Effects of Aging on the Dry Shrinkage Cracking of Lime Soils with Different Proportions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
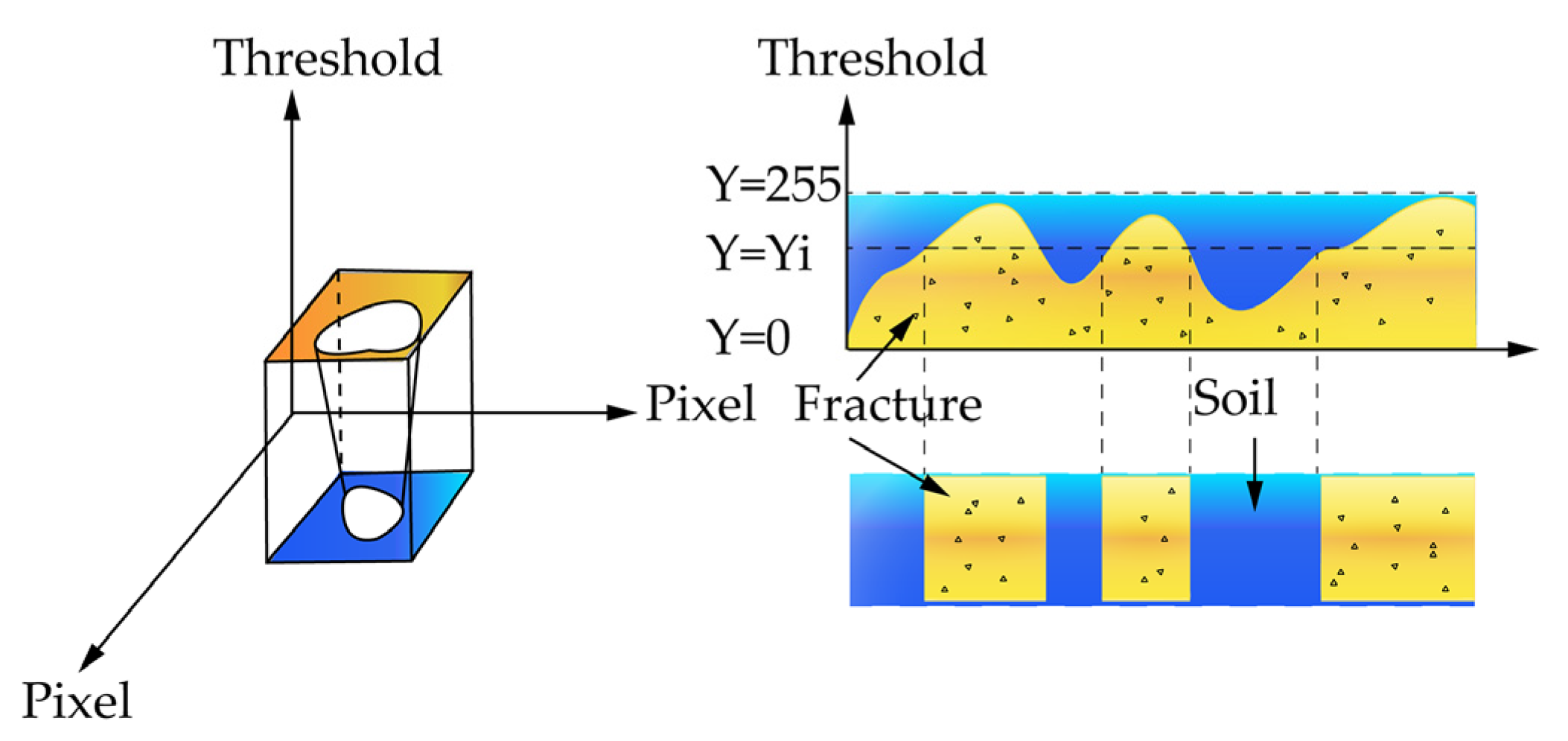
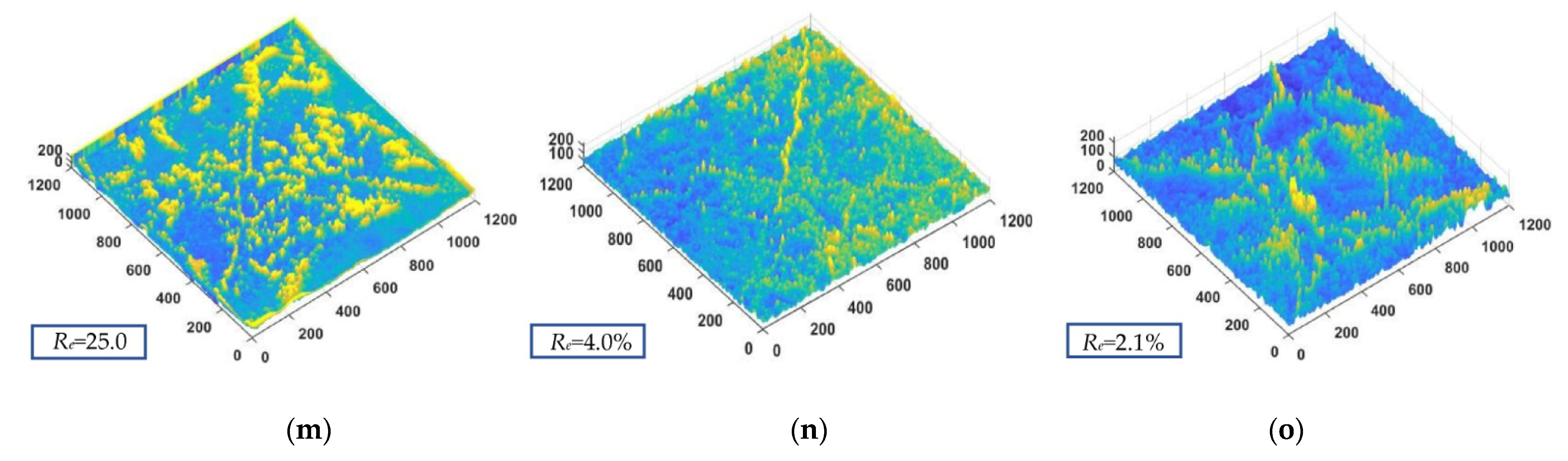
2.3. Three-Dimensional (3D) Fracture Image Processing
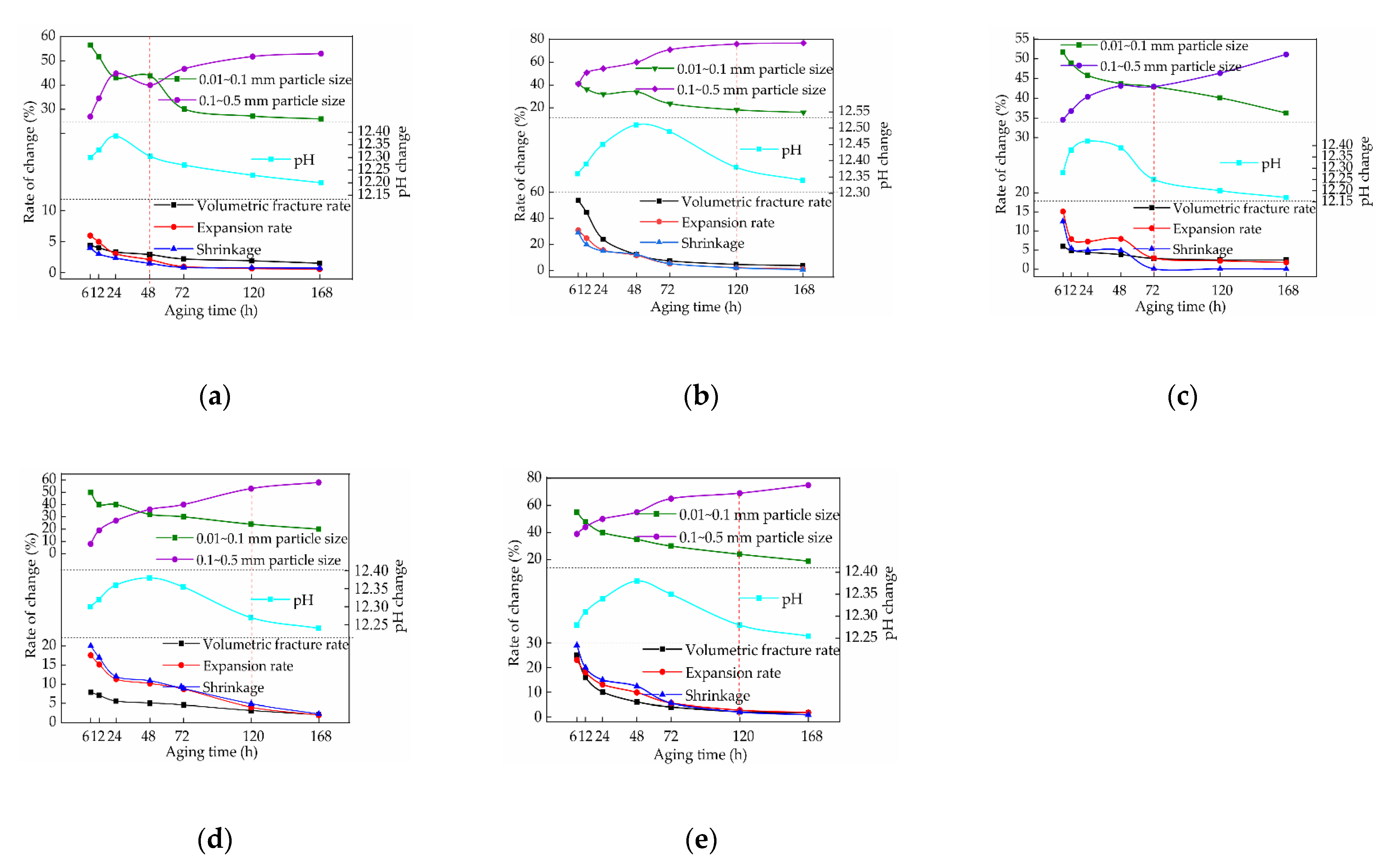
3. Results
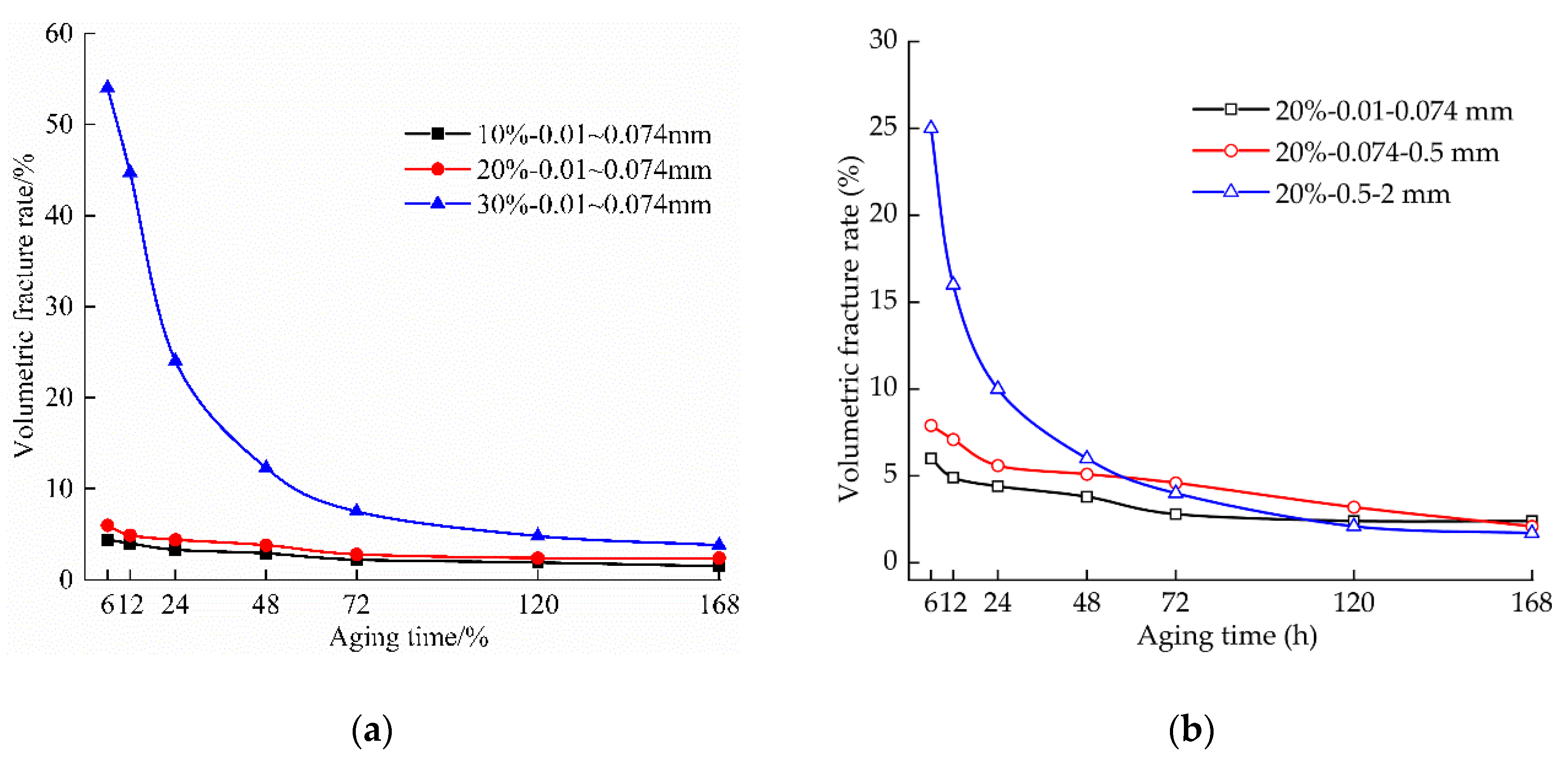
3.1. Effect of Aging on the Volume Crack Rate of the Samples
3.1.1. Effect of the Aging Time on the Volume Crack Rate of the Samples
3.1.2. Effect of the Lime Content on the Volume Crack Rate of the Samples
3.1.3. Effect of the Lime Particle Size on the Volume Crack Rate of the Samples
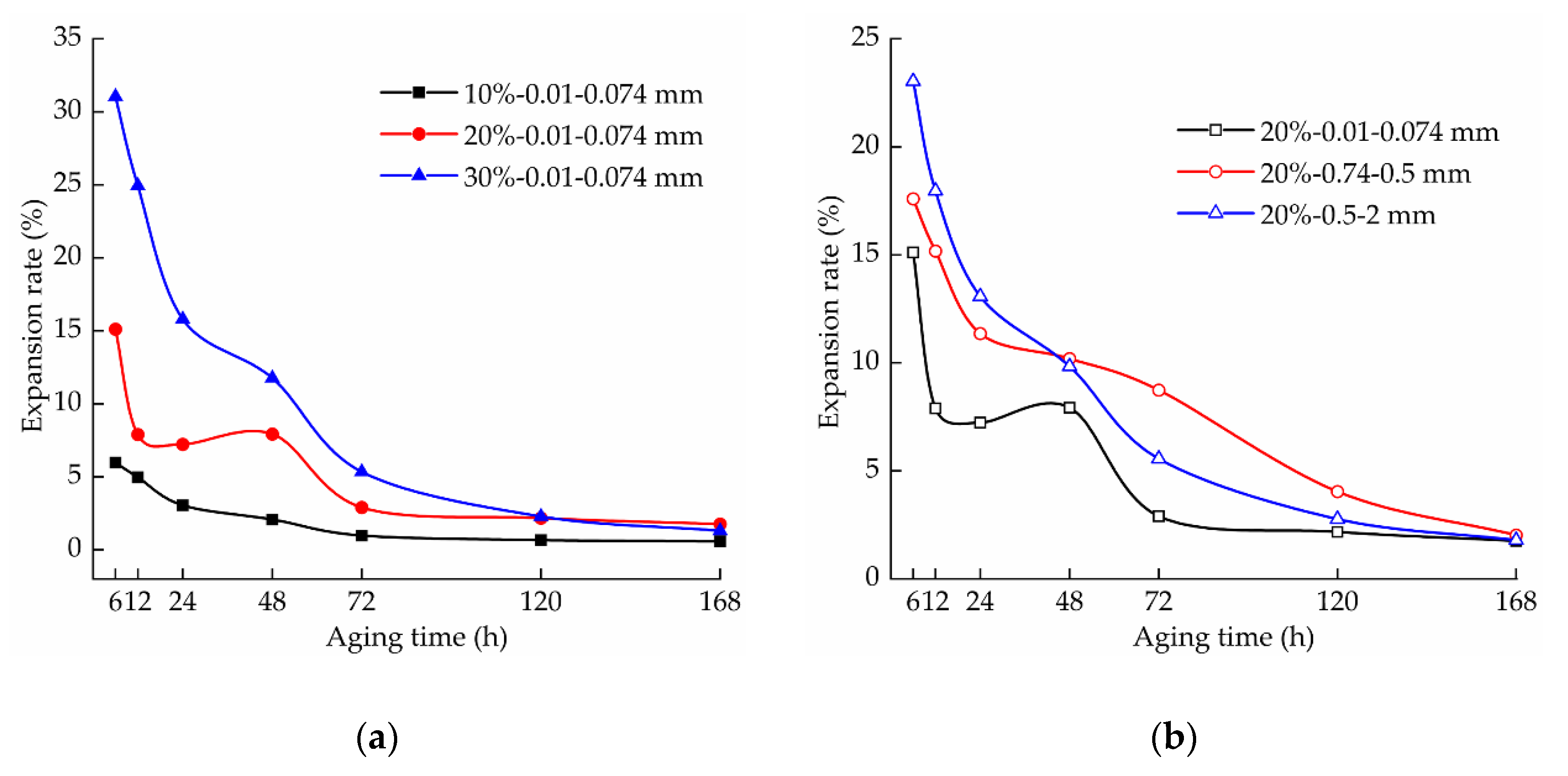
3.2. Effect of Aging on the Expansion of the Samples
3.2.1. Effect of the Aging Time on the Expansion of the Samples
3.2.2. Effect of the Lime Content on the Expansion of the Samples
3.2.3. Effect of the Lime Particle Size on the Expansion of the Samples
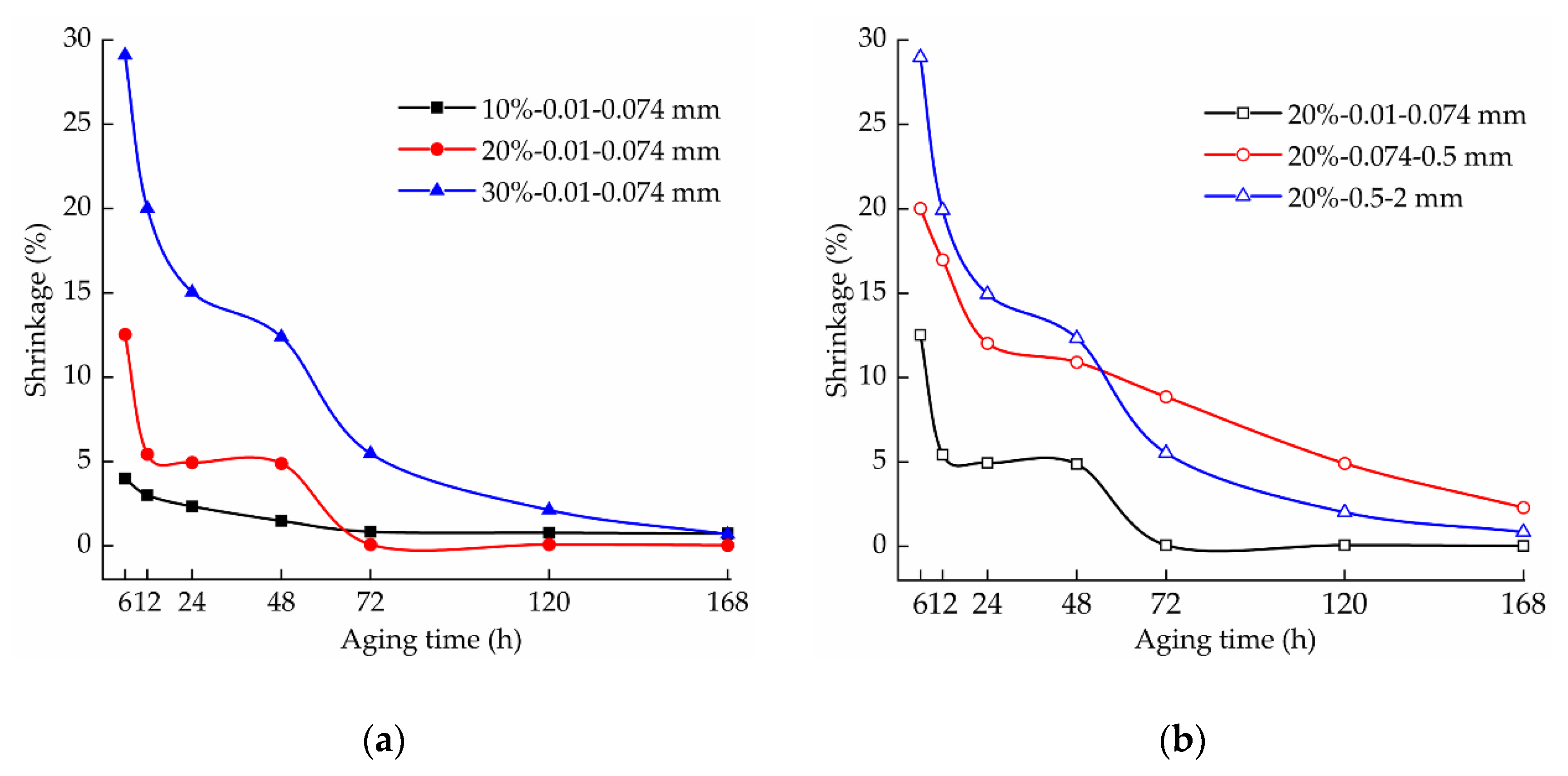
3.3. Effect of Aging on the Shrinkage of the Samples
3.3.1. Effect of Aging Time on the Shrinkage of the Samples
3.3.2. Effect of the Lime Content on the Shrinkage of the Samples
3.3.3. Effect of the Lime Particle Size on the Sample Shrinkage
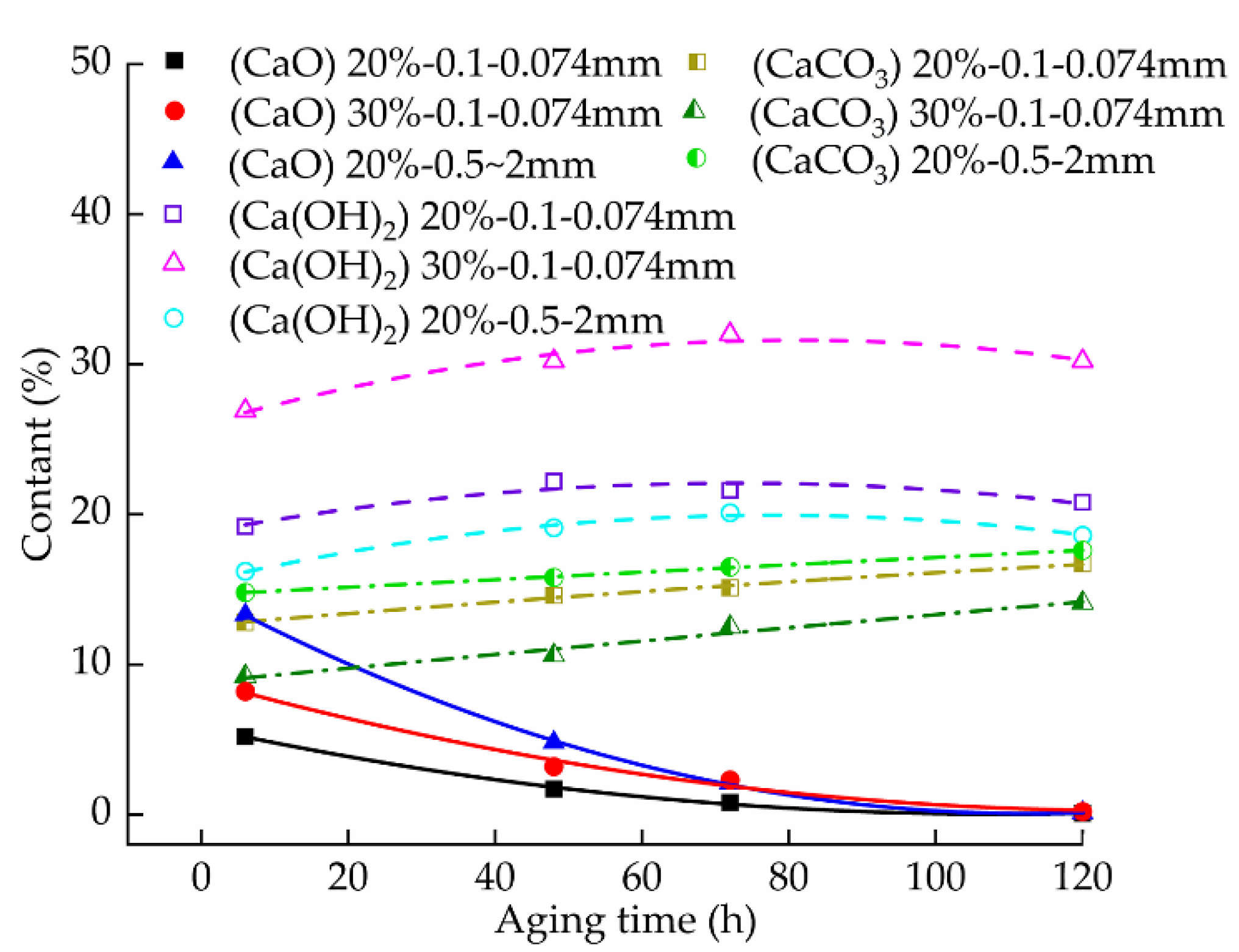
3.4. Effect of Aging on the pH of the Samples
3.4.1. Effect of the Aging Time on the pH of the Samples
3.4.2. Effect of the Lime Content on the pH of the Samples
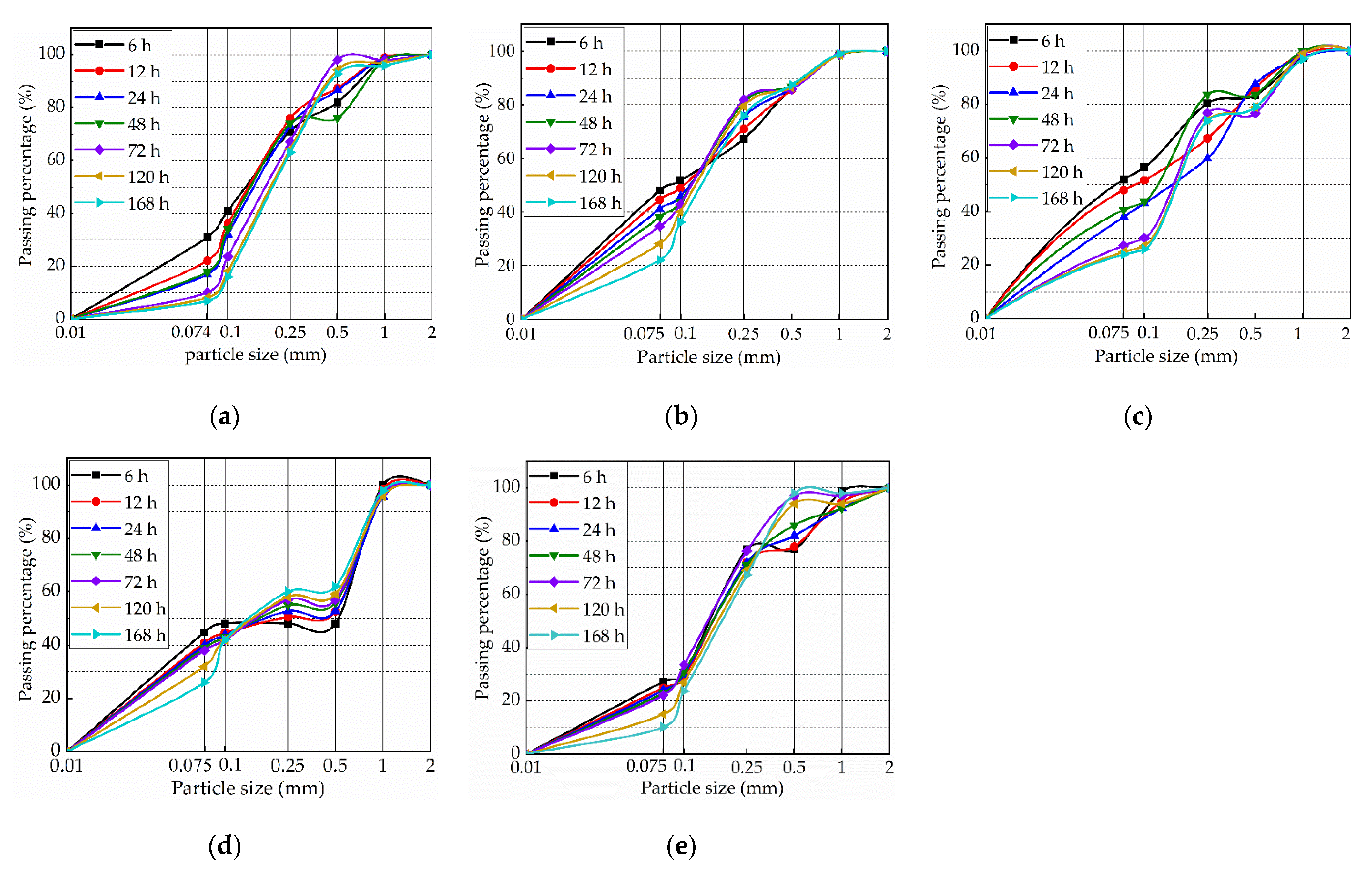
3.5. Effect of Aging on the Particle Distribution of the Samples
3.5.1. Effect of the Aging Time on the Particle Size of the Samples
3.5.2. Effect of the Lime Content on the Particle Size of the Samples
3.5.3. Effect of the Lime Particle Size on the Particle Size of the Samples
4. Discussion
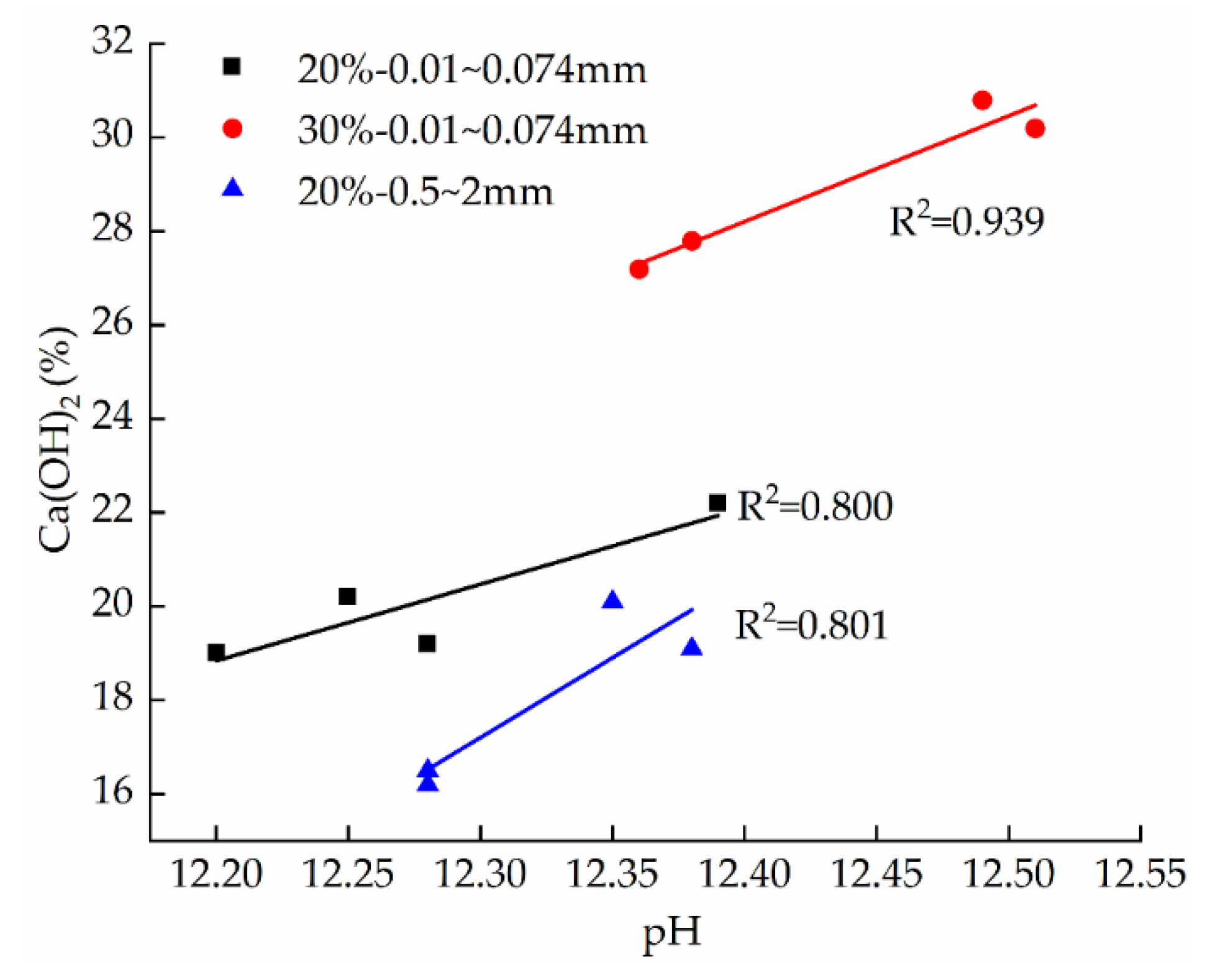
4.1. Effect of pH on the Dry Shrinkage Cracking of the Samples
4.2. Effect of the Particle Distribution on the Dry Shrinkage Cracking of the Samples
4.3. Effects of pH and Particle Size on the Reasonable Aging Time of the Lime Soil Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, G.; Germinario, C.; Grifa, C.; Ma, X. Characterization of ancient building lime mortars of Anhui Province of China: A multi-analytical approach. Archaeometry 2020, 62, 888–903. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Colantuono, A.; Vecchio, S.D.; Mascolo, G. Self-Assembled 3D Portlandite Crystals upon Aging of Lime Putty. J. Comput. Theor. Nanosci. 2017, 23, 5938–5940. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, N.; Zhang, L.; Rong, H.; Wei, C.J. Effect of modified materials on hygrothermal properties of raw soil blocks. J. Bulid. Mater. 2021, 24, 313–317. [Google Scholar]
- Giulia, G.; Costanza, C.; Manuela, C.; Dimitri, D.; Michaël, P.; Giacomo, R.; Enza, V. Multi-scale analysis of the mechanical improvement induced by lime addition on a pyroclastic soil. Eng. Geol. 2017, 221, 193–201. [Google Scholar]
- Branco, F.C.; Belgas, M.D.; Mendes, C.; Pereira, L.; Ortega, J.M. Mechanical Performance of Lime Mortar Coatings for Rehabilitation of Masonry Elements in Old and Historical Buildings. Sustainability 2021, 13, 3281. [Google Scholar] [CrossRef]
- Yue, J.W.; Lin, J.; Wang, Y.F.; Liu, T.J.; Zhang, Y.F.; Wang, S.Y.; Wang, W.Z. Study on improvement of soil water physical properties of Kaifeng imitation site. Adv. Eng. Sci. 2020, 52, 46–55. [Google Scholar]
- Cecconi, M.; Cambi, C.; Carrisi, S.; Deneele, D.; Vitale, E.; Russo, G. Sustainable Improvement of Zeolitic Pyroclastic Soils for the Preservation of Historical Sites. Appl. Sci. 2020, 10, 899. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Park, J.H.; Pham, K.V.A.; Lee, C.H.; Lee, K. Experimental Investigation of Traditional Clay Brick and Lime Mortar Intended for Restoration of Cultural Heritage Sites. Appl. Sci. 2021, 11, 6228. [Google Scholar] [CrossRef]
- Arizzi, A.; Viles, H.; Cultrone, G. Experimental testing of the durability of lime based mortars used for rendering historic buildings. Constr. Build. Mater. 2012, 28, 807–818. [Google Scholar] [CrossRef]
- Kong, D.; Chen, J.; Wan, R.; Liu, H. Study on restoration materials for historical silty earthen Sites based on lime and starch ether. Adv. Mater. Sci. Eng. 2020, 6, 2850780. [Google Scholar] [CrossRef]
- Consoli, N.C.; Marin, E.; Samaniego, R.; Filho, H.; Cristelo, N. Field and laboratory behaviour of fine-grained soil stabilized with lime. Can. Geotech. J. 2020, 57, 1–19. [Google Scholar] [CrossRef]
- Stoltz, G.; Cuisinier, O.; Masrouri, F. Multi-scale analysis of the swelling and shrinkage of a lime-treated expansive clayey soil. Appl. Clay Sci. 2012, 61, 44–51. [Google Scholar] [CrossRef]
- Cui, Y.J.; Tang, A.M.; Xu, M.N. Effects of the maximum soil aggregates size and cyclic wetting–drying on the stiffness of a lime-treated clayey soil. Géotechnique 2011, 61, 421–429. [Google Scholar]
- Zhang, S.B.; Xie, J.B.; Jiang, S.; Shi, L.F.; Wang, S.; Wu, Y.C. Study on the development law of dry shrinkage cracks in lime improved expansive soil. Contrib. Technol. 2018, 47, 1247–1249. [Google Scholar]
- Elert, K.; Rodriguez, N.C.; Pardo, E.S. Lime mortars for the conservation of historic buildings. Stud. Conserv. 2002, 47, 62–75. [Google Scholar]
- Grilo, J.; Santos, S.A.; Faria, P. Mechanical and mineralogical properties of natural hydraulic lime metakaolin mortars in different curing conditions. Constr. Build. Mater. 2014, 51, 287–294. [Google Scholar] [CrossRef]
- Cui, K.; Feng, F.; Chen, W.W.; Wang, X.H.; Cheng, F.Q. Study on physical compatibility of fissure grouting slurry stone in soil sites with quicklime as admixture. Rock. Soil. Mech. 2019, 40, 4627–4636. [Google Scholar]
- State Administration of Cultural Relics. Code for Design of Protection and Reinforcement of Soil Sites in Dry Environment: GB/T 36747—2018; State Administration of Market Supervision and Administration; China National Standardization Administration: Beijing, China, 2018; pp. 1–16.
- Mascolo, G.; Mascolo, M.C.; Vitale, A.; Marino, O. Microstructure evolution of lime putty upon aging. J. Cryst. Growth 2010, 312, 2363–2368. [Google Scholar] [CrossRef]
- Margalha, M.G.; Silva, A.S.; Maria, D.R.V.; Ball, R.J.; Allen, G.C. Microstructural Changes of Lime Putty during Aging. J. Mater. Civ. Eng. 2013, 25, 1524–1532. [Google Scholar] [CrossRef]
- Maafi, N.; Akchiche, M.; Sara, R. Wetting and Drying Compacted Soil-Lime Mixtures. In Proceedings of the International Congress and Exhibition “Sustainable Civil Infrastructures: Innovative Infrastructure Geotechnology”, Sharm El Sheikh, Egypt, 15–19 July 2017; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Wei, G.F.; Zhang, B.J.; Fang, S.Q. Study on lime aging mechanism and its application in cultural relics protection. J. Bulid. Mater. 2012, 15, 96–102. [Google Scholar]
- Beruto, D.T.; Barberis, F.; Botter, R. Calcium carbonate binding mechanism in the setting of calcium and calcium–magnesium putty lime. J. Cult. Herit. 2005, 6, 253–260. [Google Scholar] [CrossRef]
- Kang, S.H.; Wang, H.; Jong, K.; Won, K.; Yang, H. Effects of Amount of Slaking Water on Physical and Chemical Properties of Handmade Hydrated Lime used for Preservation of Architectural Heritage. J. Archttectural Inst. Korea Struct. Constr. 2019, 35, 142–149. [Google Scholar]
- Khattab, S.; Hussein, Y.A. On the Durability of fine Grained Soils Stabilized with Lime. Al-Rafadain Eng. J. 2012, 20, 85–92. [Google Scholar] [CrossRef]
- Gu, L.; Liu, R.J.; Guo, Z.W. Effect of lime digestion conditions on calcium hydroxide activity. C Power Sci. Technol. 2012, 18, 62–69. [Google Scholar]
- Sweeney, D.A.; Wong, D.; Fredlund, D.G. Effect of lime on highly plastic clay with special emphasis on aging. Transp. Res. Rec. 1988, 1190, 13–23. [Google Scholar]
- Hengique, H.M.; Santos, L.C.; Parreira, P.M. Production of milk of lime for sugar cane industry: Study of factors influencing lime slaking. Mater. Sci. Forum. 2010, 660–661, 437–442. [Google Scholar] [CrossRef]
- Pakbaz, M.S.; Farzi, M. Comparison of the effect of mixing methods (dry vs. wet) on mechanical and hydraulic properties of treated soil with cement or lime. Appl. Clay Sci. 2014, 105–106, 156–169. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Yu, B.; Zheng, A.; Fu, W.; Zhang, H.; Wan, Z. Long term carbonation effect of lime stabilized red clay strength. J. Bulid. Mater. 2013, 34, 73–79. [Google Scholar]
- Jha, A.K.; Sivapullalah, P.V. Susceptibility of strength development by lime in gypsiferous soil—A micro mechanistic study. Appl. Clay Sci. 2015, 115, 9–50. [Google Scholar] [CrossRef]
- Li, X.M.; Lu, G.Y.; Zhang, H.Y.; Yi, S.; Ren, K.S. Strength characteristics and micro mechanism of lime metakaolin improved silty sand. J. Bulid. Mater. 2020, 20, 1–14. [Google Scholar]
- Feng, H.P.; Ma, D.L.; Liu, Q.Y.; Ye, C.L. Quantitative calculation method of 3D apparent porosity of soil based on SEM images. J. Bulid. Mater. 2019, 41, 574–580. [Google Scholar]
- Moretti, L.; Confificconi, M.; Natali, S.; Andrea, A. Statistical analyses of SEM-EDS results to predict the quantity of added quicklime in a treated clayey soil. Constr. Build. Mater. 2020, 10, 2569–2574. [Google Scholar] [CrossRef]
- Jha, A.K.; Sivapullaiah, P.V. Lime Stabilization of Soil: A Physico-Chemical and Micro-Mechanistic Perspective. Indian. Geotech. J. 2019, 50, 339–347. [Google Scholar] [CrossRef]
- Priyadarshee, A.; Chandra, S.; Kumar, V. Performance of grass ash with mix of black cotton soil and lime. Innov. Infrastruct. Solut. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.J.; Cui, Y.J.; Tang, A.M.; Tang, C.S.; Benahmed, N. Changes in thermal conductivity, suction and microstructure of a compacted lime-treated silty soil during curing. Eng. Geol. 2016, 202, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.X.; He, F.J.; Zhu, J.Y. Performance and mechanism of CO2 carbonated slag-CaO- MgO-solidified soils. Acta. Geotech. 2019, 41, 2197–2206. [Google Scholar]
- Wang, Y.; Cui, Y.J.; Tang, A.M.; Tang, C.S.; Benahmed, N. Effects of aggregate size on water retention capacity and microstructure of lime-treated silty soil. Géotech. Lett. 2015, 5, 269–274. [Google Scholar] [CrossRef]









| Lime Content (%) | Lime Particle Size (mm) |
|---|---|
| 10 | 0.01–0.074 |
| 20 | |
| 30 | |
| 20 | 0.074–0.5 |
| 20 | 0.5–2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.; Chen, Y.; Zhao, L.; Wang, S.; Su, H.; Yang, X.; Gao, H.; Zhang, Y.; Li, W. Effects of Aging on the Dry Shrinkage Cracking of Lime Soils with Different Proportions. Appl. Sci. 2022, 12, 145. https://doi.org/10.3390/app12010145
Yue J, Chen Y, Zhao L, Wang S, Su H, Yang X, Gao H, Zhang Y, Li W. Effects of Aging on the Dry Shrinkage Cracking of Lime Soils with Different Proportions. Applied Sciences. 2022; 12(1):145. https://doi.org/10.3390/app12010145
Chicago/Turabian StyleYue, Jianwei, Ying Chen, Limin Zhao, Siyuan Wang, Huicong Su, Xue Yang, Huijie Gao, Yiang Zhang, and Wenhao Li. 2022. "Effects of Aging on the Dry Shrinkage Cracking of Lime Soils with Different Proportions" Applied Sciences 12, no. 1: 145. https://doi.org/10.3390/app12010145






