Characterization of Tissue Equivalent Materials Using 3D Printing for Patient-Specific DQA in Radiation Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. 3D Printer
2.2. Infill Density for the 3D Printing Materials
2.3. Fabrication of PLA/PBAT/Baso4 Mixed Filaments
2.4. CT Scan
2.5. Uniformity
3. Results
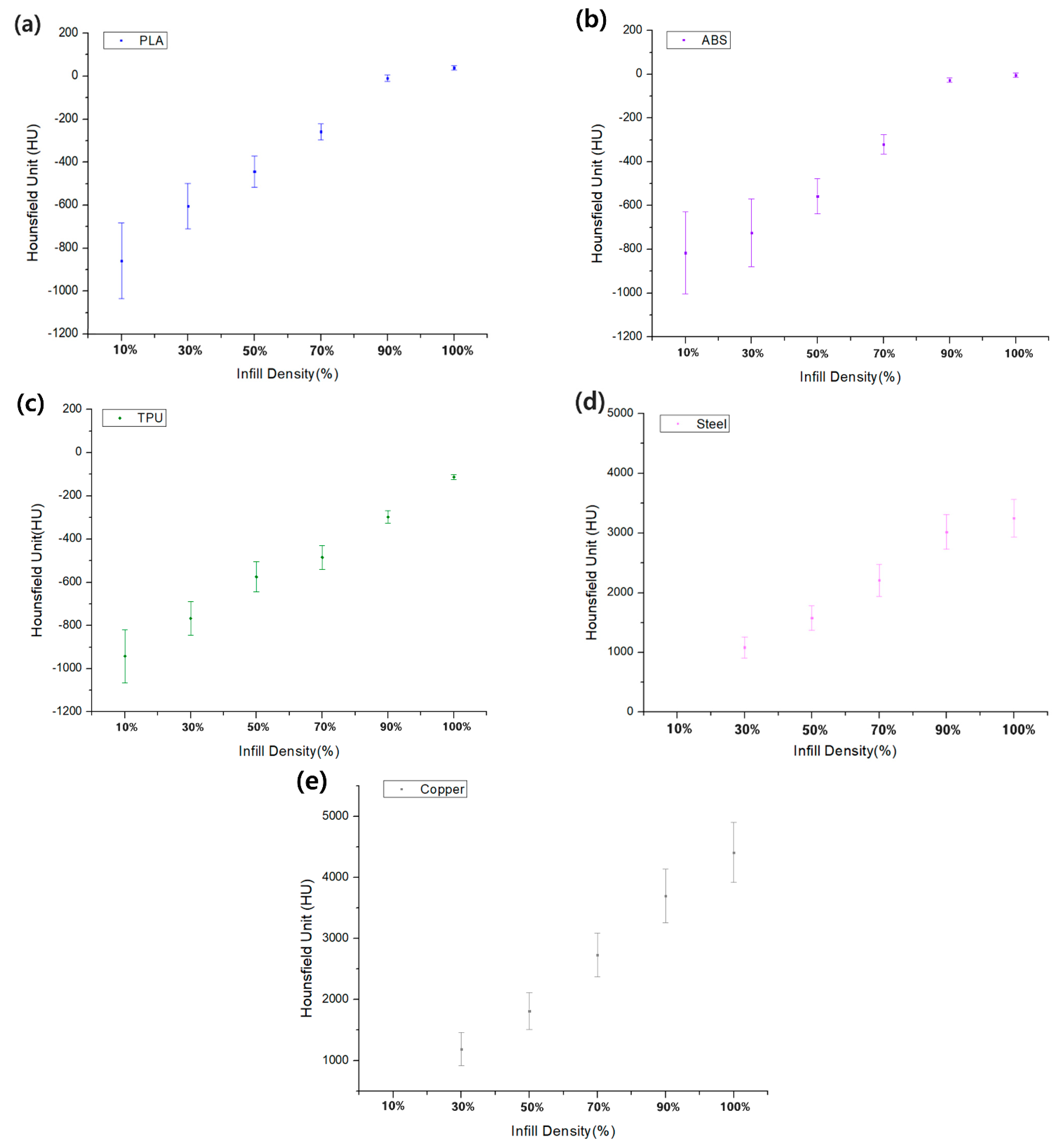
3.1. Comparison of the HU Value for Infill Density and Infill Patterns
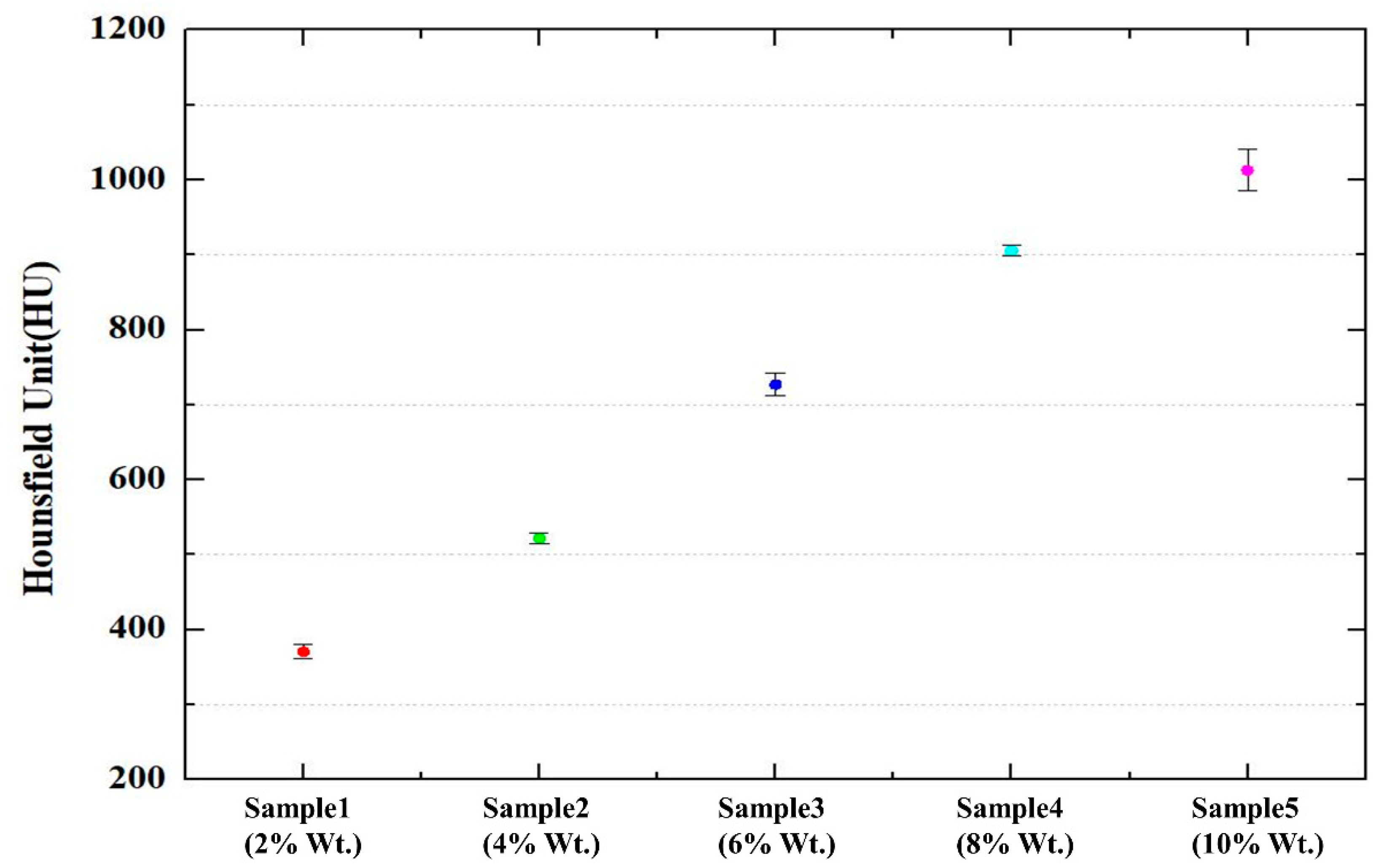
3.2. Characteristics of Mixed Filament
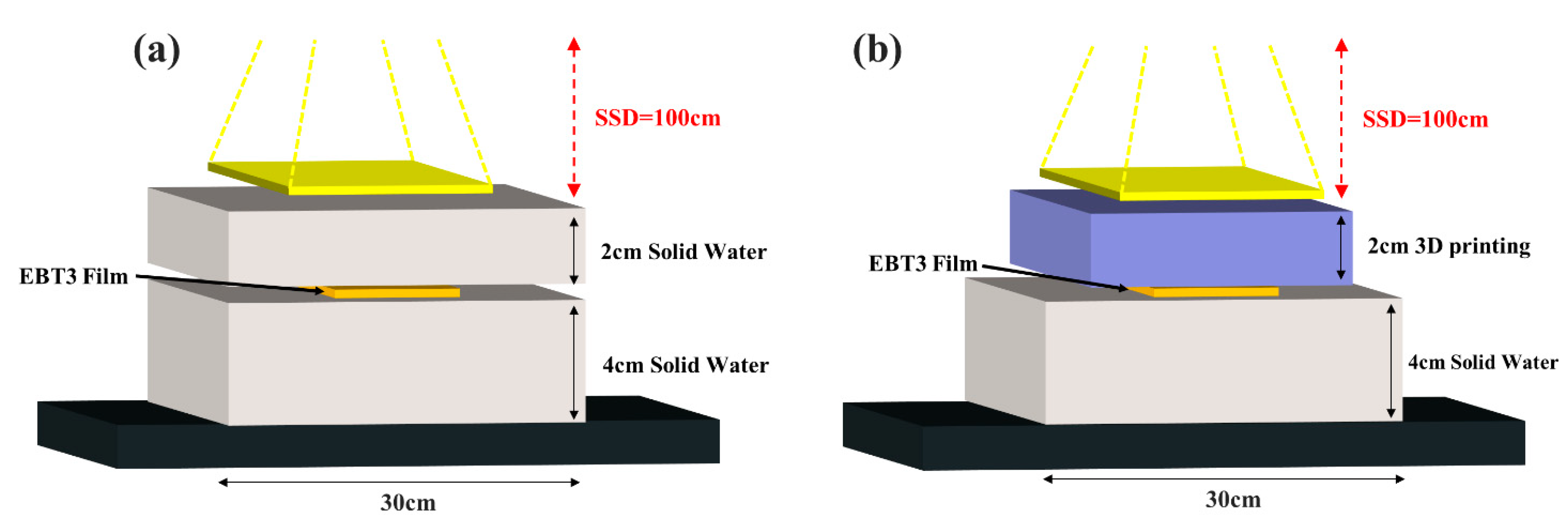
3.3. Analysis of Radiation Effect in Clinical Photon Beam Using Gafchromic Film
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haleem, A.; Javaid, M.; Suman, R.; Singh, R.P. 3D printing applications for radiology: An overview. Indian J. Radiol. Imaging 2021, 31, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Travers, S.Z. Biomodeling and 3D Printing: A Novel Radiology Subspecialty. Ann. 3D Print. Med. 2021, 4, 100038. [Google Scholar] [CrossRef]
- O’Reilly, M.; Hoff, M.; Friedman, S.D.; Jones, J.F.X.; Cross, N.M. Simulating tissues with 3d-printed and castable materials. J. Digit. Imaging 2020, 33, 1280–1291. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Zhao, H.; He, Z.; Shi, L.; He, Y.; Ju, N.; Rong, Y.; Qiu, J. Design and fabrication of a personalized anthropomorphic phantom using 3D printing and tissue equivalent materials. Quant. Imaging Med. Surg. 2019, 9, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hou, K.; Dyer, B.A.; Chen, J.; Shi, L.; Sun, Y.; Xu, L.; Zhao, H.; Li, Z.; Chen, T.; et al. Constructing customized multimodal phantoms through 3D printing: A preliminary evaluation. Front. Phys. 2021, 9, 605630. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Dyer, B.A.; Boone, J.M.; Liu, S.; Chen, T.; Zheng, F.; Zhu, Y.; Sun, Y.; Rong, Y.; et al. 3D-printed breast phantom for multi-purpose and multi-modality imaging. Quant. Imaging Med. Surg. 2019, 9, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Giron, I.; den Harder, J.M.; Streekstra, G.J.; Geleijns, J.; Veldkamp, W.J.H. Development of a 3D printed anthropomorphic lung phantom for image quality assessment in CT. Phys. Med. 2019, 57, 47–57. [Google Scholar] [CrossRef]
- Watanabe, Y. Derivation of linear attenuation coefficients from CT numbers for low-energy photons. Phys. Med. 1999, 44, 2201–2210. [Google Scholar] [CrossRef]
- Thomas, S.J. Relative electron density calibration of CT scanners for radiotherapy treatment planning. Br. J. Radiol. 1999, 72, 781–787. [Google Scholar] [CrossRef]
- Qi, Y.; He, L.; Wang, Z.; Liu, Y.; Liu, H.; Huo, W.; Xu, X.G.; Chen, Z. Evaluation of secondary dose and cancer risk for out-of-field organ in esophageal cancer IMRT in a Chinese hospital using ATOM phantom measurements. Radiat. Prot. Dosim. 2017, 177, 389–396. [Google Scholar] [CrossRef]
- Oh, S.A.; Kim, M.J.; Kang, J.S.; Hwang, H.S.; Kim, Y.J.; Kim, S.H.; Kim, S.K. Feasibility of Fabricating Variable Density Phantoms Using 3D Printing for Quality Assurance (QA) in Radiotherapy. Prog. Med. Phys. 2017, 28, 106–110. [Google Scholar] [CrossRef]
- Yea, J.W.; Park, J.W.; Kim, S.K.; Kim, D.Y.; Kim, J.G.; Seo, C.Y.; Jeong, W.H.; Jeong, M.Y.; Oh, S.A. Feasibility of a 3D-printed anthropomorphic patient-specific head phantom for patient-specific quality assurance of intensity-modulated radiotherapy. PLoS ONE 2017, 12, e0181560. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Gao, R.; Li, M.; Qiu, J. Mechanical and medical imaging properties of 3D-printed materials as tissue equivalent materials. J. Appl. Clin. Med. Phys. 2021, 23, e13495. [Google Scholar] [CrossRef] [PubMed]
- Chandekar, A.; Dinesh, K.M.; Sharma, S.; Saraogi, G.K.; Gupta, U.; Gupta, G. 3D Printing Technology: A New Milestone in the Development of Pharmaceuticals. Curr. Pharm. Des. 2019, 25, 937–945. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.F.; Thieringer, F.M. Evaluation of the dimensional accuracy of 3D-printed anatomical mandibular models using FFF, SLA, SLS, MJ, and BJ printing technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef]
- Hong, D.; Lee, S.; Kim, G.B.; Lee, S.M.; Kim, N.; Seo, J.B. Development of a CT imaging phantom of anthromorphic lung using fused deposition modeling 3D printing. Medicine 2020, 99, e18617. [Google Scholar] [CrossRef]
- Madamesila, J.; McGeachy, P.; Villarreal Barajas, J.E.; Khan, R. Characterizing 3D printing in the fabrication of variable density phantoms for quality assurance of radiotherapy. Phys. Med. 2016, 32, 242–247. [Google Scholar] [CrossRef]
- Kairn, T.; Crowe, S.B.; Markwell, T. Use of 3D Printed Materials as Tissue-Equivalent Phantoms. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Toronto, ON, Canada, 7–12 June 2015; Springer: Cham, Switzerland, 2015; pp. 728–731. [Google Scholar] [CrossRef]
- Dancewicz, O.L.; Sylvander, S.R.; Markwell, T.S.; Crowe, S.B.; Trapp, J.V. Radiological properties of 3D printed materials in kilovoltage and megavoltage photon beams. Phys. Med. 2017, 38, 111–118. [Google Scholar] [CrossRef]
- Ma, X.; Buschmann, M.; Unger, E.; Homolka, P. Classification of X-ray attenuation properties of additive manufacturing and 3D printing materials using computed tomography from 70 to 140 kVp. Front. Bioeng. Biotechnol. 2021, 9, 763960. [Google Scholar] [CrossRef]
- Kairn, T.; Crowe, S.B.; Fogg, P.; Trapp, J.V. The appearance and effects of metallic implants in CT images. Australas Phys. Eng. Sci. Med. 2013, 36, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Yartsev, S. kVCT, MVCT, and hybrid CT image studies—Treatment planning and dose delivery equivalence on helical tomotherapy. Med. Phys. 2010, 37, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Jusufbegović, M.; Pandžić, A.; Šehić, A.; Jašić, R.; Julardžija, F.; Vegar-Zubović, S.; Beganović, A. Computed tomography tissue equivalence of 3D printing materials. Radiography 2022, 28, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Q.; Liu, G. A Review of 3D Printed Bone Implants. Micromachines 2022, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.A.; Lambert, J.; Greer, P.B. CT-ED conversion on a GE LightSpeed-RT scanner: Influence of scanner settings. Australas. Phys. Eng. Sci. Med. 2008, 31, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, J.I.; Park, S.Y.; Oh, D.H.; Kim, S.T. Reliability of the gamma index analysis as a verification method of volumetric modulated arc therapy plans. Radiat. Oncol. 2018, 13, 175. [Google Scholar] [CrossRef]
- Michael, G. X-ray computed tomography. Phys. Educ. 2001, 36, 442–451. [Google Scholar] [CrossRef]
- Kairn, T.; Zahrani, M.; Cassim, N.; Livingstone, A.G.; Charles, P.H.; Crowe, S.B. Quasi-simultaneous 3D printing of muscle-, lung-and bone-equivalent media: A proof-of-concept study. Phys. Eng. Sci. Med. 2020, 43, 701–710. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Brandt, M.; Leary, M.; Kron, T. The interlace deposition method of bone equivalent material extrusion 3D printing for imaging in radiotherapy. Mat. Design 2021, 1, 109439. [Google Scholar] [CrossRef]
- Van der Walt, M.; Crabtree, T.; Albantow, C. PLA as a suitable 3D printing thermoplastic for use in external beam radiotherapy. Phys. Eng. Sci. Med. 2019, 42, 1165–1176. [Google Scholar] [CrossRef]
- Wu, J.K.; Yu, M.C.; Chen, S.H.; Liao, S.H.; Wang, Y.J. Low cost multifunctional 3D printed image quality and dose verification phantom for an image-guided radiotherapy system. PLoS ONE 2022, 17, e0266604. [Google Scholar] [CrossRef] [PubMed]



| Filament Name | Manufacturer | Extruder Temperature (°C) | Physical Density (g/cm3) |
|---|---|---|---|
| PLA | Basf Ultrafuse | 205 | 1.25 |
| ABS_Fusion+ | Basf Ultrafuse | 250 | 1.075 |
| TPU 85A | Basf Ultrafuse | 200 | 1.082 |
| Steel | ColorFabb | 210 | 3.13 |
| Copper | ColorFabb | 220 | 3.8 |
| BaSO4 Ratio (%) | PBAT Ratio (%) | PLA Ratio (%) | HU Value |
|---|---|---|---|
| 2 | 40 | 58 | 371 ± 9 |
| 4 | 40 | 56 | 522 ± 7 |
| 6 | 40 | 54 | 727 ± 15 |
| 8 | 40 | 52 | 905 ± 7 |
| 10 | 40 | 50 | 1013 ± 28 |
| Tissue | CIRS Material (HU) | Std. Dev | Filament | 3D Printing Materials (HU) | Std. Dev. |
|---|---|---|---|---|---|
| Water | 3.0 | 1.6 | ABS (100%) | −2 | 6 |
| Adipose | −63.3 | 2.9 | PLA (90%) | −60 | 8.3 |
| Breast | −35.1 | 2.5 | TPU (100%) | −32 | 8 |
| Liver | 47.1 | 2.0 | PLA (100%) | 42 | 8 |
| Lung inhale | −772.1 | 7.5 | TPU (30%) | −766 | 84 |
| Lung exhale | −492.1 | 6.1 | PLA (50%) | −490 | 53 |
| TPU (70%) | −484 | 41 | |||
| Trabecular bone | 236.9 | 7.5 | BaSO4 Mix (2%) | 371 | 9 |
| Dense bone | 898.8 | 28.4 | BaSO4 Mix (8%) | 905 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Jang, Y.J.; Kim, K.B.; Bahng, J.; Choi, S.H. Characterization of Tissue Equivalent Materials Using 3D Printing for Patient-Specific DQA in Radiation Therapy. Appl. Sci. 2022, 12, 9768. https://doi.org/10.3390/app12199768
Choi Y, Jang YJ, Kim KB, Bahng J, Choi SH. Characterization of Tissue Equivalent Materials Using 3D Printing for Patient-Specific DQA in Radiation Therapy. Applied Sciences. 2022; 12(19):9768. https://doi.org/10.3390/app12199768
Chicago/Turabian StyleChoi, Yona, Young Jae Jang, Kum Bae Kim, Jungbae Bahng, and Sang Hyoun Choi. 2022. "Characterization of Tissue Equivalent Materials Using 3D Printing for Patient-Specific DQA in Radiation Therapy" Applied Sciences 12, no. 19: 9768. https://doi.org/10.3390/app12199768





