Computational Modelling and Simulation of Fluid Structure Interaction in Aortic Aneurysms: A Systematic Review and Discussion of the Clinical Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Selection Criteria
2.3. Quality Assessment
2.4. Study Selection
3. Results
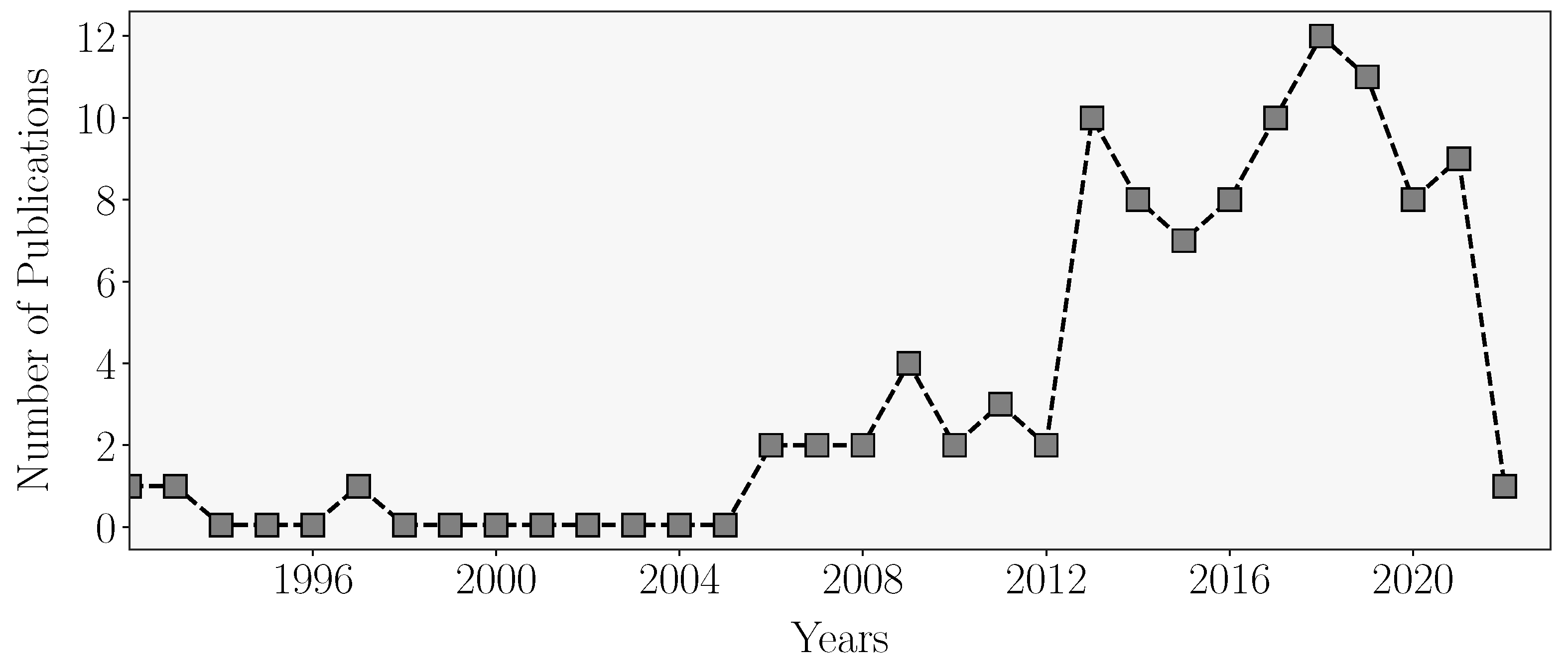
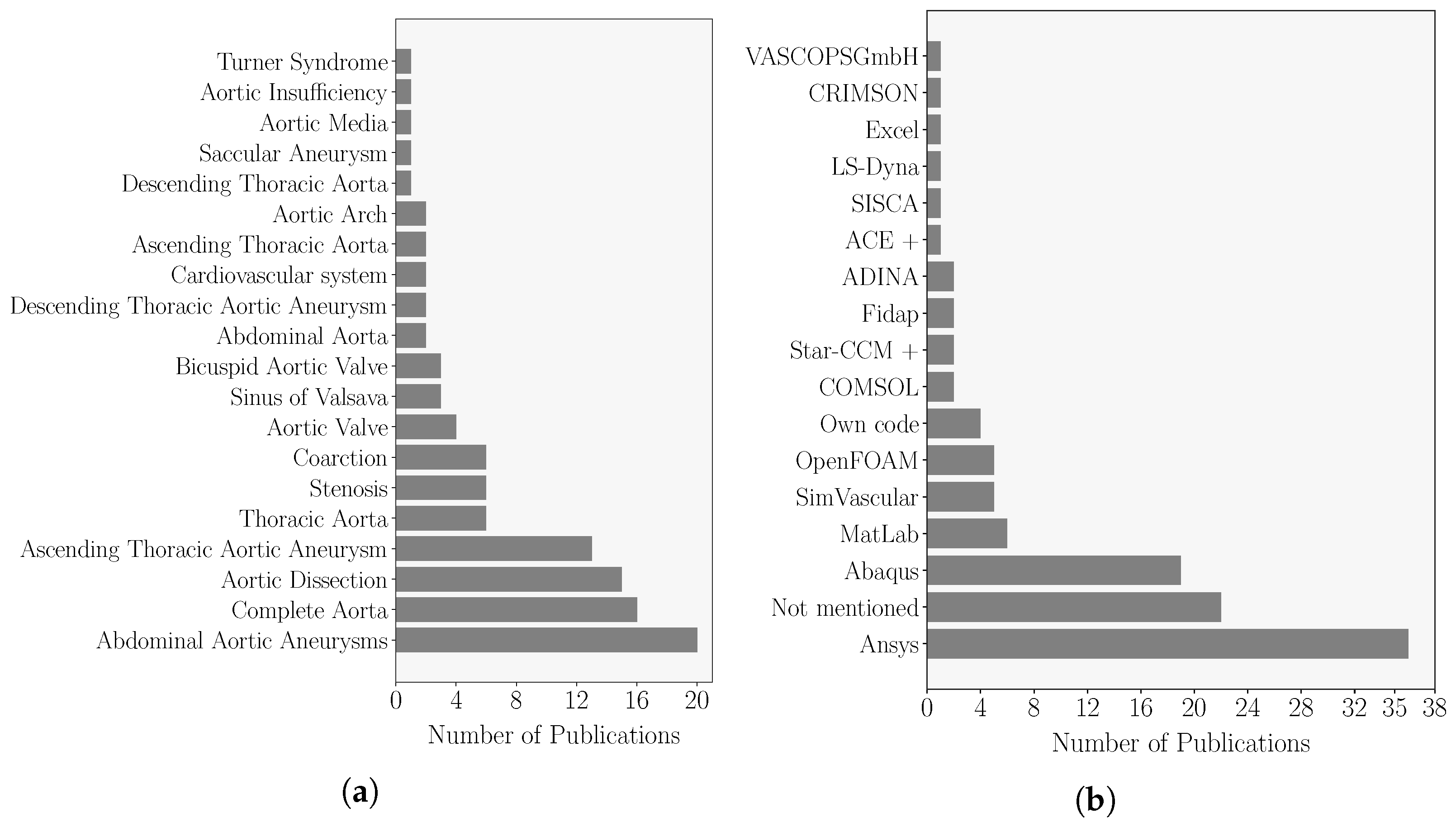
3.1. Publication Overview
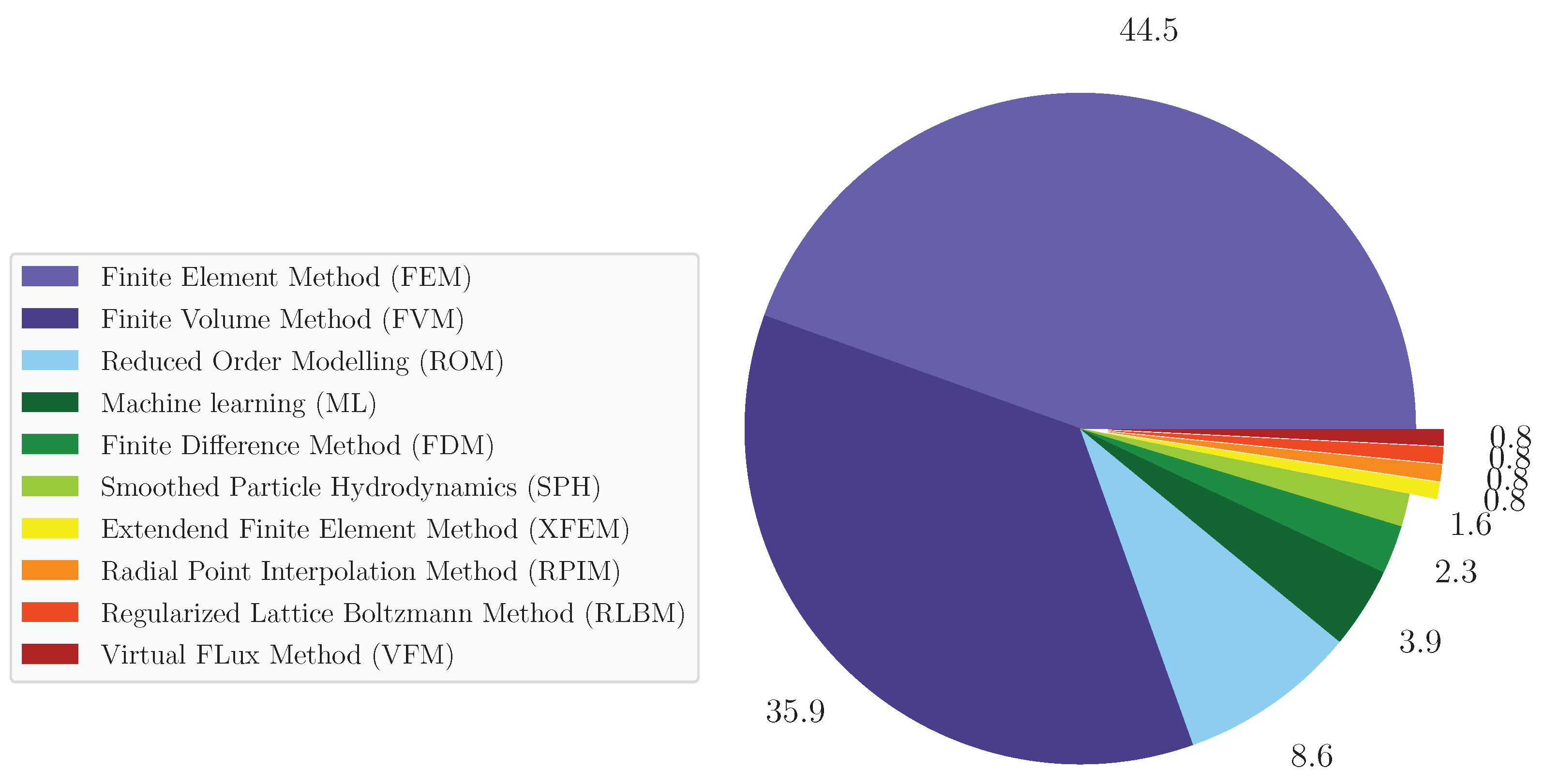
3.2. Computational Techniques to Model Diseased Aortas
3.3. Summary of the Results
- Biomechanical behaviour of healthy and diseased aortas;
- Aortic wall characterisation;
- Risk assessment strategies and diagnosing techniques;
- Numerical modelling augmentation.
3.4. Biomechanical Behaviour of Healthy and Diseased Aortas
3.4.1. Healthy Aorta
3.4.2. Aortic Aneurysms
3.4.3. Aortic Dissection
3.5. Aortic Wall Characterisation
3.5.1. Cardiac Phantom Materials
3.5.2. Wall Microstructure
3.5.3. Non-Invasive Estimation of Wall Material Properties
3.6. Risk Assessment Strategies and Diagnosing Techniques
3.6.1. Mathematical Models as Tools to Assist Clinical Diagnosis
3.6.2. Risk Estimation of Aneurysm Rupture
3.7. Numerical Modelling Augmentation
3.7.1. Relevance of Modelling Certain Physiological Features
3.7.2. Novel Numerical Techniques
3.7.3. Sensitivity Analysis
4. Discussion
5. Conclusions
- Numerical models are feasible tools to recreate the complex conditions of the biomechanical system. These models can have the potential to provide insightful data toward clinical practice.
- Among the relevant data that numerical models can provide, we have highlighted: (i) the WSS and WS-based metrics, which were strongly correlated with aortic microstructure (mys) functioning and elevated propensity to rupture; (ii) PWV due to its ability to evaluate aortic stiffness by clinical guidelines; (iii) and wall strain.
- One-dimensional modelling and ML were the next most selected techniques. One-dimensional models proved to allow quick computations of PWV on a patient-specific basis. ML presented a wide range of applications that outperformed usual methods while reducing the computation time.
- There is no consensus on the gold standard technique to model the haemodynamics and structure dynamics of the aortic wall. From the review, FEM and FVM are the two preferred techniques to perform CSM and CFD patient-specific simulations, respectively. These techniques were collectively chosen in around of the analysed works, are widely implemented in commercial and open-source computing platforms and allow the use of complex geometric models such as diseased aortas.
- Accuracy, computing time and lack of validation were the main identified contributors to the lack of application of numerical models in real-life medical applications. Accuracy issues are mostly correlated with poor selection of rheological, turbulence or wall constitutive models and difficulties in correctly assessing the in vivo patient-specific parameters for these models and patient-specific geometric models or BC. Excluding ROM, computing 3D haemodynamics or structural data can easily take days or even weeks, particularly in grid-based methods, which is not suitable for time-sensitive situations. Moreover, there is a lack of both numerical validations as assessed by the GRADE approach (only 12% of the total works presented numerical validation against in vivo patient-specific data) and extensive clinical trials.
- To date, there is still a lack of reports on the bibliography of studies on the impact of considering wall thickness and material properties’ heterogeneity, surrounding aortic structures, the internal pressure of the human body, the calibration of impedance-based outlet conditions and aortic root motion on the numerical results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAA | Abdominal Aortic Aneurysms |
| AD | Aortic Dissection |
| ATAA | Ascending Thoracic Aortic Aneurysm |
| AV | Aortic Valve |
| BAV | Bicuspid Aortic Valve |
| BC | Boundary Condition |
| CFD | Computational Fluid Dynamics |
| CSM | Computational Solid Mechanics |
| CT | Computed Tomography |
| CVD | Cardiovascular Diseases |
| DNS | Direct Numerical Simulations |
| DTAA | Descending Thoracic Aortic Aneurysms |
| FDM | Finite Difference Method |
| FEM | Finite Element Method |
| FL | False Lumen |
| FSI | Fluid–Structure Interaction |
| FVM | Finite Volume Method |
| GPU | Graphical Processing Unit |
| LES | Large Eddy Simulations |
| ML | Machine Learning |
| MRI | Magnetic Resonance Imaging |
| OSI | Oscillatory Shear Index |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PWS | Peak Wall Stress |
| PWV | Pulse Wave Velocity |
| RANS | Reynolds-Averaged Navier–Stokes |
| RLBM | Regularised Lattice Boltzmann Method |
| ROM | Reduced Order Model |
| RPIM | Radial Point Interpolation Method |
| SPH | Smoothed Particle Hydrodynamics |
| STJ | Sinotubular Junction |
| TAV | Tricuspid Aortic Valve |
| TAWSS | Time-Averaged Wall Shear Stress |
| TL | True Lumen |
| VFM | Virtual Flux Method |
| WS | Wall Stress |
| WSS | Wall Shear Stress |
| XFEM | Extended Finite Element Method |
References
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; Kouchoukos, N.T.; Lytle, B.W.; Milewicz, D.M.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J. Am. Coll. Cardiol. 2010, 55, 27–129. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed]
- Adriaans, B.P.; Wildberger, J.E.; Westenberg, J.J.; Lamb, H.J.; Schalla, S. Predictive imaging for thoracic aortic dissection and rupture: Moving beyond diameters. Eur. Radiol. 2019, 29, 6396–6404. [Google Scholar] [CrossRef]
- Franzetti, G.; Bonfanti, M.; Homer-Vanniasinkam, S.; Diaz-Zuccarini, V.; Balabani, S. Experimental evaluation of the patient-specific haemodynamics of an aortic dissection model using particle image velocimetry. J. Biomech. 2022, 134, 110963. [Google Scholar] [CrossRef]
- Koltukluoǧlu, T.; Blanco, P. Boundary control in computational haemodynamics. J. Fluid Mech. 2018, 847, 329–364. [Google Scholar] [CrossRef]
- Lucio, M.; García, M.; García, J.; Rodríguez, L.; Marcos, F. On the importance of tunica intima in the aging aorta: A three-layered in silico model for computing wall stresses in abdominal aortic aneurysms. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, A.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The ‘Digital Twin’to enable the vision of precision cardiology. Eur. Heart J. 2020, 41, 4556–4564. [Google Scholar] [CrossRef] [PubMed]
- Coorey, G.; Figtree, G.A.; Fletcher, D.F.; Redfern, J. The health digital twin: Advancing precision cardiovascular medicine. Nat. Rev. Cardiol. 2021, 18, 803–804. [Google Scholar] [CrossRef] [PubMed]
- Thunes, J.R.; Phillippi, J.A.; Gleason, T.G.; Vorp, D.A.; Maiti, S. Structural modeling reveals microstructure-strength relationship for human ascending thoracic aorta. J. Biomech. 2018, 71, 84–93. [Google Scholar] [CrossRef]
- Gültekin, O.; Hager, S.P.; Dal, H.; Holzapfel, G.A. Computational modeling of progressive damage and rupture in fibrous biological tissues: Application to aortic dissection. Biomech. Model. Mechanobiol. 2019, 18, 1607–1628. [Google Scholar] [CrossRef]
- Chen, W.; Chan, L.; Hutchins, N.; Poon, E.; Ooi, A. Direct numerical simulation of pulsatile flow in pipes. In Proceedings of the 19th Australasian Fluid Mechanics Conference, Melbourne, Australia, 8–11 December 2014. [Google Scholar]
- Febina, J.; Sikkandar, M.Y.; Sudharsan, N. Wall shear stress estimation of thoracic aortic aneurysm using computational fluid dynamics. Comput. Math. Methods Med. 2018, 2018, 7126532. [Google Scholar] [CrossRef] [PubMed]
- Mendez, V.; Di Giuseppe, M.; Pasta, S. Comparison of hemodynamic and structural indices of ascending thoracic aortic aneurysm as predicted by 2-way FSI, CFD rigid wall simulation and patient-specific displacement-based FEA. Comput. Biol. Med. 2018, 100, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wolański, W.; Gzik-Zroska, B.; Joszko, K.; Gzik, M.; Sołtan, D. Numerical analysis of blood flow through artery with elastic wall of a vessel. In Innovations in Biomedical Engineering; Gzik, M., Tkacz, E., Paszenda, Z., Pietka, E., Eds.; Springer: Cham, Switzerland, 2017; Volume 526, pp. 193–200. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W.; Kunz, R.; Craig, J.; Montori, V.M.; et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. Br. Med. J. 2008, 336, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J. What is “quality of evidence” and why is it important to clinicians? Br. Med. J. 2008, 336, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Owen, A. A numerical model of the aorta and aortic valve. In Proceedings of the 18th Annual Conference on Computers in Cardiology, Venice, Italy, 23–26 September 1991; pp. 649–652. [Google Scholar]
- Lou, Z.; Yang, W.J. A computer simulation of the non-Newtonian blood flow at the aortic bifurcation. J. Biomech. 1993, 26, 37–49. [Google Scholar] [CrossRef]
- Žáček, M.; Krause, E. Numerical simulation of the blood flow in the human cardiovascular system. J. Biomech. 1996, 29, 13–20. [Google Scholar] [CrossRef]
- Molony, D.; Broderick, S.; Callanan, A.; McGloughlin, T.; Walsh, M. Fluid–Structure Interaction in Healthy, Diseased and Endovascularly Treated Abdominal Aortic Aneurysms. In Biomechanics and Mechanobiology of Aneurysms; McGloughlin, T., Ed.; Springer: Cham, Switzerland, 2011; Volume 7, pp. 163–179. [Google Scholar] [CrossRef]
- Doyle, B.; Callanan, A.; Grace, P.; Kavanagh, E. On the influence of patient-specific material properties in computational simulations: A case study of a large ruptured abdominal aortic aneurysm. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 150–164. [Google Scholar] [CrossRef]
- Carneiro, F.; Ribeiro, V.; Teixeira, J.; Teixeira, S. Numerical study of blood fluid rheology in the abdominal aorta. WIT Trans. Ecol. Environ. 2008, 114, 169–178. [Google Scholar] [CrossRef]
- Helderman, F.; Manoch, I.; Breeuwer, M.; Kose, U.; Schouten, O.; Sambeek, M.; Poldermans, D.; Pattynama, P.; Wisselink, W.; Steen, A.; et al. A numerical model to predict abdominal aortic aneurysm expansion based on local wall stress and stiffness. Med. Biol. Eng. Comput. 2008, 46, 1121–1127. [Google Scholar] [CrossRef]
- Bäumler, K.; Vedula, V.; Sailer, A.; Seo, J.; Chiu, P.; Mistelbauer, G.; Chan, F.; Fischbein, M.; Marsden, A.; Fleischmann, D. Fluid–structure interaction simulations of patient-specific aortic dissection. Biomech. Model. Mechanobiol. 2020, 19, 1607–1628. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhu, M.; Chang, Y.; Qiao, H.; Liu, Y. The risk of stanford type-A aortic dissection with different tear size and location: A numerical study. Biomed. Eng. Online 2016, 15, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.; Boccadifuoco, A.; Celi, S.; Salvetti, M. Hemodynamics and stresses in numerical simulations of the thoracic aorta: Stochastic sensitivity analysis to inlet flow-rate waveform. Comput. Fluids 2021, 230, 173–182. [Google Scholar] [CrossRef]
- Comunale, G.; di Micco, L.; Boso, D.; Susin, F.; Peruzzo, P. Numerical models can assist choice of an aortic phantom for in vitro testing. Bioengineering 2021, 8, 101. [Google Scholar] [CrossRef]
- Liang, L.; Liu, M.; Martin, C.; Sun, W. A machine learning approach as a surrogate of finite element analysis-based inverse method to estimate the zero-pressure geometry of human thoracic aorta. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e3103. [Google Scholar] [CrossRef]
- Valente, R.; Mourato, A.; Brito, M.; Xavier, J.; Tomás, A.; Avril, S. Fluid-Structure Interaction Modeling of Ascending Thoracic Aortic Aneurysms in SimVascular. Biomechanics 2022, 2, 189–204. [Google Scholar] [CrossRef]
- Capellini, K.; Vignali, E.; Costa, E.; Gasparotti, E.; Biancolini, M.E.; Landini, L.; Positano, V.; Celi, S. Computational fluid dynamic study for aTAA hemodynamics: An integrated image-based and radial basis functions mesh morphing approach. J. Biomech. Eng. 2018, 140, 111007. [Google Scholar] [CrossRef]
- Bols, J.; Taelman, L.; De Santis, G.; Degroote, J.; Verhegghe, B.; Segers, P.; Vierendeels, J. Unstructured hexahedral mesh generation of complex vascular trees using a multi-block grid-based approach. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 663–672. [Google Scholar] [CrossRef]
- Taelman, L.; Degroote, J.; Swillens, A.; Vierendeels, J.; Segers, P. Fluid-structure interaction simulation of pulse propagation in arteries: Numerical pitfalls and hemodynamic impact of a local stiffening. Int. J. Eng. Sci. 2014, 77, 1–13. [Google Scholar] [CrossRef]
- Shang, E.K.; Nathan, D.P.; Sprinkle, S.R.; Vigmostad, S.C.; Fairman, R.M.; Bavaria, J.E.; Gorman, R.C.; Gorman, J.H., III; Chandran, K.B.; Jackson, B.M. Peak wall stress predicts expansion rate in descending thoracic aortic aneurysms. Ann. Thorac. Surg. 2013, 95, 593–598. [Google Scholar] [CrossRef]
- Ben-Or Frank, M.; Niestrawska, J.; Holzapfel, G.; deBotton, G. Micromechanically-motivated analysis of fibrous tissue. J. Mech. Behav. Biomed. Mater. 2019, 96, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, H.; Tafazzoli-Shadpour, M.; Shadmehr, M.B. Analysis of arterial wall remodeling in hypertension based on lamellar modeling. J. Am. Soc. Hypertens. 2015, 9, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Q.; Feng, J.; Yu, P.; Zhang, S.; Teng, Z.; Gillard, J.H.; Song, R.; Jing, Z. A pilot study exploring the mechanisms involved in the longitudinal propagation of acute aortic dissection through computational fluid dynamic analysis. Cardiology 2014, 128, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Nisco, G.D.; Zhang, P.; Calò, K.; Liu, X.; Ponzini, R.; Bignardi, C.; Rizzo, G.; Deng, X.; Gallo, D.; Morbiducci, U. What is needed to make low-density lipoprotein transport in human aorta computational models suitable to explore links to atherosclerosis? Impact of initial and inflow boundary conditions. J. Biomech. 2018, 68, 33–42. [Google Scholar] [CrossRef]
- Pasta, S.; Rinaudo, A.; Luca, A.; Pilato, M.; Scardulla, C.; Gleason, T.G.; Vorp, D.A. Difference in hemodynamic and wall stress of ascending thoracic aortic aneurysms with bicuspid and tricuspid aortic valve. J. Biomech. 2013, 46, 1729–1738. [Google Scholar] [CrossRef]
- Nowak, M.; Melka, B.; Rojczyk, M.; Gracka, M.; Nowak, A.; Golda, A.; Adamczyk, W.; Isaac, B.; Białecki, R.; Ostrowski, Z. The protocol for using elastic wall model in modeling blood flow within human artery. Eur. J. Mech. B. Fluids 2019, 77, 273–280. [Google Scholar] [CrossRef]
- Sotelo, J.; Dux-Santoy, L.; Guala, A.; Rodríguez-Palomares, J.; Evangelista, A.; Sing-Long, C.; Urbina, J.; Mura, J.; Hurtado, D.E.; Uribe, S. 3D axial and circumferential wall shear stress from 4D flow MRI data using a finite element method and a laplacian approach. Magn. Reson. Med. 2018, 79, 2816–2823. [Google Scholar] [CrossRef]
- Sotelo, J.; Urbina, J.; Valverde, I.; Tejos, C.; Irarrazaval, P.; Hurtado, D.E.; Uribe, S. Quantification of wall shear stress using a finite-element method in multidimensional phase-contrast MR data of the thoracic aorta. J. Biomech. 2015, 48, 1817–1827. [Google Scholar] [CrossRef]
- Suito, H.; Ueda, T.; Sze, D. Numerical simulation of blood flow in the thoracic aorta using a centerline-fitted finite difference approach. Jpn. J. Ind. Appl. Math. 2013, 30, 701–710. [Google Scholar] [CrossRef]
- He, F.; Li, X.Y. Selection of boundary conditions in numerical investigation of arterial flow. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009. [Google Scholar] [CrossRef]
- Brunet, J.; Pierrat, B.; Badel, P. A Parametric Study on Factors Influencing the Onset and Propagation of Aortic Dissection Using the Extended Finite Element Method. IEEE Trans. Biomed. Eng. 2021, 68, 2918–2929. [Google Scholar] [CrossRef]
- Fukui, T.; Morinishi, K. Blood flow simulation in the aorta with aortic valves using the regularized lattice boltzmann method with LES model. In Proceedings of the 7th Subrata Chakrabarti International Conference on Fluid Structure Interaction, Gran Canaria, Spain, 10 April 2013; Volume 129, pp. 97–107. [Google Scholar] [CrossRef]
- Aboelkassem, Y.; Savic, D.; Campbell, S. Mathematical modeling of aortic valve dynamics during systole. J. Theor. Biol. 2015, 365, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Geertsema, A.; Rakhorst, G.; Mihaylov, D.; Blanksma, P.; Verkerke, G. Development of a numerical simulation model of the cardiovascular system. Artif. Organs 1997, 21, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Bollache, E.; Kachenoura, N.; Frouin, F.; Redheuil, A.; Mousseaux, E.; Lucor, D. Numerical modeling of arterial pulse wave propagation to characterize aortic hemodynamic: Validation using magnetic resonance data. Innovation Res. Biomed. Eng. 2013, 34, 86–89. [Google Scholar] [CrossRef]
- Sazonov, I.; Khir, A.; Hacham, W.; Boileau, E.; Carson, J.; van Loon, R.; Ferguson, C.; Nithiarasu, P. A novel method for non-invasively detecting the severity and location of aortic aneurysms. Biomech. Model. Mechanobiol. 2017, 16, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Kizilova, N.; Mizerski, J. Validation of numerical models for flow simulation and wave propagation along human aorta. In Proceedings of the 23rd Fluid Mechanics Conference, Zawiercie, Poland, 9–12 September 2018; Volume 1101. [Google Scholar] [CrossRef]
- Biancolini, M.E.; Capellini, K.; Costa, E.; Groth, C.; Celi, S. Fast interactive CFD evaluation of hemodynamics assisted by RBF mesh morphing and reduced order models: The case of aTAA modelling. Int. J. Interact. Des. Manuf. 2020, 14, 1227–1238. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H. Pressure distribution over the leaflets and effect of bending stiffness on fluid-structure interaction of the aortic valve. J. Fluid Mech. 2019, 883, 1–29. [Google Scholar] [CrossRef]
- Rueckel, J.; Reidler, P.; Fink, N.; Sperl, J.; Geyer, T.; Fabritius, M.; Ricke, J.; Ingrisch, M.; Sabel, B. Artificial intelligence assistance improves reporting efficiency of thoracic aortic aneurysm CT follow-up. Eur. J. Radiol. 2021, 134, 109424. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Sun, W. A generic physics-informed neural network-based constitutive model for soft biological tissues. Comput. Methods Appl. Mech. Eng. 2020, 372, 113402. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Avril, S.; Lu, J. Prediction of local strength of ascending thoracic aortic aneurysms. J. Mech. Behav. Biomed. Mater. 2021, 115, 104284. [Google Scholar] [CrossRef]
- He, X.; Avril, S.; Lu, J. Estimating aortic thoracic aneurysm rupture risk using tension-strain data in physiological pressure range: An in vitro study. Biomech. Model. Mechanobiol. 2021, 20, 683–699. [Google Scholar] [CrossRef]
- Luo, Y.; Fan, Z.; Baek, S.; Lu, J. Machine learning-aided exploration of relationship between strength and elastic properties in ascending thoracic aneurysm. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2977. [Google Scholar] [CrossRef] [PubMed]
- Arico, C.; Alotta, G.; Zingales, M.; Napoli, E.; Monteleone, A.; Nagy, R. Numerical Simulations of the Hydrodynamics of the Abdominal Aorta Aneurysm (AAA) Using a Smoothed Particle Hydrodynamics Code with Deformable Wall Preliminary Results. In Proceedings of the 4th IEEE International Forum on Research and Technologies for Society and Industry, Palermo, Italy, 10–13 September 2018. [Google Scholar] [CrossRef]
- Aricò, C.; Sinagra, M.; Nagy, R.; Napoli, E.; Tucciarelli, T. Investigation of the hemodynamic flow conditions and blood-induced stresses inside an abdominal aortic aneurysm by means of a SPH numerical model. Int. J. Numer. Methods Biomed. Eng. 2020, 36, e3263. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Belinha, J.; Neves, J.; Vilaça, I.; Natal Jorge, R. Numerical simulation of aneurysms with Finite Element and meshless methods. In Proceedings of the 6th IEEE Portuguese Meeting on Bioengineering, Lisbon, Portugal, 22–23 February 2019. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, L.; Liu, X. A numerical study of fluid-structure coupled effect of abdominal aortic aneurysm. Bio-Med. Mater. Eng. 2015, 26, S245–S255. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.; Auer, M.; Biasetti, J. Structural and hemodynamical analysis of aortic aneurysms from computerized tomography angiography data. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering: Image Processing, Biosignal Processing, Modelling and Simulation, Munich, Germany, 7–12 September 2009; pp. 1584–1587. [Google Scholar] [CrossRef]
- Long Ko, J.K.; Liu, R.W.; Ma, D.; Shi, L.; Ho Yu, S.C.; Wang, D. Pulsatile hemodynamics in patient-specific thoracic aortic dissection models constructed from computed tomography angiography. J. X-ray Sci. Technol. 2017, 25, 233–245. [Google Scholar] [CrossRef]
- Campobasso, R.; Condemi, F.; Viallon, M.; Croisille, P.; Campisi, S.; Avril, S. Evaluation of peak wall stress in an ascending thoracic aortic aneurysm using FSI simulations: Effects of aortic stiffness and peripheral resistance. Cardiovasc. Eng. Technol. 2018, 9, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Jalalahmadi, G.; Linte, C.; Helguera, M. A numerical framework for studying the biomechanical behavior of abdominal aortic aneurysm. In Proceedings of the Medical Imaging 2017: Biomedical Applications in Molecular, Structural, and Functional Imaging, Orlando, FL, USA, 11–16 February 2017; Volume 10137. [Google Scholar] [CrossRef]
- Callaghan, F.M.; Karkouri, J.; Broadhouse, K.; Evin, M.; Fletcher, D.F.; Grieve, S.M. Thoracic aortic aneurysm: 4D flow MRI and computational fluid dynamics model. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1894–1895. [Google Scholar] [CrossRef]
- Alishahi, M.; Alishahi, M.; Emdad, H. Numerical simulation of blood flow in a flexible stenosed abdominal real aorta. Sci. Iran. 2011, 18, 1297–1305. [Google Scholar] [CrossRef]
- Chaudhari, K.; Patel, H. Hemodynamics numerical simulation of stenosis bifurcation. In Proceedings of the ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015; Volume 3. [Google Scholar] [CrossRef]
- Keshavarz-Motamed, Z.; Kadem, L. 3D pulsatile flow in a curved tube with coexisting model of aortic stenosis and coarctation of the aorta. Med. Eng. Phys. 2011, 33, 315–324. [Google Scholar] [CrossRef]
- Melka, B.; Adamczyk, W.; Rojczyk, M.; Nowak, M.; Gracka, M.; Nowak, A.; Golda, A.; Bialecki, R.; Ostrowski, Z. Numerical investigation of multiphase blood flow coupled with lumped parameter model of outflow. Int. J. Numer. Methods Heat Fluid Flow 2020, 30, 228–244. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Morbiducci, U.; Grigioni, M. Time dependent non-Newtonian numerical study of the flow field in a realistic model aortic arch. Int. J. Artif. Organs 2006, 29, 709–718. [Google Scholar] [CrossRef]
- Lantz, J.; Renner, J.; Karlsson, M. Wall shear stress in a subject specific human aorta—Influence of fluid-structure interaction. Int. J. Appl. Mech. 2011, 3, 759–778. [Google Scholar] [CrossRef]
- Marom, G.; Kim, H.S.; Rosenfeld, M.; Raanani, E.; Haj-Ali, R. Fully coupled fluid-structure interaction model of congenital bicuspid aortic valves: Effect of asymmetry on hemodynamics. Med. Biol. Eng. Comput. 2013, 51, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.H.; Fatouraee, N.; Katoozian, H.; Pashaei, A.; Camara, O.; Frangi, A. Numerical simulation of blood flow in the left ventricle and aortic sinus using magnetic resonance imaging and computational fluid dynamics. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Šeta, B.; Torlak, M.; Vila, A. Numerical simulation of blood flow through the aortic arch. In Proceedings of the International in proceedings on Medical and Biological Engineering, Sarajevo, Bosnia and Herzegovina, 16–18 March 2017; Volume 62, pp. 259–268. [Google Scholar] [CrossRef]
- Totorean, A.; Ioncica, M.; Ciocan, T.; Bernad, S.; Totorean, C.; Bernad, E. Medical IMAGE-Based Numerical Simulation of the Abdominal Aorta Flow. In Proceedings of the 10th International Conference on Energy and Environment, Bucharest, Romania, 14–15 October 2021. [Google Scholar] [CrossRef]
- Numata, S.; Itatani, K.; Kanda, K.; Doi, K.; Yamazaki, S.; Morimoto, K.; Manabe, K.; Ikemoto, K.; Yaku, H. Blood flow analysis of the aortic arch using computational fluid dynamics. Eur. J. Cardiothorac. Surg. 2016, 49, 1578–1585. [Google Scholar] [CrossRef]
- Prahl Wittberg, L.; van Wyk, S.; Fuchs, L.; Gutmark, E.; Backeljauw, P.; Gutmark-Little, I. Effects of aortic irregularities on blood flow. Biomech. Model. Mechanobiol. 2016, 15, 345–360. [Google Scholar] [CrossRef]
- Yeh, H.H.; Rabkin, S.W.; Grecov, D. Hemodynamic assessments of the ascending thoracic aortic aneurysm using fluid-structure interaction approach. Med. Biol. Eng. Comput. 2018, 56, 435–451. [Google Scholar] [CrossRef] [PubMed]
- García–Herrera, C.; Celentano, D.; Herrera, E. Modelling and numerical simulation of the in vivo mechanical response of the ascending aortic aneurysm in Marfan syndrome. Med. Biol. Eng. Comput. 2017, 55, 419–428. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, F.; Riga, C.; Bicknell, C.; Hamady, M.; Gibbs, R.; Wood, N.; Xu, X. Analysis of flow patterns in a patient-specific aortic dissection model. J. Biomech. Eng. 2010, 132. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Dillon-Murphy, D.; Figueroa, C. Computational study of anatomical risk factors in idealized models of type B aortic dissection. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 736–745. [Google Scholar] [CrossRef]
- Wan Ab Naim, W.N.; Ganesan, P.B.; Sun, Z.; Chee, K.H.; Hashim, S.A.; Lim, E. A perspective review on numerical simulations of hemodynamics in aortic dissection. Sci. World J. 2014, 2014, 652520. [Google Scholar] [CrossRef]
- Chen, H.; Peelukhana, S.; Berwick, Z.; Kratzberg, J.; Krieger, J.; Roeder, B.; Chambers, S.; Kassab, G. Editor’s Choice-Fluid-Structure Interaction Simulations of Aortic Dissection with Bench Validation. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, A.; Shahmirzadi, D.; Li, R.; Doyle, B.; Konofagou, E.; McGloughlin, T. 3D-printed tissue-mimicking phantoms for medical imaging and computational validation applications. 3D Print. Addit. Manuf. 2014, 1, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Thunes, J.R.; Pal, S.; Fortunato, R.N.; Phillippi, J.A.; Gleason, T.G.; Vorp, D.A.; Maiti, S. A structural finite element model for lamellar unit of aortic media indicates heterogeneous stress field after collagen recruitment. J. Biomech. 2016, 49, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Thunes, J.R.; Fortunato, R.N.; Gleason, T.G.; Vorp, D.A. Computational modeling of the strength of the ascending thoracic aortic media tissue under physiologic biaxial loading conditions. J. Biomech. 2020, 108, 109884. [Google Scholar] [CrossRef]
- Wang, R.; Yu, X.; Gkousioudi, A.; Zhang, Y. Effect of Glycation on Interlamellar Bonding of Arterial Elastin. Exp. Mech. 2021, 61, 81–94. [Google Scholar] [CrossRef]
- Ban, E.; Cavinato, C.; Humphrey, J.D. Differential propensity of dissection along the aorta. Biomech. Model. Mechanobiol. 2021, 20, 895–907. [Google Scholar] [CrossRef]
- Azadani, A.N.; Chitsaz, S.; Matthews, P.B.; Jaussaud, N.; Leung, J.; Tsinman, T.; Ge, L.; Tseng, E.E. Comparison of mechanical properties of human ascending aorta and aortic sinuses. Ann. Thorac. Surg. 2012, 93, 87–94. [Google Scholar] [CrossRef]
- Yang, A.S.; Wen, C.Y.; Tseng, L.Y.; Chiang, C.C.; Tseng, W.Y.; Yu, H.Y. An innovative numerical approach to resolve the pulse wave velocity in a healthy thoracic aorta model. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 461–473. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Sun, W. Estimation of in vivo mechanical properties of the aortic wall: A multi-resolution direct search approach. J. Mech. Behav. Biomed. Mater. 2018, 77, 649–659. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Sulejmani, F.; Lou, X.; Iannucci, G.; Chen, E.; Leshnower, B.; Sun, W. Identification of in vivo nonlinear anisotropic mechanical properties of ascending thoracic aortic aneurysm from patient-specific CT scans. Sci. Rep. 2019, 9, 12983. [Google Scholar] [CrossRef]
- Farzaneh, S.; Trabelsi, O.; Avril, S. Inverse identification of local stiffness across ascending thoracic aortic aneurysms. Biomech. Model. Mechanobiol. 2019, 18, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Seo, J.H.; Mittal, R. Computational modelling and analysis of haemodynamics in a simple model of aortic stenosis. J. Fluid Mech. 2018, 851, 23–49. [Google Scholar] [CrossRef]
- Pasta, S.; Agnese, V.; Gallo, A.; Cosentino, F.; Di Giuseppe, M.; Gentile, G.; Raffa, G.M.; Maalouf, J.F.; Michelena, H.I.; Bellavia, D.; et al. Shear stress and aortic strain associations with biomarkers of ascending thoracic aortic aneurysm. Ann. Thorac. Surg. 2020, 110, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Cartier, R.; Mongrain, R. Derivation of a simplified relation for assessing aortic root pressure drop incorporating wall compliance. Med. Biol. Eng. Comput. 2015, 53, 241–251. [Google Scholar] [CrossRef]
- Babbs, C. Noninvasive measurement of cardiac stroke volume using pulse wave velocity and aortic dimensions: A simulation study. Biomed. Eng. Online 2014, 13, 137. [Google Scholar] [CrossRef]
- Badeli, V.; Ranftl, S.; Melito, G.; Reinbacher-Köstinger, A.; Von Der Linden, W.; Ellermann, K.; Biro, O. Bayesian inference of multi-sensors impedance cardiography for detection of aortic dissection. Int. J. Comput. Math. Electr. Electron. Eng. 2021, 41, 824–839. [Google Scholar] [CrossRef]
- Pasta, S.; Gentile, G.; Raffa, G.; Bellavia, D.; Chiarello, G.; Liotta, R.; Luca, A.; Scardulla, C.; Pilato, M. In silico shear and intramural stresses are linked to aortic valve morphology in dilated ascending aorta. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 254–263. [Google Scholar] [CrossRef]
- Bopp, M.; Bauer, A.; Wegt, S.; Jakirlic, S.; Tropea, C.; Krafft, A.; Shokina, N.; Hennig, J. A computational and experimental study of physiological pulsatile flow in an aortic aneurysm. In Proceedings of the 11th International Symposium on Turbulence and Shear Flow Phenomena, Southampton, UK, 30 July–2 August 2019. [Google Scholar]
- Bauer, A.; Wegt, S.; Bopp, M.; Jakirlic, S.; Tropea, C.; Krafft, A.; Shokina, N.; Hennig, J.; Teschner, G.; Egger, H. Comparison of wall shear stress estimates obtained by laser Doppler velocimetry, magnetic resonance imaging and numerical simulations. Exp. Fluids 2019, 60, 112. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Ismail, Y.; Dong, H.; Lou, X.; Iannucci, G.; Chen, E.P.; Leshnower, B.G.; Elefteriades, J.A.; Sun, W. Computation of a probabilistic and anisotropic failure metric on the aortic wall using a machine learning-based surrogate model. Comput. Biol. Med. 2021, 137, 104794. [Google Scholar] [CrossRef]
- Doyle, B.; Corbett, T.; Callanan, A.; Walsh, M.; Vorp, D.; McGloughlin, T. An experimental and numerical comparison of the rupture locations of an abdominal aortic aneurysm. J. Endovasc. Ther. 2009, 16, 322–335. [Google Scholar] [CrossRef]
- Condemi, F.; Campisi, S.; Viallon, M.; Troalen, T.; Xuexin, G.; Barker, A.; Markl, M.; Croisille, P.; Trabelsi, O.; Cavinato, C.; et al. Fluid-and biomechanical analysis of ascending thoracic aorta aneurysm with concomitant aortic insufficiency. Ann. Biomed. Eng. 2017, 45, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Marom, G.; Haj-Ali, R.; Raanani, E.; Schäfers, H.J.; Rosenfeld, M. A fluid-structure interaction model of the aortic valve with coaptation and compliant aortic root. Med. Biol. Eng. Comput. 2012, 50, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, M.; Sherwood, J.M.; Karimpour, M.; Agu, O.; Balabani, S.; Díaz-Zuccarini, V. Aortic dissection simulation models for clinical support: Fluid-structure interaction vs. rigid wall models. Biomed. Eng. Online 2015, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Khanafer, K.; Bull, J.; Upchurch, G., Jr.; Berguer, R. Turbulence Significantly Increases Pressure and Fluid Shear Stress in an Aortic Aneurysm Model under Resting and Exercise Flow Conditions. Ann. Vasc. Surg. 2007, 21, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mourato, A.; Brito, M.; Xavier, J.; Gil, L.; Tomás, A. On the RANS modelling of the patient-specific thoracic aortic aneurysm. In Proceedings of the 9th Portuguese Congress on Biomechanics, Biomechanics, Porto, Portugal, 19–20 February 2021; pp. 98–102. [Google Scholar] [CrossRef]
- Berguer, R.; Bull, J.; Khanafer, K. Refinements in mathematical models to predict aneurysm growth and rupture. Ann. N. Y. Acad. Sci. 2006, 1085, 110–116. [Google Scholar] [CrossRef]
- Lantz, J.; Ebbers, T.; Engvall, J.; Karlsson, M. Numerical and experimental assessment of turbulent kinetic energy in an aortic coarctation. J. Biomech. 2013, 46, 1851–1858. [Google Scholar] [CrossRef]
- Stevens, S.; Lakin, W.; Goetz, W. A differentiable, periodic function for pulsatile cardiac output based on heart rate and stroke volume. Math. Biosci. 2003, 182, 201–211. [Google Scholar] [CrossRef]
- Attaran, S.; Niroomand-oscuii, H.; Ghalichi, F. A novel, simple 3D/2D outflow boundary model for blood flow simulations in compliant arteries. Comput. Fluids 2018, 174, 229–240. [Google Scholar] [CrossRef]
- Santis, G.D.; De Beule, M.; Van Canneyt, K.; Segers, P.; Verdonck, P.; Verhegghe, B. Full-hexahedral structured meshing for image-based computational vascular modeling. Med. Eng. Phys. 2011, 33, 1318–1325. [Google Scholar] [CrossRef]
- Matthys, K.; Alastruey, J.; Peiró, J.; Khir, A.; Segers, P.; Verdonck, P.; Parker, K.; Sherwin, S. Pulse wave propagation in a model human arterial network: Assessment of 1-D numerical simulations against in vitro measurements. J. Biomech. 2007, 40, 3476–3486. [Google Scholar] [CrossRef]
- Salvucci, F.; Perazzo, C.; Barra, J.; Armentano, R. Assessment of pulsatile wall shear stress in compliant arteries: Numerical model, validation and experimental data. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, Minneapolis, MN, USA, 3–6 September 2009; pp. 2847–2850. [Google Scholar] [CrossRef]
- Bollache, E.; Kachenoura, N.; Redheuil, A.; Frouin, F.; Mousseaux, E.; Recho, P.; Lucor, D. Descending aorta subject-specific one-dimensional model validated against in vivo data. J. Biomech. 2014, 47, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Hackstein, U.; Krickl, S.; Bernhard, S. Estimation of ARMA-model parameters to describe pathological conditions in cardiovascular system models. Inf. Med. Unlocked 2020, 18, 100310. [Google Scholar] [CrossRef]
- Boccadifuoco, A.; Mariotti, A.; Capellini, K.; Celi, S.; Salvetti, M.V. Uncertainty quantification applied to hemodynamic simulations of thoracic aorta aneurysms: Sensitivity to inlet conditions. In Quantification of Uncertainty: Improving Efficiency and Technology; D’Elia, M., Gunzburger, M., Rozza, G., Eds.; Springer: Cham, Switzerland, 2020; pp. 171–192. [Google Scholar] [CrossRef]
- Antonuccio, M.; Mariotti, A.; Celi, S.; Salvetti, M. Effects of the Distribution in Space of the Velocity-Inlet Condition in Hemodynamic Simulations of the Thoracic Aorta. In Proceedings of the 8th International Work-Conference on Bioinformatics and Biomedical Engineering, Granada, Spain, 6–8 May 2020. [Google Scholar] [CrossRef]

| Numerical Techniques | WSS | BP | PWV | WS | Strain | Advantages | Limitations |
|---|---|---|---|---|---|---|---|
| Finite Element Method | ✪ | ✪ | ✪ | ✪ | ✪ | Wide implementation in computing platforms. Ability to handle complex geometries. Preferred to develop CSM models | Requires mesh generation and is more computationally demanding than FVM and FDM |
| Finite Volume Method | ✪ | ✪ | ✪ | ✔ | ✔ | Wide implementation in commercial and open-source software. Preferred to perform CFD simulations | It induces complexity in mesh generation and requires more interpolation algorithms |
| Finite Difference Method | ✔ | ✔ | ✔ | ✔ | ✔ | Easier to implement efficient simulations in rectangular or box-shaped computational domains | Challenging to implement in complex geometric models, particularly for geometries with curved shapes |
| Extended Finite Element Method | ✔ | ✔ | ✔ | ✪ | ✪ | Useful to study crack propagation. It does not require mesh refinement or adaptative meshes | Not recommended for crack branching problems, 3D domains and highly heterogeneous or non-linear media |
| 0D | ✖ | ✪ | ✖ | ✖ | ✖ | Possibility of coupling with 3D models in order to mimic the flow resistance imposed by the downstream vasculatures | Only describes the global behaviour of the system |
| 1D | ✔ | ✔ | ✪ | ✖ | ✖ | Allow quick computations of PWV | Provide incomplete descriptions of the aortic haemodynamics |
| 2D | ✔ | ✔ | ✪ | ✖ | ✔ | Easier to implement than 3D simulations and quicker to compute results | As computer processing and 3D solvers have increased, these simulations have lost relevance |
| Virtual FLux Method | ✪ | ✪ | ✔ | ✖ | ✖ | Resorts to GPU, which allows quicker computations. Enables flow calculation in curved surfaces using Cartesian grids | Challenging to implement patient-specific geometries |
| Regularised Lattice Boltzmann Method | ✪ | ✪ | ✖ | ✖ | ✖ | Designed to run on parallel architectures. Simple implementation and fixed regular grid | Stability problems in particular in heterogeneous media and thermal applications |
| Radial Point Interpolation Method | ✔ | ✔ | ✪ | ✪ | ✪ | Does not require mesh generation | Still a newly developed technology that requires further development |
| Smooth Particle Hydrodynamics | ✔ | ✪ | ✪ | ✪ | ✪ | Meshless and GPU-based method that allows for quicker computations | It does not capture well flow viscous features. Hard to implement inlet and outlet BC |
| Machine Learning | ✪ | ✪ | ✪ | ✪ | ✪ | Wide range of possible applications. Regarding numerical modelling, it is mainly applied to pre- and post-processing tasks | Hard to implement. Highly impacted by the quality of the training data sets |
| First Author | Year | Simulation | Solver | Geometry | Boundary Conditions | Flow | Wall | Main Remarks | Aim of the Work | GRADE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| J. Brunet | 2021 | CSM (XFEM) | Abaqus | AD (I) | LP; Axial DF | - | - | HGO | The propagation of AD is more likely to occur in larger and deeper tears | Study the effect of tear orientation on AD progression | Low |
| A. Totorean | 2021 | CFD (FEM; FVM) | SimVas-cular; Fluent | AAA (PS-CT) | I:CV (0.25 m s−1); O: 0P | L | N | - | Downstream of the abdominal bifurcation, recirculation zones, supraphysiological WSS and long particle residence time were found | Assess geometrical parameters’ influence on AAA and its branches’ haemodynamics | Low |
| B. Melka | 2020 | CFD (FVM) | Fluent | Coarction (I) | I:FR; O:WM | L | MM-E-E | - | One-phased and MM-EE rheological models produced similar results for high-velocity regimens | Investigate the influence of coarction on aortic haemodynamics | Low |
| Y. Chen | 2019 | FSI (FEM; FVM) | Ansys | Aortic Valve (I) | I:PoT; O:— | L | N | NH; S-VK | High bending stiffness causes a reduction in opening area of the AV; low bending stiffness may induce flapping oscillations | Assess the effect of the leaflets’ bending stiffness on FSI simulation | Low |
| R. Campobasso | 2018 | FSI (FEM; FVM) | Ansys | ATAA (PS-MRI) | I:VF; O:FR & WM | L | C | LEI | Stiffer aortas usually present abnormal WS distributions; increased peripheral resistance is correlated with increase in WSS | Evaluate the haemodynamic and structural changes in ATAA behaviour for different wall material properties | High |
| H. Yeh | 2017 | FSI (FEM) | COMSOL | ATAA (PS-Echo) | I:FR; O:CP | L | N | HGO | Significant differences in wall strain, blood pressure, WSS and turbulence were found between normative and hypertensive loading | Analysis of ATAA biomechanics under hypertensive and normative conditions | Low |
| C. García Herrera | 2017 | CSM (FEM) | House-hold code | ATAA (I) | LP (120–160 mmHg); PrS; DF | - | - | D | The stress field in MFS patients was mainly circumferentially oriented, almost uniform and presented higher WS magnitude | Numerically characterise ATAA biomechanics in MFS patients | Low |
| G. Jalalahmadi | 2017 | CSM (FEM) | Ansys | AAA (PS-CT/MRI; I) | LP (120 mmHg) | - | - | N-H; R-V | PWS increased with maximum diameter, asymmetry and turtuosity and decreased with wall thickening | Evaluation of the influence of different geometrical features on PWS | Low |
| J. Long | 2017 | CFD (FVM) | CFX | AD (PS-CT) | I:FR; O:PoT | N | - | WSS in the FL were significantly higher; peak vortical flow appeared at aortic tear initiation points | Further investigate the pathogenesis and progression of AD | Low | |
| B. Šeta | 2017 | CFD (FVM) | Star-CCM+ | Aortic Arch (PS-CT) | I:VP; O:FR | L | C-Y | - | The flow on the aortic arch presented higher velocity near the inner wall, and clockwise helical and recirculating flow | Investigate the haemodynamics in the aortic arch | Low |
| Y. Shi | 2016 | CFD (FVM) | Fluent | AD (PS-CT) | I:PoT; O:PoT | L | N | - | Pressure and WSS seem to be well correlated with tear location | Evaluate the influence of tear location and tear size on AD haemodynamics | Low |
| S. Numata | 2016 | CFD (FVM) | Fluent | TAA(PS-CT) | I:CV (); O:WM | RNG | N | - | Abnormal WSS was correlated with intimal layer (mal)functioning; high OSI was correlated with regions of high probability of rupture | Assess the haemodynamic variations between healthy and TAA patients | Low |
| L. Prahl | 2016 | CFD (FVM) | - | TS (PS-MRI) | I:FR; O:CP | L | Q | - | Anatomical variations contributed to increased WSS and recirculation zones where the n-N effects were significant | Assess how anatomical changes in TS patients influence aortic haemodynamics | Low |
| S. Ahmed | 2016 | CFD (FEM) | CRIMSON | AD (I) | I:FR; O:WM | L | N | - | AD induces increased blood pressure; the FL location is only impactful on blood pressure and velocities on the inner curvature | Evaluate AD haemodynamic alterations associated with anatomical variations | Low |
| H. Chen | 2016 | FSI (FEM) | Abaqus | AD (I) | I:FR; O:PoT | EM | N | LEI | Distally to the flap, lower velocities and higher flow turbulence were found | Assess the haemodynamic and structural features of a flap–blood interaction | Moderate |
| F. Callaghan | 2015 | CFD (FVM) | CFX | TAA (PS-MRI) | I:FR; O:FR; WM | SST | N | - | Vortices were formed at the entrance of the SA, which caused flow turbulence and recirculation zones | Study the haemodynamics on SA | High |
| K. Chaudhari | 2015 | CFD (FVM) | Fluent | Abdominal Stenosis (I) | - | L | N | - | As the blockage increases, the mean flow velocity and WSS magnitude also increase; the changes were significant for arterial blockage above 50% | Evaluate the haemodynamics on abdominal stenosis of different blockage degrees | Low |
| Y. Cong | 2015 | FSI (FEM; FVM) | Ansys | AAA (I) | I:PoT; O:CP | L | N | LEI | AAA haemodynamics were characterised by the formation of two vortices, which were appointed as the dominant aspect of fluid dynamics | Study the influence of vessel dilation and aspect ratio on the haemodynamics | Low |
| M. Moosavi | 2014 | FSI (FEM) | ADINA | Sinus of Valsava (PS-MRI) | I:FR; O:PoT | L | N | LEI | Vortices are formed in the LV and travel towards aorta; higher pressure and WSS were found in the aorta | Compute the haemodynamics inside the LV and aortic sinuses | High |
| T. Fukui | 2013 | FSI (RLBM; VFM) | Own code | Sinus of Valsava (I) | I:FR; O:PoT | LES | N | - | The formation of two vortices near the aortic root was evidenced and correlated with high WSS and diminished blood supply to the myocardium | Assess WSS distributions on sinus of Valsava patients | Low |
| H. Suito | 2013 | CFD (FDM) | - | TA (PS-CT) | I:FR; O:0P | L | N | - | All the tested geometries produce a swirled flow on the TA | Investigate effect of aortic torsion on the blood flow | Low |
| G. Marom | 2013 | FSI | Abaqus | BAV (I) | I:PoT; O:PoT | L | N | LEI | BAV caused more turbulence near the aortic root, and higher velocity and WSS | Evaluate the influence of BAV on blood flow | Moderate |
| S. Pasta | 2013 | FSI (FEM; FVM) | Ansys | AD (PS-CT) | I:VF; O:FR | L | N | RV | In BAV patients, an intrinsic disturbed blood flow that contributes to an asymmetric and elevated WS distribution was evidenced | Study the main differences in haemodynamics and WS between BAV and TAV patients | Low |
| Y. Zhang | 2013 | CFD (FVM) | Fluent | AD (PS-CT) | I:FR; O:0P | L | N | - | The pressure imbalance between the TL and FL is responsible for the delamination of aortic wall | Elucidate the mechanisms of longitudinal propagation of AD | Low |
| E. Shang | 2013 | CSM (FEM) | Abaqus | DTAA (PS-CT) | LP (120 mmHg) | - | - | RV | Positive correlation between PWS and aneurysm growth | Presented new correlations between PWS and aneurysm growth | Low |
| D. Molony | 2011 | FSI (FEM; FVM) | Abaqus; Fluent | AAA (PS-CT) | I:FR; O:PoT | L | N | RV | Aneurysm induced WSS magnitude increase (3-fold) and contributed to disturbed flow patterns | Evaluate the differences in haemodynamics between healthy subjects and AAA | Low |
| M. Alishahi | 2011 | FSI (FEM; FVM) | Ansys | Stenosis (PS-CT) | I:FR; O:PoT | L | PL | LEI | Wall deformation was low but still produced significantly different numerical results | Investigate the effects of pulsatile flow and flexible walls on aortic flow | Low |
| J. Lantz | 2011 | FSI (FEM; FVM) | Ansys | Aorta (PS-MRI) | I:FR; O:WM; EP | N | LEI | The model accurately estimated flow patterns, presenting close agreement with MRI data for lower-velocity regimens; WSS is highly influenced by wall motion | Evaluate the flow dynamics on healthy aorta, in particular WSS distributions | High | |
| Z. Cheng | 2010 | CFD (FVM) | CFX | AD (PS-CT) | I:FR; O:0P | Q | - | Disturbed flow and strong recirculation within both the TL and FL were found | Study the flow in AD and assess the crucial features to aneurysm dilation | Low | |
| Z. Keshavarz-Motamed | 2010 | CFD (FVM) | Fluent | Stenosis; Coarction (I) | I:FR; O:— | N | - | The combination of coarction and stenosis highly influenced WSS and pressure distributions and induced the formation of secondary flow patterns | Study the effect of pulsatile flow on haemodynamics of stenosed and coarcted aortas | Low | |
| T. Gasser | 2009 | CFD (FVM); CSM (FEM) | CFX; VASCOPS | AAA (PS-CT) | I:FR; O:PoT | L | C-Y | - | Significant haemodynamic and structural differences were found between healthy subjects and AAA patients | Study haemodynamic and structural features of AAA | Low |
| C. Gaudio | 2006 | CFD (FVM) | Fluent | Aortic Arch (I) | I:FR; O:FR | L | PL | - | Modelling the aortic root motion influenced the numerical results; intimal thickening occurred in low OSI and WSS regions | Show the potential of numerical tools to recreate the haemodynamics of the aortic arch | Low |
| First Author | Year | Simulation | Solver | Geometry | Boundary Conditions | Flow | Wall | Main Remarks | Aim of the Work | GRADE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G. Comunale | 2021 | FSI (FEM) | Abaqus | TA (PS-MRI) | I:FP; O:CP | L | N | O; HGO | Geometrical factors highly influence the haemodynamic patterns; wall compliance was the dominant factor for the overall aortic mechanical behaviour | Assess the viability of using in vitro silicone models to mimic vascular tissue | Moderate |
| E. Ban | 2021 | CSM (FEM) | - | AD (RVol) | Fluid injection | - | - | N-H; F | The maximum pressure prior to tearing is highly dependent on local geometry and material properties | Assess which location of the aortic wall is more likely to dissect | Low |
| R. Wang | 2020 | CSM (FEM) | Abaqus | AD (RVol) | UT | - | - | HGO | Glucose-treated elastin presented increased peeling force, energy release rate and interlamellar strength | Evaluate the effect of elastin glycation on AD progression | Moderate |
| S. Maiti | 2020 | CSM (FEM) | Own code | AD (RVol) | UT | - | - | LEI | In biaxial conditions, the rupture is likely to occur in any direction; organisation and fibre properties are the main contributors to aortic strength | Assess the relevant changes in aortic wall microstructure during rupture | Low |
| S. Farzaneh | 2019 | CSM (FEM) | Abaqus | ATAA (PS-CT) | DF; LP (80 mmHg) | - | - | HGO | Extensional stiffness (higher in the ascending aorta) and high rupture risk were statistically correlated | Develop a technique to non-invasively identify aortic wall mechanical properties | High |
| O. Gültekin | 2019 | CSM (FEM) | - | AD (RVol) | DF; LP (80–600 mmHg); Tor | – | - | HGO | AD development is consequence of in-plane shear stresses created by the heterogeneity of aortic wall properties and FL propagation occurs mainly due to the secondary blood flow | Study the mechanisms that explain the beginning and progression of AD | Low |
| M. Frank | 2019 | CSM (FEM) | - | Aortic Media (RVol) | BT | - | - | Y; NH | The developed model captured the overall response only for physiological pressure ranges | Present a new micromechanically based wall constitutive model | Moderate |
| J. Thunes | 2017 | CSM (FEM) | MatLab | Aorta (RVol) | Axial DF | - | - | N-H; LEI | Collagen fibres present higher strength and stiffness but lower failure stretch when compared to elastin; collagen breakage is the governing tissue failure mechanism | Study the microstructural response of the aortic wall to rupture | Moderate |
| J. Thunes | 2016 | CSM (FEM) | MatLab | Aorta (RVol) | Axial DF | - | - | N-H; LEI | Collagen fibres oriented in loading direction were more solicited | Study the response of aortic wall microstructure under UT conditions | Low |
| H. Taghizadeh | 2015 | CSM (FEM) | Own-Code | Aorta (RVol) | LP (100–160 mmHg) | - | - | HGO | Elastin fibres have a dominant role for lower strains | Study microstructure components of the aortic wall under hypertensive conditions | Low |
| A. Cloonan | 2014 | FSI (FEM) | Abaqus | AAA (PS-CT) | I:FR; O:RF | L | N | HI | The materials presented similar mechanical properties to vascular tissue; PWV results were consistent with FSI simulation findings | Assess whether certain phantom materials are good replicates of vascular tissue | Moderate |
| S. Yang | 2014 | FSI (FVM) | ACE + | TA (PS-MRI) | I:FR; O:FR | L | N | LEI | The proposed method may be used to evaluate whether the considered material parameters are suitable | Present a new method for arterial wall compliance assessment | Low |
| A. Azadani | 2012 | CSM (FEM) | Matlab | AS (I) | BT | - | - | 2D F | Ascending aorta (more compliant) and AS tissue presented significantly different material properties | Evaluate ATAA and AS material properties differences | Moderate |
| F. Helderman | 2008 | CSM (FEM) | - | AAA (PS-CT) | LP | - | - | LEI | Aneurysmal growth was correlated with elevated aortic stiffness | Evaluate the aortic microstructure alterations promoted by abnormal WS distributions | Low |
| First Author | Year | Simulation | Solver | Geometry | Boundary Conditions | Flow | Wall | Main Remarks | Aim of the Work | GRADE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| V. Badeli | 2021 | CSM (FEM) | COMSOL | AD (I) | Specific Voltage | - | - | - | The use of Bayesian approach outperformed regular multi-sensor impedance cardiography methodology | Intended to simulate the propagation of trans-thoracic electric pulses | Low |
| M. Liu | 2021 | CSM (FEM) | - | ATAA (PS-CT) | LP (80–120 mmHg); PrS | - | - | HGO | The developed ML tool outperformed other rupture risk estimation methods | Identify the locations where the aortic rupture is more likely to occur | Low |
| S. Pasta | 2020 | CFD (FVM) | Fluent | ATAA (PS-CT) | I:CV; O:WM | L | C | - | A combination of haemodynamic, wall parameters and circulating biomarkers may be a suitable method to assess the risk of acute complications | Investigate the relation between WSS and aortic wall strain and the presence of biomarkers | Low |
| A. Bauer | 2019 | CFD (FVM) | Open- FOAM | AAA (I) | I:FR; O:0G | L | N | - | Laser Doppler velocimetry is the preferred technique to estimate WSS | Evaluate the gold-standard method to estimate WSS | Moderate |
| M. Bopp | 2019 | CFD (FVM) | Open- FOAM | AAA (I) | I:FR; O:0G | RANS | N | - | Magnetic resonance velocimetry captured better the flow turbulence and better predicted WSS when compared to WSS | Present validated numerical descriptions of AAA blood flow to improve MRV efficiency | Moderate |
| N. Kizilova | 2018 | CFD (2D) | - | Aorta | I:FR; O:Imp | L | K-V | LEI | The developed model found good agreement with in vivo data and outperformed regular rigid wall simulations | Develop a validated realistic model of the aorta that is able to evaluate PWV | High |
| C. Zhu | 2018 | CFD (FVM) | - | AS (I) | I:CV; O:— | DNS | N | - | Murmur source location is not correlated with stenosis degree and is not coincident with stenosis location | Enhance auscultation by studying post-stenosis haemodynamics | Low |
| J. Sotelo | 2018 | CFD (FEM) | MatLab | Aorta (PS-MRI) | I:PoT; O:PoT | L | N | - | The circumferential component of WSS presented good correlation with disturbed flow | Implement a method to estimate WSS different components | Low |
| F. Condemi | 2017 | CFD (FVM) | Fluent | ATAA; AI (PS-MRI) | I:VF; O:FR; WM | L | C | - | Inflow jet impingement against the aortic wall creates a non-homeostatic WSS distribution | Study the role of altered haemodynamics in aortic rupture risk | High |
| I. Sazonov | 2017 | CFD (1D) | - | AAA | I:FR; O:RF | L | N | LEI | Aortic compliance alterations may be a good monitoring metric for diagnosing aneurysms that can be estimated via ultra-sound measurements | Develop a new diagnosis technique for aneurysms based on 1D modelling | Moderate |
| S. Pasta | 2016 | FSI (FEM; FVM) | Abaqus; Fluent | BAV (PS-CT) | I:FR; O:WM | L | N | P | BAV patients showed increased WSS and WS when compared to TAV patients | Evaluation of new ascending aortic dilatation risk predictors | Low |
| H. Mohammadi | 2015 | CFD (2D) | LS-Dyna | AS | I:PoT; O:0P | L | N | LEI | Systolic transvalvular pressure gradient is useful to evaluate the degree of stenosis | Improve the diagnosis efficiency of aortic stenosis | Low |
| J. Sotelo | 2015 | CFD (FEM) | MatLab | Aorta (PS-MRI) | I:PoT; O:PoT | L | N | - | Wall deformation highly impacted the WSS magnitude | Develop a new technique to evaluate WSS distributions in the aorta | Low |
| C. Babbs | 2014 | CFD (0D) | Excel | CS | I:PoT; O:R | - | - | F | The proposed methodology presented good estimation when constant compliance was applied | Develop a new technique to measure cardiac stroke volume | Low |
| E. Shang | 2013 | CSM (FEM) | Abaqus | DTAA (PS-CT) | LP (120 mmHg) | - | - | RV | Positive correlations between PWS and aneurysm growth were found | Study the rupture of aneurysms | Low |
| B. Doyle | 2009 | CSM (FEM) | Abaqus | AAA (I) | DF | - | - | O | The numerical results accurately predicted the sites of rupture | Simulate the rupture in aneurysms using in silico and in vitro experiments | Moderate |
| First Author | Year | Simulation | Solver | Geometry | BC Conditions | Flow | Wall | Main Remarks | Aim of the Work | GRADE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R. Valente | 2022 | FSI (FEM) | SimVas- cular | ATAA (PS-CT) | I:FR; O:WM | L | N | N-H | The developed method contributed to improved convergence | Develop a novel method to separately mesh the fluid and solid domains | Low |
| A. Mariotti | 2021 | FSI (FEM) | SimVas- cular | TA (PS-MRI) | I:VP-MRI; O:WM | L | N | LEI | WSS presented significant differences for different inlet flow wave forms | Evaluate the numerical results’ sensitivity to the inlet flow rate waveform | Low |
| A. Mourato | 2021 | CFD (FVM) | Open- FOAM | ATAA (PS-CT) | I:FR; O:PoT | SST | CY | - | Turbulence and n-N behaviour of blood is not relevant to be modelled in ATAA | Evaluate the relevance of modelling turbulence and n-N behaviour | Low |
| K. Baumler | 2020 | FSI (FEM) | SimVas- cular | AD (PS-CTA) | I:VP; O:WM; PrS; EP | L | N | N-H | The inclusion of two-way FSI, PrS and adequate wall and flap material properties improved the numerical results’ agreement with in vivo data | Assess the relevance of modelling realistic wall and dissection flap deformation on AD | High |
| U. Hackstein | 2020 | CFD (0D) | SISCA | AAA | I:PoT; O:-PoT | L | N | - | Certain estimated model parameters presented significant differences between healthy and diseased subjects | Estimate coefficients of 0D models using ARMA approach, which may assist in clinical diagnosis | Low |
| M. Biancolini | 2020 | CFD (FVM) | Fluent | ATAA (PS-MRI) | I:VF; O:FR; WM | L | C-Y | - | ROM and RBF mesh morphing allow one to explore new results interactively and almost in real time | Assess the relevance of using mesh morphing and ROM techniques | Low |
| M. Lucio | 2020 | CSM (FEM) | Abaqus | AAA (I) | LP (120 mmHg); DF | - | - | HGO | Intima presents early exponential stiffening, which contributes to the load bearing of the aortic wall | Evaluate the intima layer’s load-bearing effect on aged aortas | Low |
| J. Silva | 2019 | CSM (RPIM) | - | AAA (I) | LP | - | - | LEI | FEM and RPIM models produced similar results | Test the viability of using RPIM methods | Low |
| M. Nowak | 2019 | FSI (FEM; FVM) | Ansys | Coarction (PS-MRI) | I:VP; O:OP | L | C | LEI | FSI simulations increase in relevance for decreased wall stiffness | Assess the relevance of resorting to FSI simulations | High |
| C. Aricò | 2019 | CFD (SPH) | - | AAA (PS-CT) | I:VP; O:PoT; DF | L | N | - | Moving wall simulations produced more realistic haemodynamics | Present a novel tool to predict AAA haemodynamics | Low |
| G. de Nisco | 2018 | CFD (FVM) | - | ATAA (PS-MRI) | I:VP; O:FR | L | N | - | Stroke volume and cardiac cycle duration presented significant influence on the numerical results | Explore the sensitivity of the blood-to-wall LDL transfer to inlet BC | Low |
| K. Capellini | 2018 | CFD (FVM) | Fluent | ATAA (PS-MRI) | I:VP; O:WM | L | N | - | Significant haemodynamic changes appeared only for a 60% increase in aneurysm diameter | Address the effects of geometrical changes on haemodynamics | Low |
| C. Àrico | 2018 | CFD (SPH) | - | AAA (I) | I:FR; O:PoT; DF | L | N | - | The model was able to accurately reproduce the biomechanical behaviour of the aorta | Present a novel technique to perform FSI simulations using SPH and moving BC | Low |
| S. Attaran | 2018 | FSI (FEM) | ADINA | Aorta (I) | I:FR; O:2D | L | N | LEI | The developed BC is able to accurately model pulse wave reflection | Present a new model to apply as an outlet BC | Low |
| T. Koltukluoǧlu | 2018 | CFD (FEM) | Open- FOAM | Aorta (PS-MRI) | - | L | N | - | The presented technique outperformed classical CFD approaches | Present an inverse method to estimate in vivo BC | Moderate |
| V. Mendez | 2018 | FSI (FEM; FVM) | Ansys | ATAA (PS-MRI/CT) | I:FR; O:WM | L | N | HGO | Both WSS and WS presented significant differences between the simplest approaches and FSI models | Assess if FSI formulation is more accurate than CFD or CSM analysis | Low |
| J. Febina | 2018 | CFD (FVM) | Star-CCM+ | SA (PS-CT) | I:FR; O:FR | L | N | - | For modelling SA assuming pulsatile, laminar and n-N flow are key factors | Provide insights into SA modelling in adequate numerical settings | Low |
| L. Liang | 2018 | CSM (FEM) | Abaqus | TA (I) | LP (10–16 kPa) | - | - | HGO | FEM models can successfully train ML tools with considerable reduction in computational time | Estimate the reference configuration of human TA resorting to ML tools | Low |
| W. Wolánski | 2017 | FSI (FEM; FVM) | Ansys | Aorta (PS-CT) | I:FR; O:FR | L | N | NH | Rigid wall simulations overestimate pressure and WSS-driven metrics | Assess how the intramural pressure changes with wall stiffness | Low |
| J. Bols | 2016 | FSI (FEM) | Abaqus; Fluent | Coarction (PS) | I:FR; O:FR; WM | L | N | PH | Automated mesh generation can be useful to reduce the reporting time | Reduce the computational effort of grid generation | Low |
| M. Alimohammadi | 2015 | FSI (FEM; FVM) | Ansys | AD (PS-CT) | I:FR; O:WM | SST | C-Y | RV | FSI models have increased accuracy, but for AD, the required computational time can be excessive | Assess the haemodynamic changes and the stresses in the flap–blood interaction | Low |
| L. Taelman | 2014 | FSI (FEM; FVM) | Abaqus; Fluent | Coarction (I) | I:PoT; O:RF | L | N | MR | Numerical dissipation and diffusion are mainly determined by spatial and time discretisation | Study the effects of coarction on pulse wave reflection | Low |
| W. Chen | 2014 | CFD (FVM) | Open- FOAM | Aorta(I) | I:FR; O:— | DNS | N | - | No significant changes were found using DNS for turbulence modelling | Study the impact of modelling the turbulence of blood flow | Low |
| E. Bollache | 2014 | CFD (1D) | - | DTA | I:FR; O:WM | L | N | - | PWV, total arterial resistance and compliance were calculated and validated against MRI data | Evaluate the application of 1D modelling on cardiovascular medicine | High |
| Y. Aboelkassem | 2014 | CFD (0D) | - | AV | - | - | - | - | No relevant conclusions were presented besides the fact that it was suggested as realistic | Mathematical model that recreates AV dynamics | Low |
| E. Bollache | 2013 | CFD (1D) | - | Aorta | I:FR; O:WM | L | N | - | Numerical results closely reproduced velocity, PWV and area changes | Evaluate the efficiency of using 1D models to estimate PWV | High |
| J. Lantz | 2013 | CFD (FVM) | CFX | Coarction (PS-MRI) | I:VP; O:FR; PoT | LES | N | - | TKE may be a good indicator of abnormal or pathophysiological flow conditions | Compare the results of CFD and MRI data | High |
| G. Marom | 2012 | FSI (FEM) | Abaqus | BAV (I) | I:PoT; O:PoT | L | N | CFN | The CSM simulation overestimated by 30% the coaptation area, 55% the contact pressure and 170% the closure time | Evaluate the difference in modelling AV with CSM or FSI | Low |
| H. Suito | 2013 | CFD (FDM) | - | TA (SemiPS-CT) | I:FR; O:0P | L | N | - | All the tested geometries produce a swirled flow somewhere on the TA | Investigate the blood flow in TA, focusing on the effect of torsion | Low |
| B. Doyle | 2012 | CSM (FEM) | Abaqus | AAA (PS-CT) | LP (120 mmHg) | - | - | PH | Using patient-specific parameters had significant impact on the results | Evaluate the impact of considering patient-specific material properties | Low |
| F. He | 2009 | CFD (FDM) | - | Aorta (I) | I:VP; O:PoT | L | N | - | Under transient conditions, inlet and outlet BC significantly altered the numerical results | Assess the sensitivity of numerical results to the chosen BC | Low |
| F. Salvucci | 2009 | CFD (1D) | MatLab | Aorta | VP; PoT | L | N | - | The developed model presented good agreement with in vivo data | Novel method to estimate WSS distribution | High |
| F. Carneiro | 2008 | CFD (FVM) | Fluent | Abdominal aorta (I) | I:CV (0.234 m s−1); O:— | N; C-Y | - | Small differences in WSS were found between different rheological models | Explore the haemodynamics in the bifurcation of the abdominal aorta | Moderate | |
| K. Matthys | 2007 | CFD (1D) | - | Aorta | I:FR; O:FR; PoT | L | N | - | Energy losses at bifurcations had a secondary effect on the blood flow and wall viscoelasticity might have a significant effect on PWV | Explore the effects on PWV of energy loss and fluid inertia | Moderate |
| K. Khanafer | 2007 | CFD (FEM) | Fidap | AAA (I) | I:FR; O:0G | n-N | - | It is relevant to model n-N behaviour and turbulence in AD | Evaluate the influence of pulsatile, turbulent and n-N flow | Low | |
| R. Berguer | 2006 | CFD (FEM) | Fidap | AAA (I) | I:FR; O:0G | n-N | - | High blood pressure and turbulence were postulated as contributors to aneurysm rupture | Same as Khanafer et al. [109] | Low | |
| A. Geertsema | 1997 | CFD (0D) | - | CS | - | - | - | - | The model was able to simulate haemodynamic waveforms for different heart frequencies and heart diseases | Developed a new numerical model of the cardiovascular system | High |
| A. Owen | 1992 | CFD (1D) | - | AV | PoT | L | N | - | Blood pressure, PWV and wall properties presented good agreement with theory and empirical data | Develop a model that estimates the haemodynamics of the AV | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourato, A.; Valente, R.; Xavier, J.; Brito, M.; Avril, S.; de Sá, J.C.; Tomás, A.; Fragata, J. Computational Modelling and Simulation of Fluid Structure Interaction in Aortic Aneurysms: A Systematic Review and Discussion of the Clinical Potential. Appl. Sci. 2022, 12, 8049. https://doi.org/10.3390/app12168049
Mourato A, Valente R, Xavier J, Brito M, Avril S, de Sá JC, Tomás A, Fragata J. Computational Modelling and Simulation of Fluid Structure Interaction in Aortic Aneurysms: A Systematic Review and Discussion of the Clinical Potential. Applied Sciences. 2022; 12(16):8049. https://doi.org/10.3390/app12168049
Chicago/Turabian StyleMourato, André, Rodrigo Valente, José Xavier, Moisés Brito, Stéphane Avril, José César de Sá, António Tomás, and José Fragata. 2022. "Computational Modelling and Simulation of Fluid Structure Interaction in Aortic Aneurysms: A Systematic Review and Discussion of the Clinical Potential" Applied Sciences 12, no. 16: 8049. https://doi.org/10.3390/app12168049









