Phytotoxicity and Accumulation of Antibiotics in Water Lettuce (Pistia stratiotes) and Parrot Feather (Myriophyllum aquaticum) Plants under Hydroponic Culture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibiotics and Chemicals
2.2. Experimental Design
2.3. Assay of Phytotoxicity Parameters of Water Plants
2.4. Antibiotic Analysis in Water and Plant Samples
2.5. Bioconcentration Factor
3. Results and Discussion
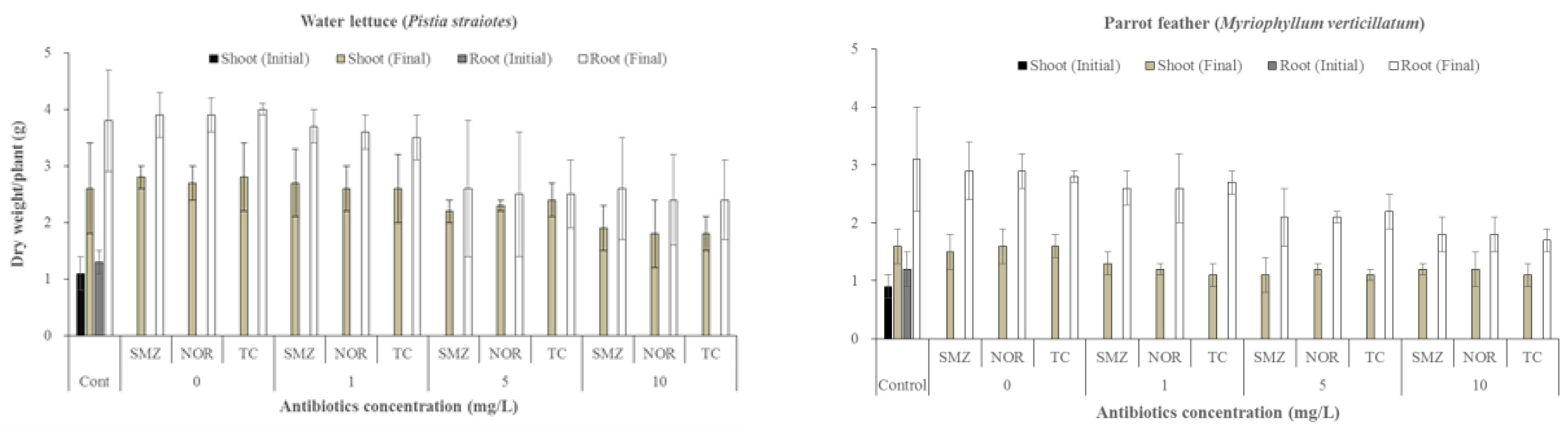
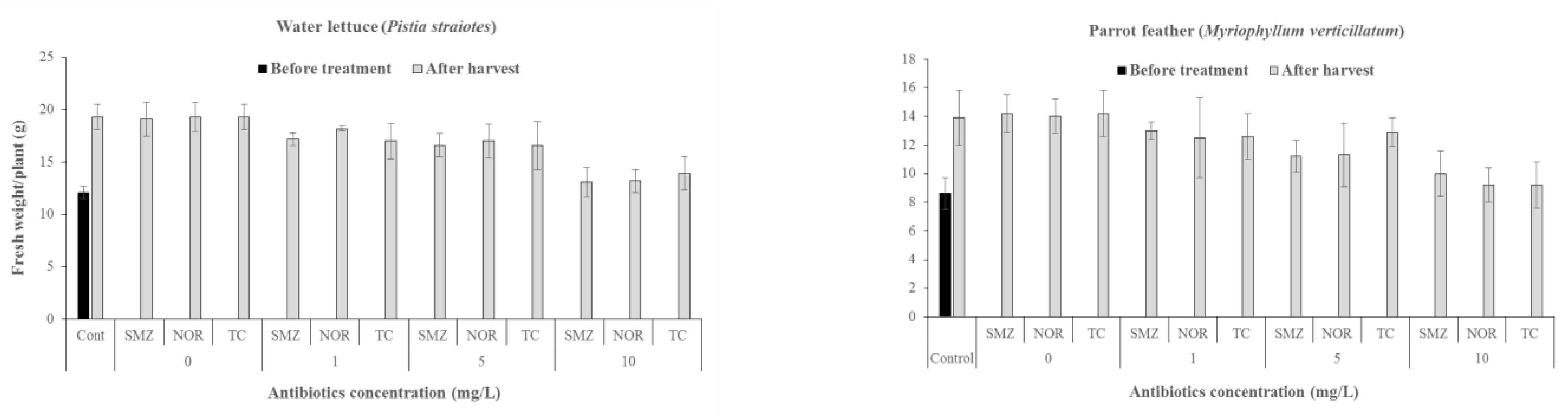
3.1. Effects of Antibiotics on Growth of Water Plants
3.2. Effects of Antibiotics on Chlorophyll Content
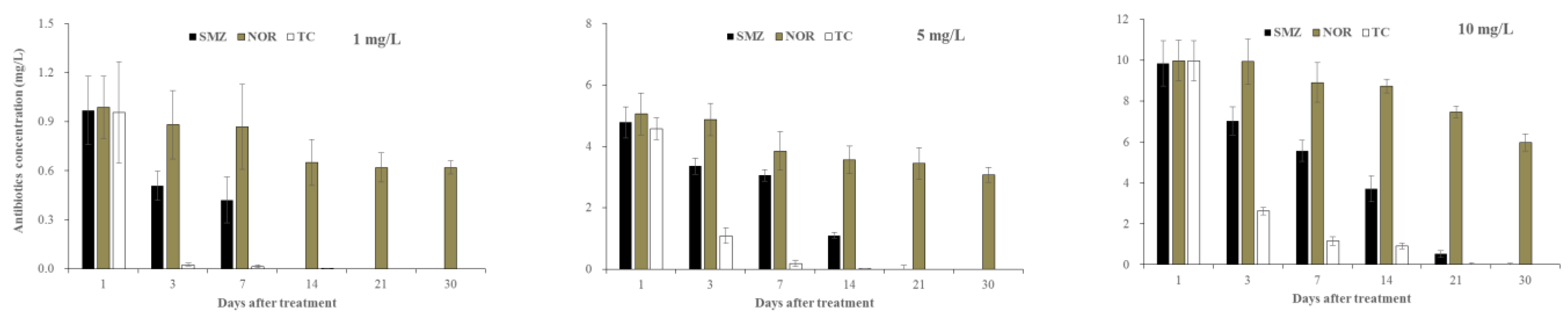
3.3. Antibiotic Residues in Aqueous Solution
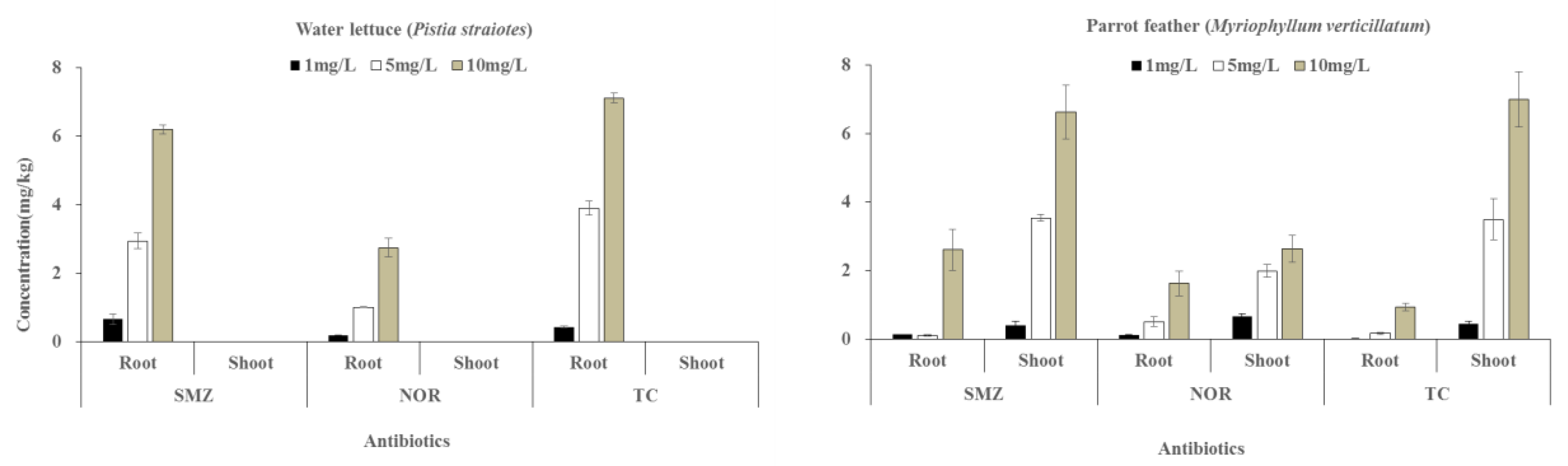
3.4. Uptake Translocation of Antibiotics into Water Lettuce and Parrot Feather
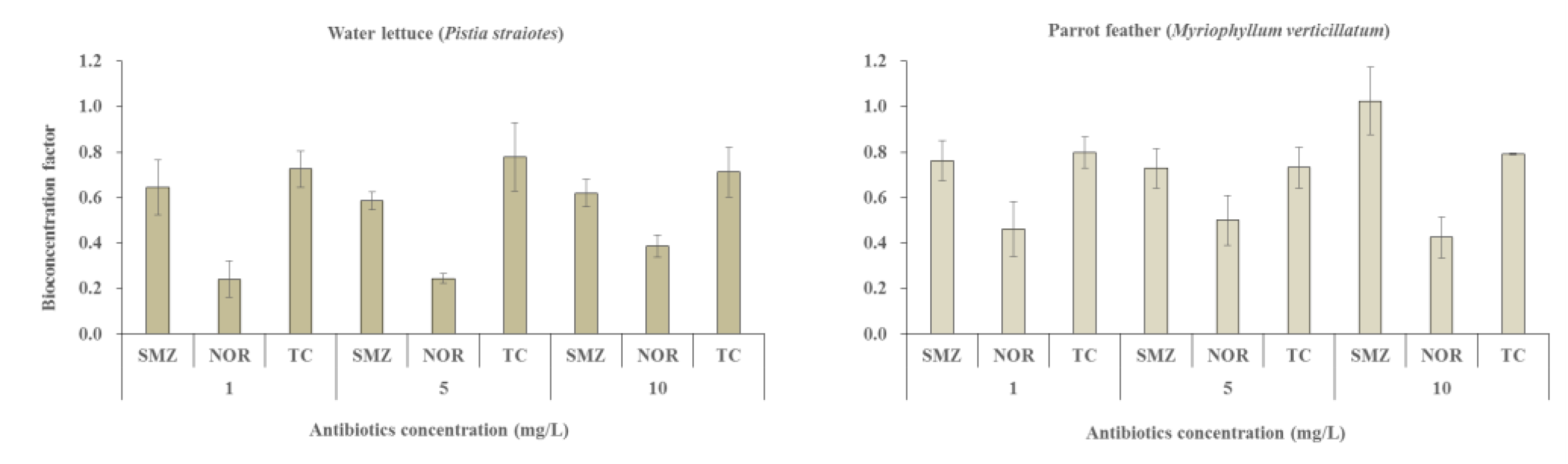
3.5. Bioconcentration Factor
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Paz-Alberto, A.M. Phytoremediation: A green technology to remove environmental pollutants. Am. J. Clim. Chang. 2013, 2, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Milena, M.; Anna, W.; Elbieta, S. Phytoremediation techniques in wastewater treatment. Environ. Biotechnol. 2015, 11, 10–13. [Google Scholar]
- Du, L.F.; Liu, W.K. Occurrence, fate, and ecotoxicity of antibiotics in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Grassi, M.; Luigi, R.; Anna, F. Endocrine disruptors compounds, pharmaceuticals and personal care products in urban wastewater: Implications for agricultural reuse and their removal by adsorption process. Environ. Sci. Pollut. Res. 2013, 20, 3618–3628. [Google Scholar] [CrossRef] [PubMed]
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563, 366–376. [Google Scholar] [CrossRef]
- Gujarathi, N.P.; Bryan, J.H.; James, C.L. Phytoremediation potential of Myriophyllum aquaticum and Pistia stratiotes to modify antibiotic growth promoters, tetracycline, and oxytetracycline, in aqueous wastewater systems. Int. J. Phytoremediation 2015, 7, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.G.; Fletcher, J.; Solomon, K.R.; Sibley, P.K. Effects of ten antibiotics on seed germination and root elongation in three plant species. Arch. Environ. Contam. Toxicol. 2011, 60, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Dordio, A.V.; Duarte, C.; Barreiros, M.; Carvalho, A.; Pinto, A.; Costa, C.T. Toxicity and removal efficiency of pharmaceutical metabolite clofibric acid by Typha spp. Bioresour. Technol. 2009, 100, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.C.; Hanamoto, S.; Yamashita, N.; Huang, X.; Tanaka, H. Antibiotics removal in biological sewage treatment plants. Pollution 2016, 2, 131–139. [Google Scholar]
- Jathwa, A.I.; Muna, Y.A.A. Detection of tetracycline, doxycycline, chlortetracycline, and oxytetracycline antibiotics in Nineveha drug wastewater. Al-Nahrain J. Eng. Sci. 2012, 15, 215–221. [Google Scholar]
- Huang, X.D.; El-Alawi, Y.; Penrose, D.M.; Glick, B.R.; Greenberg, B.M. Responses of three grass species to creosote during phytoremediation. Environ. Pollut. 2004, 130, 453–463. [Google Scholar] [CrossRef]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetlands plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Huang, C.; Renew, J.; Sivieby, K. Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. Water Res. 2001, 120, 30–40. [Google Scholar]
- Boonsaner, M.; Hawker, D.W. Accumulation of oxytetracycline and norfloxacin from saline soil by soybeans. Sci. Total Environ. 2010, 408, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Michelini, L.; Meggio, F.; La Rocca, N.; Ferro, S.; Ghisi, R. Accumulation and effects of sulfadimethoxine in Salix fragilis L. plants: A preliminary study to phytoremediation purposes. Int. J. Phytoremediation 2012, 14, 388–402. [Google Scholar] [CrossRef]
- Herklotz, P.A.; Gurung, P.; Heuvel, B.V.; Kinney, C.A. Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere 2010, 78, 1416–1421. [Google Scholar] [CrossRef]
- Dettenmaier, E.M.; Doucette, W.J.; Bugbee, B. Chemical hydrophobicity and uptake by plant roots. Environ. Sci. Technol. 2009, 43, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, N.; Riffat, N.M.; Muhamamd, A.; Nasrullah, K.; Muhammad, F.S. Hyperaccumulators of heavy metals of industrial areas of Islamabad and Rawalpindi. Pak. J. Bot. 2011, 43, 1925–1933. [Google Scholar]
- Ramesh, P.P.; Chu, L.L.; Kim, T.S.; Sohng, J.K. Bioconversion of tetracycline antibiotics to novel glucoside derivatives by single-vessel multienzymatic glycosylation. J. Microbiol. Biotechnol. 2018, 28, 298–304. [Google Scholar]
- Kim, D.W.; Thomas, M.H.; Kim, B.S.; Laura, K.S.; Kellie, A.W.; John, B.S. Modification of norfloxacin by a Microbacterium sp. strain isolated from a wastewater treatment plant. Appl. Environ. Microbiol. 2011, 77, 6100–6108. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, T.W.; Liu, Y.T.; Deng, Y.; Hsieh, Y.C.; Tan, C.C.; Wang, S.L.; Huang, S.T.; Tzou, Y.M. Removal of sulfamethazine antibiotics using cow manure-based carbon adsorbents. Int. J. Environ. Sci. Technol. 2016, 13, 973–984. [Google Scholar] [CrossRef] [Green Version]

| Antibiotics | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (eV) |
|---|---|---|---|
| NOR | 152 | 110.0 | 16 |
| TC | 445 | 410.0 | 25 |
| SMZ | 279 | 186.1 | 24 |
| Antibiotics | Retention Time | R2 | LOQ (ng/g) | Recovery (%) | |
|---|---|---|---|---|---|
| Plant | Water | ||||
| NOR | 3.52 | 0.99 | 2.59 | 80.1 ± 2.8 | 91.2 ± 3.2 |
| TC | 7.49 | 0.99 | 5.17 | 74.1 ± 3.6 | 84.6 ± 2.0 |
| SMZ | 7.68 | 0.99 | 1.54 | 79.9 ± 2.2 | 88.7 ± 3.2 |
| Item | DAT | Inhibition Rates of Chlorophyll (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 mg/L | 5 mg/L | 10 mg/L | ||||||||||
| SMZ | NOR | TC | SMZ | NOR | TC | SMZ | NOR | TC | SMZ | NOR | TC | ||
| Chl a | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −3.1 ± 0.1 | −1.8 ± 0.2 | 0 | −10 | −9 | |
| 7 | 0 | 0 | 0 | −6.9 ± 0.6 | −3.0 ± 0.1 | −1.2 ± 0.1 | −6.5 ± 0.3 | −10.5 ± 0.6 | −12.9 ± 0.3 | −21.3 ± 3.1 | −24.4 ± 3.1 | −32.9 ± 1.4 | |
| 14 | −3.8 ± 0.2 | −2.2 ± 0.1 | −1.6 ± 0.2 | −8.5 ± 0.4 | −3.3 ± 0.2 | −6.5 ± 0.2 | −15.5 ± 1.1 | −15.3 ± 1.5 | −10.3 ± 1.1 | −30.0 ± 2.0 | −32.5 ± 2.9 | −39.8 ± 2.0 | |
| 21 | −3.9 ± 0.5 | −3.8 ± 0.4 | −3.7 ± 0.3 | −13.1 ± 0.9 | −8.2 ± 0.6 | −6.0 ± 0.6 | −25.6 ± 5.6 | −16.3 ± 1.1 | −16.6 ± 2.0 | −35.3 ± 3.1 | −30.1 ± 6.9 | −42.2 ± 1.3 | |
| 30 | −4.6 ± 0.3 | −2.6 ± 0.3 | −3.2 ± 0.3 | −15.3 ± 1.2 | −10.1 ± 1.9 | −14.9 ± 1.9 | −31.3 ± 6.2 | −26.1 ± 5.6 | −31.6 ± 2.3 | −42.9 ± 3.9 | −43.6 ± 3.4 | −50.4 ± 3.5 | |
| Chl b | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | −1.9 ± 0.3 | −3.0 ± 0.3 | −3.4 ± 0.3 | −2.3 ± 0.1 | −3.8 ± 0.9 | −3.2 ± 0.2 | −4.2 ± 0.2 | −5.2 ± 0.3 | −5.3 ± 0.4 | |
| 7 | 0 | 0 | 0 | −9.6 ± 0.6 | −8.9 ± 1.2 | −9.3 ± 0.3 | −14.9 ± 2.5 | −19.8 ± 1.1 | −16.5 ± 1.5 | −20.3 ± 1.4 | −16.4 ± 0.9 | −14.4 ± 1.0 | |
| 14 | −1.2 ± 0.1 | 0 | 0 | −11.4 ± 0.2 | −15.2 ± 1.9 | −16.3 ± 1.0 | −32.4 ± 3.5 | −25.3 ± 2.5 | −29.9 ± 3.2 | −33.4 ± 1.2 | −31.6 ± 1.2 | −22.1 ± 0.6 | |
| 21 | −2.3 ± 0.1 | −2.2 ± 0.1 | −3.8 ± 0.1 | −25.6 ± 0.9 | −21.3 ± 3.6 | −18.8 ± 1.9 | −36.0 ± 5.2 | −29.1 ± 3.3 | −33.3 ± 3.0 | −36.8 ± 2.2 | −32.0 ± 2.9 | −30.9 ± 2.9 | |
| 30 | −3.7 ± 0.2 | −3.7 ± 0.4 | −4.3 ± 0.3 | −26.2 ± 0.5 | −22.5 ± 4.1 | −21.6 ± 3.5 | −41.3 ± 5.1 | −32.5 ± 3.6 | −39.8 ± 4.2 | −36.9 ± 2.5 | −34.1 ± 3.3 | −33.2 ± 3.6 | |
| Tot Chl | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | −2.2 ± 0.1 | −3.9 ± 0.6 | −3.3 ± 1.0 | −3.3 ± 0.6 | −4.3 ± 0.1 | −4.1 ± 0.2 | |
| 7 | 0 | 0 | 0 | −5.4 ± 0.4 | −6.1 ± 0.3 | −5.1 ± 0.1 | −5.6 ± 0.1 | −12.1 ± 0.1 | −9.8 ± 0.3 | −18.2 ± 0.9 | −26.3 ± 0.9 | −29.6 ± 0.9 | |
| 14 | 0 | −1.3 ± 0.2 | −1.1 ± 0.1 | −9.3 ± 0.2 | −12.9 ± 0.3 | −11.1 ± 0.6 | −24.6 ± 0.6 | −21.3 ± 3.3 | −20.2 ± 1.3 | −26.1 ± 1.5 | −30.9 ± 0.6 | −28.2 ± 0.3 | |
| 21 | −3.4 ± 0.2 | −3.2 ± 0.2 | −4.3 ± 0.2 | −13.9 ± 1.3 | −13.3 ± 0.9 | −12.9 ± 0.2 | −29.1 ± 0.9 | −25.5 ± 3.6 | −26.6 ± 1.3 | −41.6 ± 1.9 | −38.2 ± 1.5 | −33.6 ± 1.9 | |
| 30 | −3.6 ± 0.5 | −3.1 ± 0.1 | −5.2 ± 0.5 | −16.8 ± 1.0 | −15.5 ± 1.9 | −16.3 ± 0.5 | −36.1 ± 0.2 | −35.6 ± 5.2 | −39.5 ± 3.0 | −42.2 ± 1.8 | −42.6 ± 2.9 | −45.2 ± 2.1 | |
| Item | DAT | Inhibition Rates of Chlorophyll (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 mg/L | 5 mg/L | 10 mg/L | ||||||||||
| SMZ | NOR | TC | SMZ | NOR | TC | SMZ | NOR | TC | SMZ | NOR | TC | ||
| Chl a | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1.0 ± 0.2 | −1.1 ± 0.1 | −1.9 ± 0.3 | −3.7 ± 0.1 | −3.1 ± 0.1 | |
| 7 | 0 | 0 | 0 | −2.1 ± 0.1 | −2.3 ± 0.4 | −1.6 ± 0.2 | −2.9 ± 0.1 | −9.3 ± 0.4 | −3.2 ± 0.1 | −5.5 ± 1.1 | −20.5 ± 1.1 | −26.1 ± 2.9 | |
| 14 | −1.2 ± 0.1 | −1.6 ± 0.1 | −1.1 ± 0.1 | −3.9 ± 0.2 | −5.1 ± 0.1 | −5.3 ± 0.3 | −9.3 ± 0.6 | −11.4 ± 0.9 | −9.8 ± 0.2 | −11.4 ± 1.3 | −24.4 ± 1.9 | −26.9 ± 3.9 | |
| 21 | −3.0 ± 0.3 | −3.1 ± 0.1 | −3.2 ± 0.1 | −7.4 ± 0.3 | −11.6 ± 0.9 | −7.2 ± 0.1 | −13.2 ± 2.3 | −19.1 ± 1.3 | −19.3 ± 0.6 | −18.9 ± 2.6 | −29.6 ± 2.6 | −31.2 ± 2.5 | |
| 30 | −4.1 ± 0.9 | −3.2 ± 0.2 | −4.3 ± 0.2 | −9.5 ± 0.9 | −15.9 ± 1.2 | −13.3 ± 0.6 | −19.9 ± 1.9 | −26.0 ± 3.4 | −26.4 ± 1.9 | −29.6 ± 2.9 | −32.5 ± 2.5 | −34.9 ± 3.4 | |
| Chl b | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | −2.2 ± 0.1 | −1.1 ± 0.2 | −2.1 ± 0.2 | −2.1 ± 0.1 | −6.5 ± 0.1 | −4.1 ± 0.9 | −6.6 ± 0.2 | |
| 7 | 0 | 0 | 0 | −2.1 ± 0.6 | −3.6 ± 0.4 | −6.0 ± 0.5 | −3.2 ± 0.5 | −12.9 ± 0.5 | −15.9 ± 2.2 | −16.2 ± 0.3 | −16.0 ± 1.1 | −10.1 ± 1.8 | |
| 14 | 0 | 0 | 0 | −6.3 ± 0.2 | −10.1 ± 1.2 | −10.1 ± 0.2 | −19.6 ± 1.1 | −21.4 ± 0.9 | −19.1 ± 2.1 | −29.9 ± 0.9 | −30.9 ± 1.9 | −20.9 ± 1.1 | |
| 21 | 0 | −2.5 ± 0.2 | −4.6 ± 0.6 | −9.1 ± 0.9 | −19.9 ± 3.0 | −13.6 ± 1.2 | −30.2 ± 1.9 | −41.2 ± 2.9 | −32.2 ± 1.5 | −29.1 ± 1.1 | −39.2 ± 2.1 | −41.2 ± 2.0 | |
| 30 | 0 | −1.3 ± 0.1 | −2.1 ± 0.1 | −19.9 ± 0.5 | −26.3 ± 2.9 | −20.1 ± 2.1 | −36.6 ± 3.2 | −45.9 ± 4.1 | −29.1 ± 2.9 | −42.0 ± 1.9 | −39.8 ± 2.5 | −41.0 ± 2.9 | |
| Tot Chl | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | −2.9 ± 0.1 | −3.1 ± 0.2 | −3.0 ± 0.1 | −3.1 ± 0.1 | −3.6 ± 0.6 | −4.9 ± 0.4 | |
| 7 | 0 | 0 | 0 | −5.0 ± 0.3 | −6.0 ± 0.1 | −5.9 ± 0.3 | −5.2 ± 0.3 | −12.9 ± 0.9 | −8.1 ± 0.6 | −17.1 ± 0.3 | −25.4 ± 1.8 | −23.5 ± 0.2 | |
| 14 | 0 | −1.1 ± 0.1 | −1.5 ± 0.2 | −9.2 ± 0.1 | −12.1 ± 0.2 | −12.2 ± 0.5 | −23.1 ± 0.2 | −30.1 ± 1.5 | −21.4 ± 0.6 | −23.2 ± 2.1 | −31.2 ± 2.9 | −29.7 ± 3.2 | |
| 21 | −3.9 ± 0.6 | −3.3 ± 0.5 | −4.4 ± 0.3 | −13.1 ± 0.6 | −12.6 ± 0.9 | −13.0 ± 0.5 | −29.9 ± 1.5 | −33.1 ± 2.6 | −25.6 ± 3.5 | −40.9 ± 3.3 | −37.1 ± 2.2 | −30.3 ± 2.1 | |
| 30 | −3.3 ± 0.2 | −3.9 ± 0.2 | −5.9 ± 0.2 | −14.1 ± 0.5 | −13.3 ± 2.1 | −16.1 ± 0.9 | −44.2 ± 1.3 | −33.9 ± 3.8 | −41.1 ± 2.9 | −41.5 ± 2.9 | −42.5 ± 4.2 | −42.9 ± 4.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-J.; Son, J.-G. Phytotoxicity and Accumulation of Antibiotics in Water Lettuce (Pistia stratiotes) and Parrot Feather (Myriophyllum aquaticum) Plants under Hydroponic Culture Conditions. Appl. Sci. 2022, 12, 630. https://doi.org/10.3390/app12020630
Park Y-J, Son J-G. Phytotoxicity and Accumulation of Antibiotics in Water Lettuce (Pistia stratiotes) and Parrot Feather (Myriophyllum aquaticum) Plants under Hydroponic Culture Conditions. Applied Sciences. 2022; 12(2):630. https://doi.org/10.3390/app12020630
Chicago/Turabian StylePark, Young-Jae, and Jae-Gwon Son. 2022. "Phytotoxicity and Accumulation of Antibiotics in Water Lettuce (Pistia stratiotes) and Parrot Feather (Myriophyllum aquaticum) Plants under Hydroponic Culture Conditions" Applied Sciences 12, no. 2: 630. https://doi.org/10.3390/app12020630





