Screening of Plants and Indigenous Bacteria to Improve Arsenic Phytoextraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Soil Characterization and Evaluation of As Bioavailability
2.3. Phytotoxicity Tests
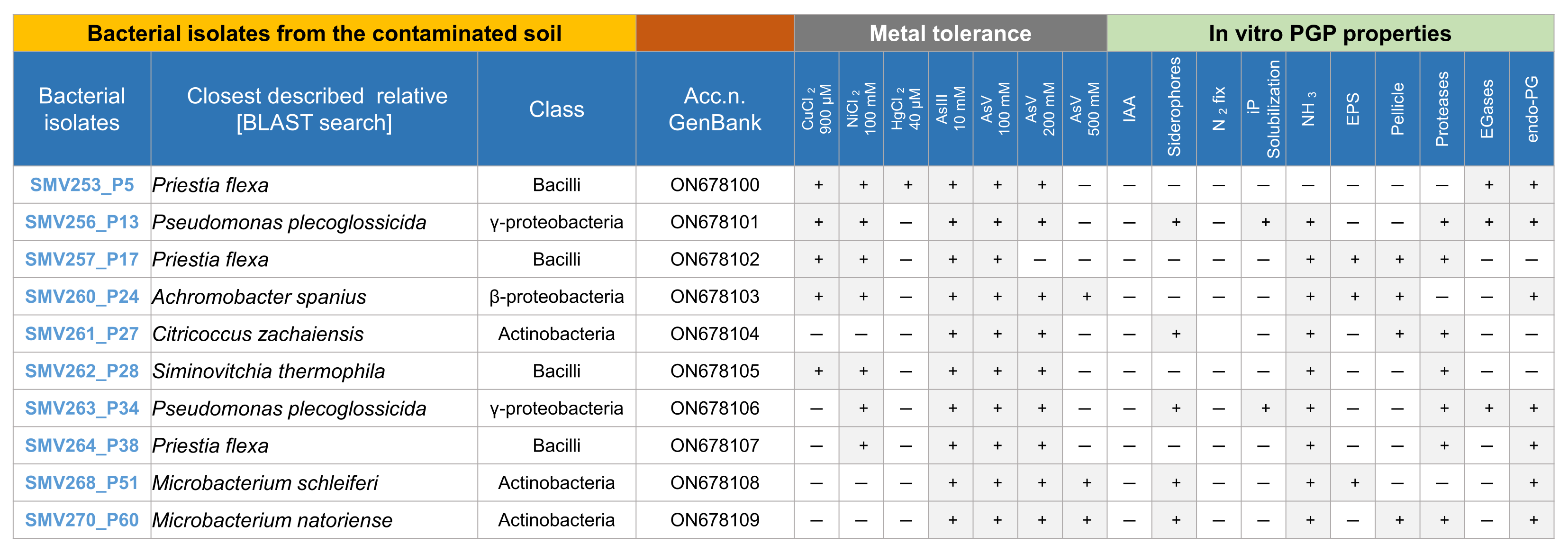
2.4. Isolation of Arsenic-Tolerant Bacteria
2.5. Characterization of Bacteria Isolates
2.6. Isolation of Hemp Endophytes
2.7. Preparation of Bacterial Consortia
2.8. Preparation of Microcosms
- All 4 selected soil types were investigated, using about 300 g of soil for each microcosm.
- Sowing involved all 3 plant species, in particular 0.4 g of B. juncea seeds, 12 seeds of C. sativa, and 5 seeds of Z. mays, by germinating the seeds on moistened cotton and then transplanting them at the appropriate time.
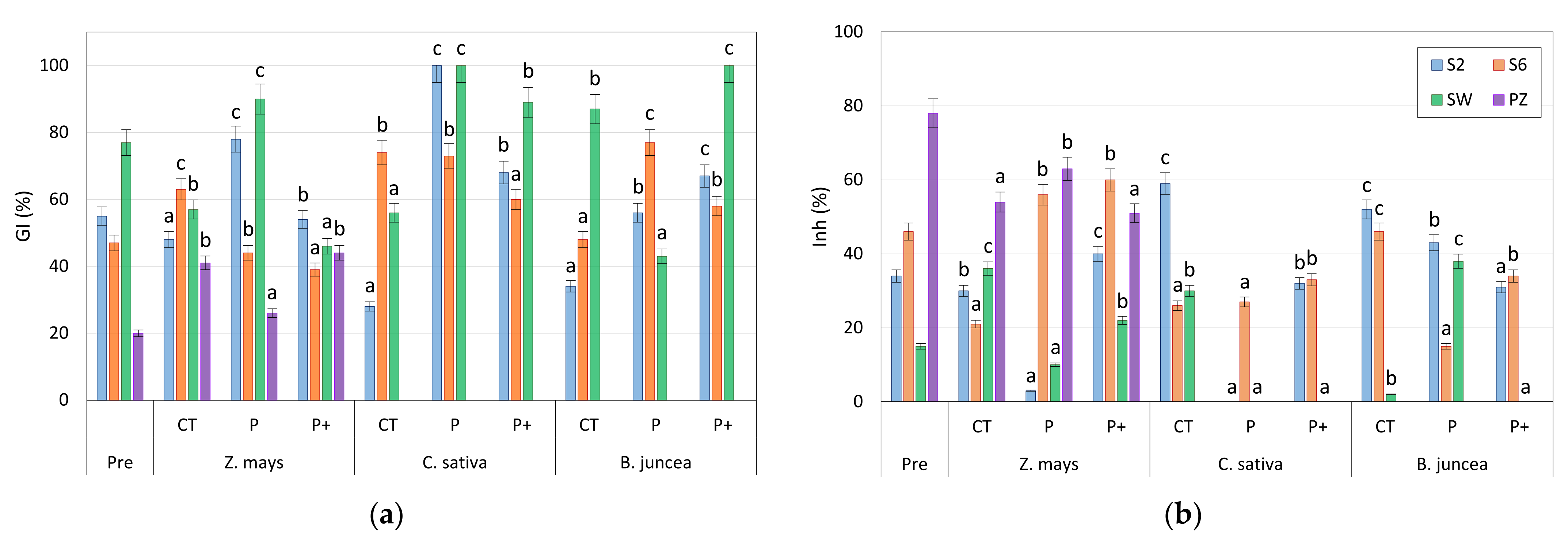
- Three different treatments were prepared for each soil and each plant, namely a control (CT), a treatment with 0.05 M KH2PO4 (P), and a treatment with the further addition of growth-promoting bacteria (P+).
- Two soils, S2 and SW, representative of the external and internal area, respectively, were investigated, and the amount of soil used for each microcosm was about 300 g.
- Three plant species, replicating the quantities and methods used for the first series of tests.
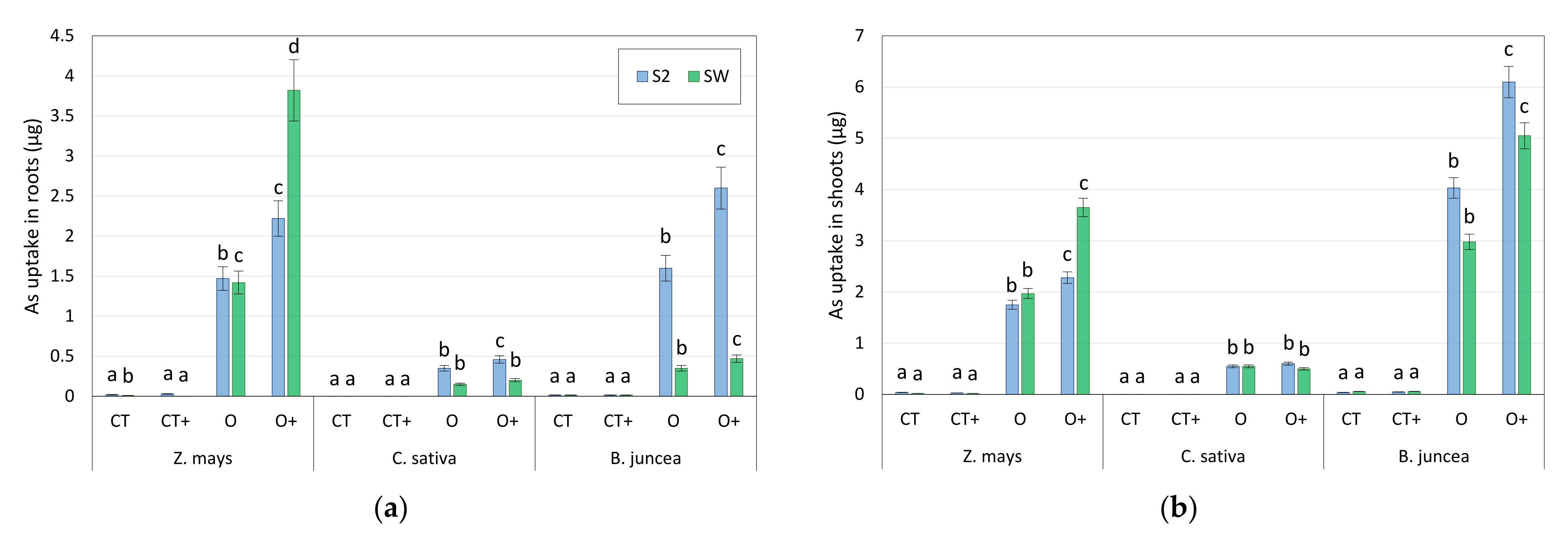
- For each soil and each plant, four different treatments were investigated, namely a control (CT), a treatment with 0.2 M KHC2O4 (O), and the same treatments with further addition of growth-promoting bacteria (CT+ and O+).
- Two soils, S2 and SW, as for the second set, and the amount of soil used for each microcosm was about 300 g.
- Two plant species (hemp and corn), replicating the quantities and methods used for previous tests.
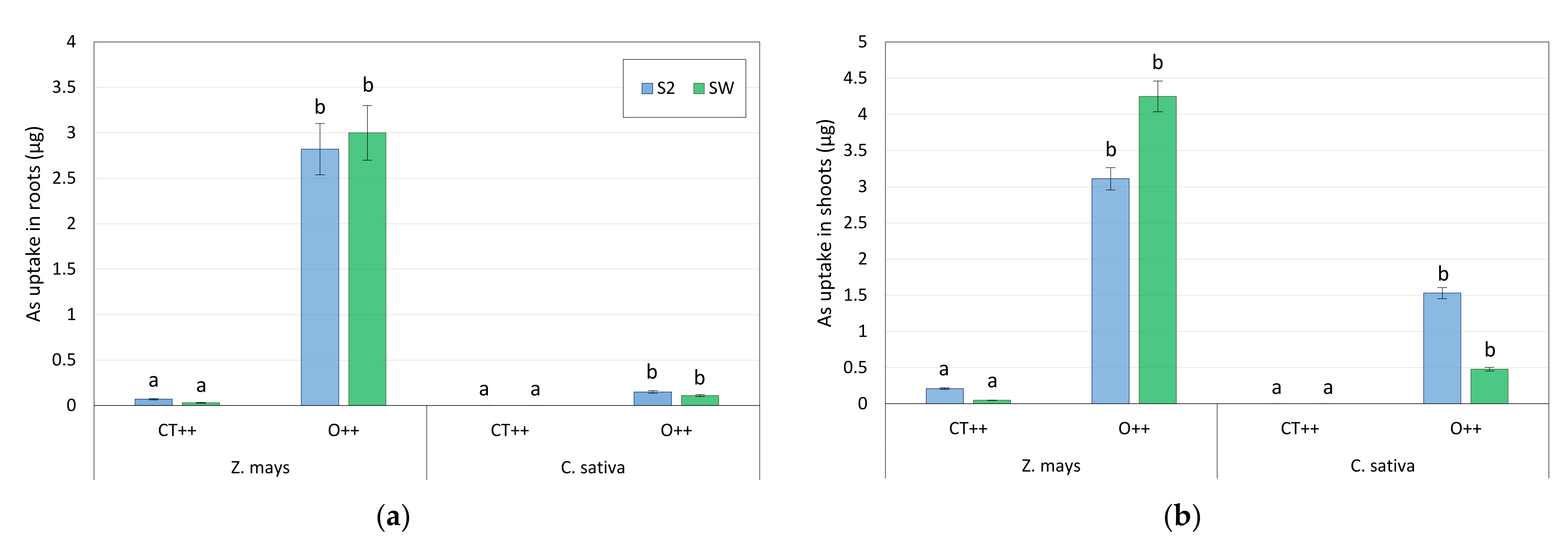
- For each soil and each plant, two different treatments were investigated, namely a control and a treatment with 0.2 M KHC2O4, both with the addition of growth-promoting bacteria from the second set of isolates, namely CT++ and O++, respectively.
2.9. Arsenic Analysis
2.10. Quality Assurance and Quality Control
2.11. Statistical Analysis
3. Results and Discussion
3.1. Soil Analysis
3.2. Phytotoxicity Test
3.3. Microcosm Tests with Phosphate
3.4. Microcosm Tests with Oxalate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vocciante, M.; Meshalkin, V. An accurate inverse model for the detection of leaks in sealed landfills. Sustainability 2020, 12, 5598. [Google Scholar] [CrossRef]
- Cirrincione, L.; La Gennusa, M.; Peri, G.; Rizzo, G.; Scaccianoce, G. The landfilling of municipal solid waste and the sustainability of the related transportation activities. Sustainability 2022, 14, 5272. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Landi, M. Red-leafed species for urban “greening” in the age of global climate change. J. For. Res. 2021, 32, 151. [Google Scholar] [CrossRef]
- Cachada, A.; Rocha-Santos, T.A.P.; Duarte, A.C. Soil and Pollution: An Introduction to the Main Issues. In Soil Pollution: From Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T.A.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Bioresour. Technol. 2008, 99, 5296. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal of polyethylene glycols from wastewater: A comparison of different approaches. Chemosphere 2021, 273, 129725. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Reverberi, A.P. A novel graphene-based sorbent for oil spill cleanup. Materials 2022, 5, 609. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Soda, O.; Fabiano, B. A sustainable, top-down mechanosynthesis of carbohydrate-functionalized silver nanoparticles. React. Chem. Eng. 2022, 7, 888–897. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95. [Google Scholar] [CrossRef]
- Pavel, L.V.; Gavrilescu, M. Overview of ex situ decontamination techniques for soil cleanup. Environ. Eng. Manag. J. 2008, 7, 815–834. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Wang, J.; Ma, S.; Wang, G.G.; Xu, L.; Fu, Z.; Song, J.; Zhang, J. Arbuscular mycorrhizal fungi communities associated with wild plants in a coastal ecosystem. J. For. Res. 2021, 32, 683. [Google Scholar] [CrossRef]
- Vocciante, M.; Dovì, V.G.; Ferro, S. Sustainability in ElectroKinetic Remediation processes: A critical analysis. Sustainability 2021, 13, 770. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal and recovery of heavy metals from tannery sludge subjected to plasma pyro-gasification process. J. Clean. Prod. 2020, 273, 123166. [Google Scholar] [CrossRef]
- Song, Y.; Kirkwood, N.; Maksimović, Č.; Zhen, X.; O’Connor, D.; Jin, Y.; Hou, D. Nature based solutions for contaminated land remediation and brownfield redevelopment in cities: A review. Sci. Total Environ. 2019, 663, 568. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, M.; Franchi, E.; Fusini, D.; Vocciante, M.; Barbafieri, M.; Pedron, F.; Rosellini, I.; Petruzzelli, G. Soil remediation: Towards a resilient and adaptive approach to deal with the ever-changing environmental challenges. Environments 2022, 9, 18. [Google Scholar] [CrossRef]
- Hou, D.; Bolan, N.S.; Tsang, D.C.W.; Kirkham, M.B.; O’Connor, D. Sustainable soil use and management: An interdisciplinary and systematic approach. Sci. Total Environ. 2020, 729, 138961. [Google Scholar] [CrossRef]
- O’Connor, D.; Zheng, X.; Hou, D.; Shen, Z.; Li, G.; Miao, G.; O’Connell, S.; Guo, M. Phytoremediation: Climate change resilience and sustainability assessment at a coastal brownfield redevelopment. Environ. Int. 2019, 130, 104945. [Google Scholar] [CrossRef]
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Franchi, E.; Petruzzelli, G.; Ferro, S. Enhancements in phytoremediation technology: Environmental assessment including different options of biomass disposal and comparison with a consolidated approach. J. Environ. Manag. 2019, 237, 560. [Google Scholar] [CrossRef]
- Vocciante, M.; De Follis D’Auris, A.; Franchi, E.; Petruzzelli, G.; Ferro, S. CO2 footprint analysis of consolidated and innovative technologies in remediation activities. J. Clean. Prod. 2021, 297, 126723. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Franchi, E.; Samà, C.; Gila, L.; Zanardi, S.; Palmery, S.; Proto, A.; et al. New light on phytoremediation: The use of luminescent solar concentrators. Appl. Sci. 2021, 11, 1923. [Google Scholar] [CrossRef]
- Conte, A.; Chiaberge, S.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M.; Franchi, E.; Pietrini, I. Dealing with complex contamination: A novel approach with a combined bio-phytoremediation strategy and effective analytical techniques. J. Environ. Manag. 2021, 288, 112381. [Google Scholar] [CrossRef] [PubMed]
- Cirrincione, L.; Marvuglia, A.; Scaccianoce, G. Assessing the effectiveness of green roofs in enhancing the energy and indoor comfort resilience of urban buildings to climate change: Methodology proposal and application. Build. Environ. 2021, 205, 108198. [Google Scholar] [CrossRef]
- Cirrincione, L.; La Gennusa, M.; Marino, C.; Nucara, A.; Marvuglia, A.; Peri, G. Passive Components for Reducing Environmental Impacts of Buildings: Analysis of an Experimental Green Roof. In Proceedings of the 2020 IEEE 20th Mediterranean Electrotechnical Conference, Palermo, Italy, 16–18 June 2020; pp. 494–499. [Google Scholar] [CrossRef]
- Pandey, V.C.; Gajic, G.; Sharma, P.; Roy, M. Adaptive Phytoremediation Practices for Sustaining Ecosystem Services. In Adaptive Phytoremediation Practices: Resilience to Climate Change; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–225. [Google Scholar] [CrossRef]
- Brunetti, G.; Papagrigoriou, I.A.; Šimůnek, J.; Stumpp, C. Green roofs for domestic wastewater treatment: Experimental and numerical analysis of nitrogen turnover. J. Hydrol. 2021, 603, 127132. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Reddy, D.H.K.; Yun, Y.S. Improving the quality of runoff from green roofs through synergistic biosorption and phytoremediation techniques: A review. Sustain. Cities Soc. 2019, 46, 101381. [Google Scholar] [CrossRef]
- Prigioniero, A.; Zuzolo, D.; Niinemets, Ü.; Guarino, C. Nature-based solutions as tools for air phytoremediation: A review of the current knowledge and gaps. Environ. Pollut. 2021, 277, 116817. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef]
- Napoli, G.; Corrao, R.; Scaccianoce, G.; Barbaro, S.; Cirrincione, L. Public and private economic feasibility of green areas as a passive energy measure: A case study in the Mediterranean city of Trapani in southern Italy. Sustainability 2022, 14, 2407. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Sheoran, V.; Singh Sheoran, A.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Applicability of a Freundlich-like model for plant uptake at an industrial contaminated site with a high variable arsenic concentration. Environments 2017, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Petruzzelli, G.; Grifoni, M.; Barbafieri, M.; Rosellini, I.; Pedron, F. Sorption: Release Processes In Soil-The Basis of Phytoremediation Efficiency. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 91–112. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant’s environmental stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Abbaszadeh-Dahaji, P.; Omidvari, M.; Ghorbanpour, M. Increasing Phytoremediation Efficiency of Heavy Metal-Contaminated Soil Using PGPR for Sustainable Agriculture. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D.K., Varma, A., Narendra, T., Eds.; Springer: Singapore, 2017; pp. 187–204. [Google Scholar] [CrossRef]
- Pietrini, I.; Grifoni, M.; Franchi, E.; Cardaci, A.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Enhanced lead phytoextraction by endophytes from indigenous plants. Soil Syst. 2021, 5, 55. [Google Scholar] [CrossRef]
- ISO 18763:2016; Soil Quality–Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. ISO—International Organization for Standardization: Geneva, Switzerland, 2016.
- Picchi, C.; Giorgetti, L.; Morelli, E.; Landi, M.; Rosellini, I.; Grifoni, M.; Franchi, E.; Petruzzelli, G.; Barbafieri, M. Cannabis sativa L. and Brassica juncea L. grown on arsenic-contaminated industrial soil: Potentiality and limitation for phytoremediation. Environ. Sci. Pollut. Res. 2022, 29, 15983. [Google Scholar] [CrossRef]
- Franchi, E.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Improved arsenic phytoextraction by combined use of mobilizing chemicals and autochthonous soil bacteria. Sci. Total Environ. 2019, 655, 328. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L. Methods of Soil Analysis, Part 3. In Chemical Methods; Soil Science Society of America Book Series; Soil Science Society of America: Madison, WI, USA, 1998. [Google Scholar]
- Barbafieri, M.; Pedron, F.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Assisted phytoremediation of a multi-contaminated soil: Investigation on arsenic and lead combined mobilization and removal. J. Environ. Manag. 2017, 203, 316. [Google Scholar] [CrossRef] [PubMed]






| Mean | |
|---|---|
| pH | 8.3 ± 0.2 |
| EC (μS cm−1) | 585 ± 10 |
| Clay (%) | 33.5 ± 1.8 |
| Silt (%) | 18.6 ± 1.1 |
| Sand (%) | 47.9 ± 0.6 |
| CEC (Cmol(+) kg−1) | 23.3 ± 0.7 |
| S2 | S6 | SW | PZ | |
|---|---|---|---|---|
| Total | 40.5 ± 2.0 | 29.3 ± 1.7 | 24.9 ± 1.5 | 67.2 ± 2.1 |
| KH2PO4 0.05 M | 1.00 ± 0.04 | 0.99 ± 0.05 | 0.52 ± 0.05 | 1.50 ± 0.07 |
| KH2PO4 0.1 M | 1.30 ± 0.07 | --- | --- | 2.20 ± 0.11 |
| K4P2O7 0.1 M | 0.20 ± 0.01 | --- | --- | 2.53 ± 0.12 |
| EDTA 1% | 0.80 ± 0.07 | --- | --- | 8.20 ± 0.80 |
| K2S2O3 0.27 M | 0.10 ± 0.01 | --- | --- | 0.30 ± 0.02 |
| KHC2O4 0.2 M | 1.88 ± 0.13 | 5.99 ± 0.30 | 14.1 ± 0.42 | 42.9 ± 0.9 |
| Soil | Treatment | Z. mays | C. sativa | B. juncea | |||
|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | ||
| S2 | CT | 0.10 ± 0.01a | 0.05 ± 0.01a | 0.05 ± 0.01a | 0.03 ± 0.01a | 0.11 ± 0.01a | 0.05 ± 0.01a |
| P | 0.77 ± 0.07b | 0.38 ± 0.03b | 0.19 ± 0.02b | 0.15 ± 0.03b | 1.27 ± 0.11b | 0.61 ± 0.06b | |
| P+ | 0.82 ± 0.07b | 0.35 ± 0.04b | 0.21 ± 0.02b | 0.16 ± 0.02b | 1.22 ± 0.10b | 0.62 ± 0.05b | |
| S6 | CT | 0.12 ± 0.02a | 0.06 ± 0.01a | 0.03 ± 0.01a | 0.02 ± 0.01a | 0.21 ± 0.03a | 0.10 ± 0.01a |
| P | 0.47 ± 0.05b | 0.23 ± 0.03b | 0.14 ± 0.02b | 0.11 ± 0.02b | 0.85 ± 0.08c | 0.41 ± 0.04c | |
| P+ | 0.57 ± 0.05b | 0.24 ± 0.02b | 0.12 ± 0.01b | 0.09 ± 0.01b | 0.69 ± 0.06b | 0.35 ± 0.03b | |
| SW | CT | 0.07 ± 0.01a | 0.04 ± 0.01a | 0.09 ± 0.01a | 0.03 ± 0.01a | 0.18 ± 0.02a | 0.11 ± 0.01a |
| P | 0.29 ± 0.03b | 0.15 ± 0.02b | 0.19 ± 0.02b | 0.09 ± 0.01b | 0.39 ± 0.04b | 0.41 ± 0.05b | |
| P+ | 0.40 ± 0.04c | 0.16 ± 0.01b | 0.25 ± 0.03c | 0.10 ± 0.01b | 0.34 ± 0.03b | 0.38 ± 0.04b | |
| PZ | CT | 0.12 ± 0.02a | 0.07 ± 0.01a | --- | --- | --- | --- |
| P | 0.21 ± 0.03b | 0.11 ± 0.01b | --- | --- | --- | --- | |
| P+ | 0.25 ± 0.03b | 0.10 ± 0.01b | --- | --- | --- | --- | |
| Soil | Treatment | Z. mays | C. sativa | B. juncea | |||
|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | ||
| S2 | CT | 0.10 ± 0.02a | 0.05 ± 0.01a | 0.03 ± 0.01a | 0.02 ± 0.01a | 0.15 ± 0.02a | 0.07 ± 0.01a |
| CT+ | 0.11 ± 0.01a | 0.04 ± 0.01a | 0.04 ± 0.01a | 0.01 ± 0.01a | 0.13 ± 0.01a | 0.08 ± 0.01a | |
| O | 4.24 ± 0.34b | 2.08 ± 0.10b | 5.10 ± 0.61b | 3.96 ± 0.20b | 10.5 ± 0.95b | 5.06 ± 0.30b | |
| O+ | 5.02 ± 0.35b | 2.13 ± 0.09b | 5.03 ± 0.45b | 3.85 ± 0.12b | 12.9 ± 1.03b | 6.57 ± 0.26c | |
| SW | CT | 0.07 ± 0.01a | 0.04 ± 0.01a | 0.03 ± 0.01a | 0.01 ± 0.01a | 0.18 ± 0.02a | 0.11 ± 0.01a |
| CT+ | 0.06 ± 0.01a | 0.04 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.16 ± 0.01a | 0.11 ± 0.01a | |
| O | 6.07 ± 0.55b | 3.19 ± 0.16b | 5.32 ± 0.48b | 2.54 ± 0.15c | 3.87 ± 0.43b | 4.03 ± 0.28b | |
| O+ | 13.2 ± 0.53c | 5.22 ± 0.21c | 4.96 ± 0.40b | 1.98 ± 0.08b | 4.42 ± 0.31b | 4.97 ± 0.25b | |
| Soil | Treatment | Z. mays | C. sativa | ||
|---|---|---|---|---|---|
| Roots | Shoots | Roots | Shoots | ||
| S2 | CT++ | 0.21 ± 0.02a | 0.26 ± 0.01a | 0.12 ± 0.01a | 0.04 ± 0.01a |
| O++ | 8.01 ± 0.72b | 4.11 ± 0.16b | 11.4 ± 0.91b | 5.57 ± 0.22b | |
| SW | CT++ | 0.11 ± 0.01a | 0.07 ± 0.01a | 0.11 ± 0.01a | 0.05 ± 0.01a |
| O++ | 15.6 ± 1.09b | 6.69 ± 0.27b | 7.82 ± 0.63b | 3.85 ± 0.19b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchi, E.; Barbafieri, M.; Petruzzelli, G.; Ferro, S.; Vocciante, M. Screening of Plants and Indigenous Bacteria to Improve Arsenic Phytoextraction. Appl. Sci. 2022, 12, 7267. https://doi.org/10.3390/app12147267
Franchi E, Barbafieri M, Petruzzelli G, Ferro S, Vocciante M. Screening of Plants and Indigenous Bacteria to Improve Arsenic Phytoextraction. Applied Sciences. 2022; 12(14):7267. https://doi.org/10.3390/app12147267
Chicago/Turabian StyleFranchi, Elisabetta, Meri Barbafieri, Gianniantonio Petruzzelli, Sergio Ferro, and Marco Vocciante. 2022. "Screening of Plants and Indigenous Bacteria to Improve Arsenic Phytoextraction" Applied Sciences 12, no. 14: 7267. https://doi.org/10.3390/app12147267







