] [CrossRef] [Green Version]
- Heicklen, J.; Olszyna, K.J. Reaction of ozone with carbon disulfide. J. Phys. Chem. 1970, 74, 4188–4198. [Google Scholar] [CrossRef]
- Yan, X.; Sun, Y.; Zhu, T.; Fan, X. Conversion of carbon disulfide in air by non-thermal plasma. J. Hazard. Mater. 2013, 261, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Raman and Infrared Spectra of Crystalline Carbon Disulfide. J. Chem. Phys. 1965, 42, 815. [Google Scholar] [CrossRef]
- Wang, D.; Mittauer, K.; Reynolds, N. Raman scattering of carbon disulfide: The temperature effect. Am. J. Phys. 2009, 77, 1130–1134. [Google Scholar] [CrossRef]
- Hoffmann, G.G.G.; De With, G.; Loos, J.J. Micro-Raman and Tip-Enhanced Raman Spectroscopy of Carbon Allotropes. Macromol. Symp. 2008, 265, 1–11. [Google Scholar] [CrossRef]
- Miranda, A.M.; Castilho-Almeida, E.W.; Ferreira, E.H.M.; Moreira, G.F.; Achete, C.A.; Armond, R.A.; Dos Santos, H.F.; Jorio, A. Line shape analysis of the Raman spectra from pure and mixed biofuels esters compounds. Fuel 2014, 115, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Bock, P.; Gierlinger, N. Infrared and Raman spectra of lignin substructures: Coniferyl alcohol, abietin, and coniferyl aldehyde. J. Raman Spectrosc. 2019, 50, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Howell, N.K.; Arteaga, G.; Nakai, S.; Li-Chan, E.C.Y. Raman Spectral Analysis in the C−H Stretching Region of Proteins and Amino Acids for Investigation of Hydrophobic Interactions. J. Agric. Food Chem. 1999, 47, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.M.; Milosavljević, V.; Žekić, A.; Romčević, N.; Daniels, S. Raman scattering analysis of silicon dioxide single crystal treated by direct current plasma discharge. Appl. Phys. Lett. 2011, 98, 051503. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Roy, M.; Mandal, B.P.; Dey, G.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2008, 19, 075103. [Google Scholar] [CrossRef]
- Spizzirri, P.; Fang, J.; Rubanov, S.; Gauja, E.; Prawer, S. Nano-Raman spectroscopy of silicon surfaces. arXiv 2010, arXiv:1002.2692. [Google Scholar]
- Hyatt, H.A.; Cherlow, J.M.; Fenner, W.R.; Porto, S.P.S. Cross section for the Raman effect in molecular nitrogen gas. J. Opt. Soc. Am. 1973, 63, 1604–1606. [Google Scholar] [CrossRef]
- Odhner, J.H.; Romanov, D.A.; Levis, R.J. Filament-based stimulated Raman spectroscopy. In Proceedings of the SPIE LASE, San Francisco, CA, USA, 17 February 2010; SPIE—International Society for Optical Engineering: Washington, DC, USA, 2010; p. 75820M. [Google Scholar]
- Dwivedi, N.; Yeo, R.J.; Satyanarayana, N.; Kundu, S.; Tripathy, S.; Bhatia, C.S. Understanding the Role of Nitrogen in Plasma-Assisted Surface Modification of Magnetic Recording Media with and without Ultrathin Carbon Overcoats. Sci. Rep. 2015, 5, 7772. [Google Scholar] [CrossRef] [Green Version]
- Pathem, B.K.; Guo, X.-C.; Rose, F.; Wang, N.; Komvopoulos, K.; Schreck, E.; Marchon, B. Carbon Overcoat Oxidation in Heat-Assisted Magnetic Recording. IEEE Trans. Magn. 2013, 49, 3721–3724. [Google Scholar] [CrossRef]
- Goohpattader, P.S.; Dwivedi, N.; Rismani-Yazdi, E.; Satyanarayana, N.; Yeo, R.J.; Kundu, S.; Bhatia, C. Probing the role of C+ ion energy, thickness and graded structure on the functional and microstructural characteristics of ultrathin carbon films (<2 nm). Tribol. Int. 2015, 81, 73–88. [Google Scholar]
- Sun, D.; Ban, R.; Zhang, P.-H.; Wu, G.-H.; Zhang, J.-R.; Zhu, J.-J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Ye, J.; He, F.; Nie, J.; Cao, Y.; Yang, H.; Ai, X. Sulfur/carbon nanocomposite-filled polyacrylonitrile nanofibers as a long life and high capacity cathode for lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 7406–7412. [Google Scholar] [CrossRef]
- Wang, X.; Gao, T.; Han, F.; Ma, Z.; Zhang, Z.; Li, J.; Wang, C. Stabilizing high sulfur loading Li–S batteries by chemisorption of polysulfide on three-dimensional current collector. Nano Energy 2016, 30, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Gerin, P.; Dengis, P.; Rouxhet, P. Performance of XPS analysis of model biochemical compounds. J. Chim. Phys. 1995, 92, 1043–1065. [Google Scholar] [CrossRef]
- Rich, A.L.; Patel, J.T. Carbon Disulfide (CS2) Mechanisms in Formation of Atmospheric Carbon Dioxide (CO2) Formation from Unconventional Shale Gas Extraction and Processing Operations and Global Climate Change. Environ. Health Insights 2015, 9, EHI.S15667. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, S.-J. In-Situ Photo-Dissociation and Polymerization of Carbon Disulfide with Vacuum Ultraviolet Microplasma Flat Lamp for Organic Thin Films. Appl. Sci. 2021, 11, 2597. https://doi.org/10.3390/app11062597
Kim J, Park S-J. In-Situ Photo-Dissociation and Polymerization of Carbon Disulfide with Vacuum Ultraviolet Microplasma Flat Lamp for Organic Thin Films. Applied Sciences. 2021; 11(6):2597. https://doi.org/10.3390/app11062597
Chicago/Turabian StyleKim, Jinhong, and Sung-Jin Park. 2021. "In-Situ Photo-Dissociation and Polymerization of Carbon Disulfide with Vacuum Ultraviolet Microplasma Flat Lamp for Organic Thin Films" Applied Sciences 11, no. 6: 2597. https://doi.org/10.3390/app11062597





