Quantitative Neutron Dark-Field Imaging of Milk: A Feasibility Study
Abstract
:1. Introduction
2. Material and Method
2.1. Samples
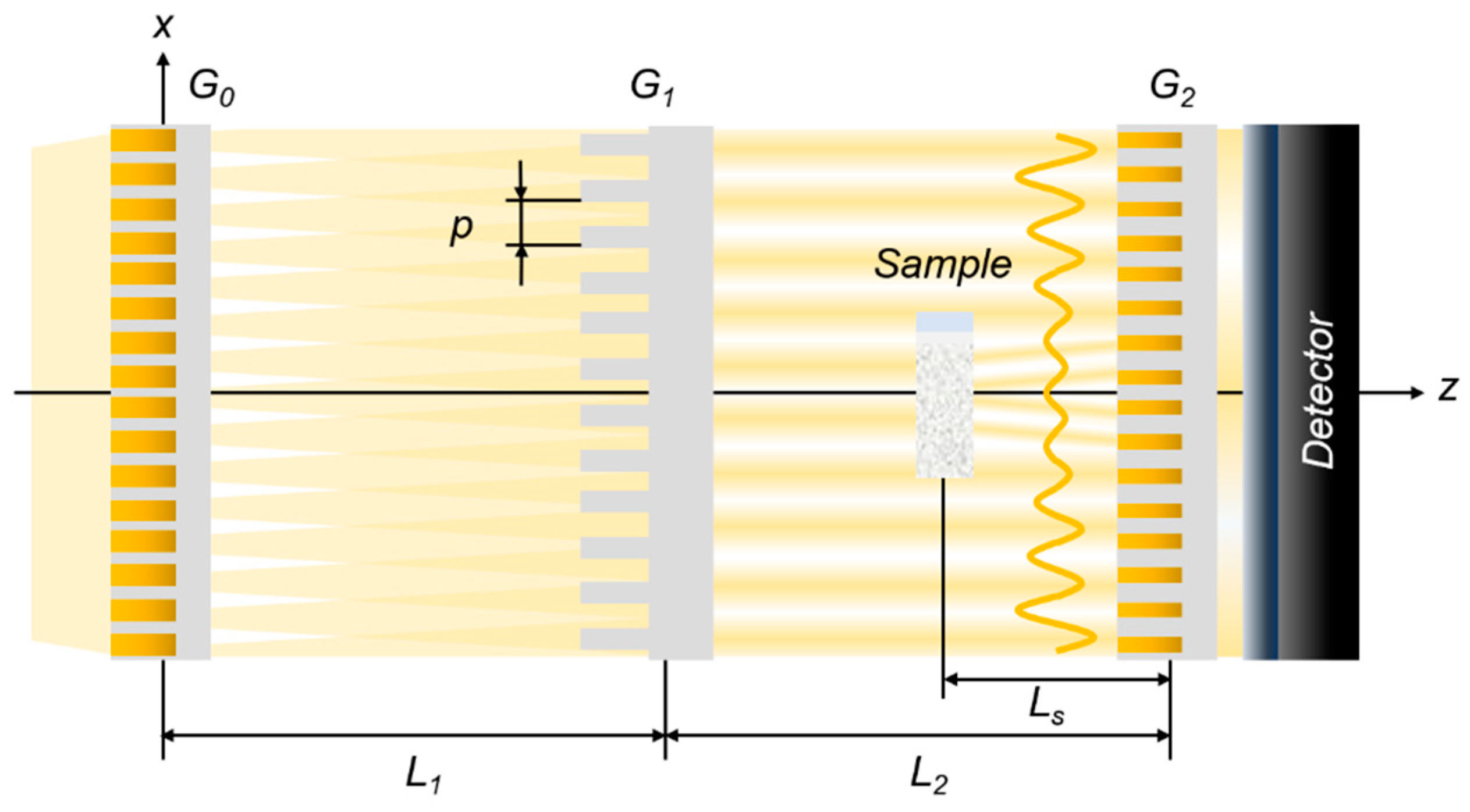
2.2. Dark-Field Contrast Imaging
2.3. Data Acquisition
2.4. Data Analyses
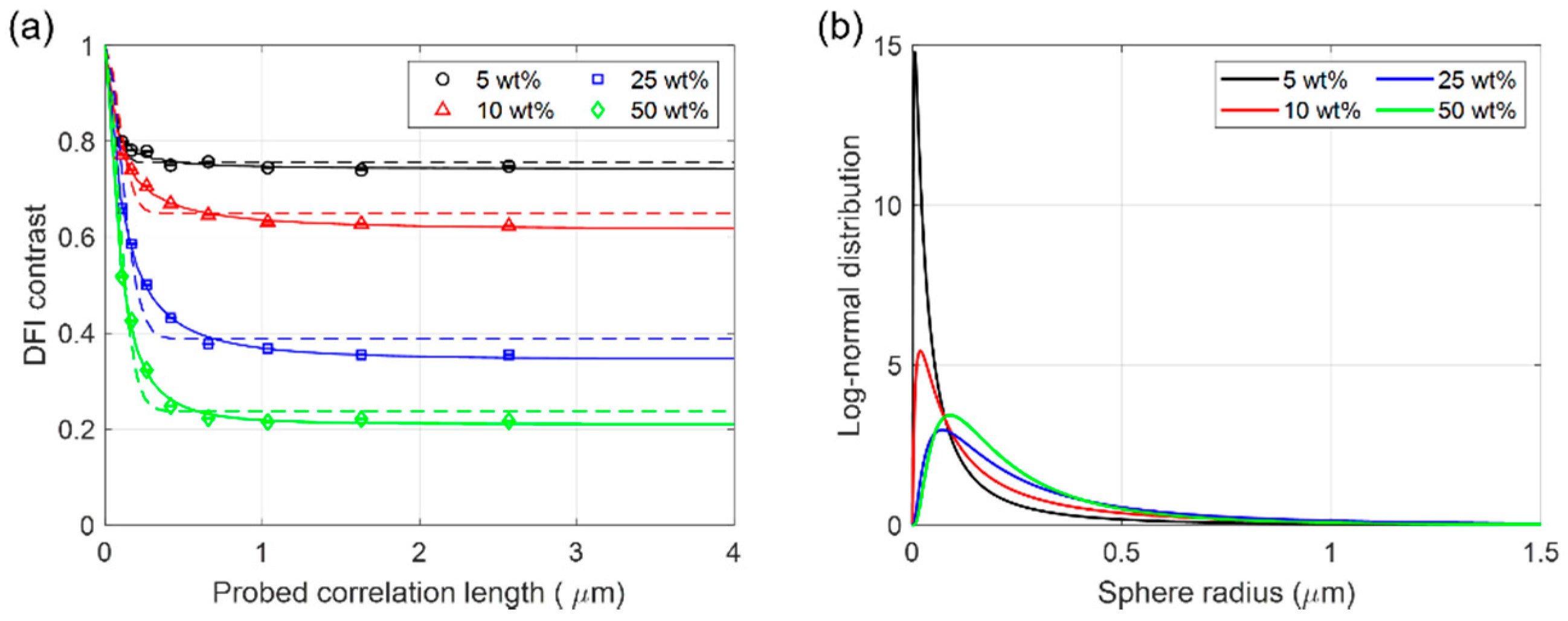
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). Food Outlook—Biannual Report on Global Food Markets; FAO: Rome, Italy, 2015; ISBN 3906570541. [Google Scholar]
- Ong, L.; Dagastine, R.R.; Kentish, S.E.; Gras, S.L. The effect of milk processing on the microstructure of the milk fat globule and rennet induced gel observed using confocal laser scanning microscopy. J. Food Sci. 2010, 75, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.; Dagastine, R.R.; Kentish, S.E.; Gras, S.L. Microstructure of milk gel and cheese curd observed using cryo scanning electron microscopy and confocal microscopy. LWT Food Sci. Technol. 2011, 44, 1291–1302. [Google Scholar] [CrossRef]
- McMahon, D.J.; Oommen, B.S. Supramolecular structure of the casein micelle. J. Dairy Sci. 2008, 91, 1709–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhardt, R.; Doster, W.; Friedrich, J.; Kulozik, U. Size distribution of pressure-decomposed casein micelles studied by dynamic light scattering and AFM. Eur. Biophys. J. 2006, 35, 503–509. [Google Scholar] [CrossRef]
- Stothart, P.H.; Cebula, D.J. Small-angle neutron scattering study of bovine casein micelles and sub-micelles. J. Mol. Biol. 1982, 160, 391–395. [Google Scholar] [CrossRef]
- Tromp, R.H.; Bouwman, W.G. A novel application of neutron scattering on dairy products. Food Hydrocoll. 2007, 21, 154–158. [Google Scholar] [CrossRef]
- Van Heijkamp, L.F.; De Schepper, I.M.; Strobl, M.; Hans Tromp, R.; Heringa, J.R.; Bouwman, W.G. Milk gelation studied with small-angle neutron scattering techniques and monte carlo simulations. J. Phys. Chem. A 2010, 114, 2412–2426. [Google Scholar] [CrossRef]
- Adams, C.P.; Callaghan-Patrachar, N.; Peyronel, F.; Barker, J.; Pink, D.A.; Marangoni, A.G. Small and ultra-small angle neutron scattering studies of commercial milk. Food Struct. 2019, 21, 100120. [Google Scholar] [CrossRef]
- Sandra, S.; Cooper, C.; Alexander, M.; Corredig, M. Coagulation properties of ultrafiltered milk retentates measured using rheology and diffusing wave spectroscopy. Food Res. Int. 2011, 44, 951–956. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Gilbert, E.P. Neutron scattering: A natural tool for food science and technology research. Trends Food Sci. Technol. 2009, 20, 576–586. [Google Scholar] [CrossRef]
- Wood, K.; Mata, J.P.; Garvey, C.J.; Wu, C.M.; Hamilton, W.A.; Abbeywick, P.; Bartlett, D.; Bartsch, F.; Baxter, P.; Booth, N.; et al. QUOKKA, the pinhole small-angle neutron scattering instrument at the OPAL Research Reactor, Australia: Design, performance, operation and scientific highlights. J. Appl. Crystallogr. 2018, 51, 294–314. [Google Scholar] [CrossRef]
- Rehm, C.; De Campo, L.; Brûlé, A.; Darmann, F.; Bartsch, F.; Berry, A. Design and performance of the variable-wavelength Bonse-Hart ultra-small-angle neutron scattering diffractometer KOOKABURRA at ANSTO. J. Appl. Crystallogr. 2018, 51, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rekveldt, M.T. Novel SANS instrument using neutron spin echo. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1996, 114, 366–370. [Google Scholar] [CrossRef]
- Smith, G.N. Revisiting neutron scattering data from deuterated milk. Food Hydrocoll. 2021, 113, 106511. [Google Scholar] [CrossRef]
- De Kruif, C.G. The structure of casein micelles: A review of small-angle scattering data. J. Appl. Crystallogr. 2014, 47, 1479–1489. [Google Scholar] [CrossRef]
- Shih, W.-H.; Shih, W.Y.; Kim, S.-I.; Liu, J.; Aksay, I.A.A. Scaling behavior ofthe elastic properties of colloidal gels. Phys. Rev. A 1990, 42, 4772. [Google Scholar] [CrossRef]
- Strobl, M.; Grünzweig, C.; Hilger, A.; Manke, I.; Kardjilov, N.; David, C.; Pfeiffer, F. Neutron dark-field tomography. Phys. Rev. Lett. 2008, 101, 123902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobl, M. General solution for quantitative dark-field contrast imaging with grating interferometers. Sci. Rep. 2014, 4, 7243. [Google Scholar] [CrossRef] [Green Version]
- Strobl, M.; Betz, B.; Harti, R.P.; Hilger, A.; Kardjilov, N.; Manke, I.; Gruenzweig, C. Wavelength-dispersive dark-field contrast: Micrometre structure resolution in neutron imaging with gratings. J. Appl. Crystallogr. 2016, 49, 569–573. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Grünzweig, C.; Bunk, O.; Frei, G.; Lehmann, E.; David, C. Neutron phase imaging and tomography. Phys. Rev. Lett. 2006, 96, 215505. [Google Scholar] [CrossRef] [Green Version]
- Strobl, M.; Harti, R.P.; Gruenzweig, C.; Woracek, R.; Plomp, J.; Grünzweig, C. Plomp Small Angle Scattering in Neutron Imaging—A Review. J. Imaging 2018, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Valsecchi, J.; Kim, J.; Lee, S.W.; Strobl, M. Symmetric Talbot-Lau neutron grating interferometry and incoherent scattering correction for quantitative dark-field imaging. Sci. Rep. 2019, 9, 18973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobl, M.; Tremsin, A.S.; Hilger, A.; Wieder, F.; Kardjilov, N.; Manke, I.; Bouwman, W.G.; Plomp, J. TOF-SEMSANS—Time-of-flight spin-echo modulated small-angle neutron scattering. J. Appl. Phys. 2015, 112, 014503. [Google Scholar] [CrossRef] [Green Version]
- Strobl, M.; Wieder, F.; Duif, C.P.; Hilger, A.; Kardjilov, N.; Manke, I.; Bouwman, W.G. Using a grating analyser for SEMSANS investigations in the very small angle range. Phys. B Condens. Matter 2012, 407, 4132–4135. [Google Scholar] [CrossRef]
- Andersson, R.; Van Heijkamp, L.F.; De Schepper, I.M.; Bouwman, W.G. Analysis of spin-echo small-angle neutron scattering measurements. J. Appl. Crystallogr. 2008, 41, 868–885. [Google Scholar] [CrossRef]
- Morgano, M.; Peetermans, S.; Lehmann, E.H.; Panzner, T.; Filges, U. Neutron imaging options at the BOA beamline at Paul Scherrer Institut. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 754, 46–56. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.H.; Lim, C.H.; Kim, T.; Ahn, C.W.; Cho, G.; Lee, S.W. Fabrication and characterization of the source grating for visibility improvement of neutron phase imaging with gratings. Rev. Sci. Instrum. 2013, 84, 063705. [Google Scholar] [CrossRef]
- Uca, O.; Bouwman, W.G.; Rekveld, M.T. Model calculations for the spin-echo small-angle neutron-scattering correlation function. J. Appl. Crystallogr. 2003, 36, 109–116. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; March, N.H. Structure factor and direct correlation function for a classical hard sphere fluid. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1967, 297, 336–350. [Google Scholar] [CrossRef]
- Lazzari, S.; Nicoud, L.; Jaquet, B.; Lattuada, M.; Morbidelli, M. Fractal-like structures in colloid science. Adv. Colloid Interface Sci. 2016, 235, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bakker, J.H.; Washington, A.L.; Parnell, S.R.; van Well, A.A.; Pappas, C.; Bouwman, W.G. Analysis of SESANS data by numerical Hankel transform implementation in SasView. J. Neutron Res. 2020, 22, 57–70. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Holt, C. Casein micelle structure, functions and interactions. In Advanced Dairy Chemistry—Proteins, 1, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 233–276. [Google Scholar]
- Andoyo, R.; Guyomarc’h, F.; Burel, A.; Famelart, M.-H. Spatial arrangement of casein micelles and whey protein aggregate in acid gels: Insight on mechanisms. Food Hydrocoll. 2015, 51, 118–128. [Google Scholar] [CrossRef]
- Andoyo, R.; Lestari, V.D.; Mardawati, E.; Nurhadi, B. Fractal Dimension Analysis of Texture Formation of Whey Protein-Based Foods. Int. J. Food Sci. 2018, 2018, 7673259. [Google Scholar] [CrossRef] [PubMed]




| Effective wavelength (Å) | 4.1 | |
| Fractional Talbot order | 1 | |
| Inter-grating distance (mm) | G0 − G1 (L1) | 1420 |
| G1 − G2 (L2) | 1420 | |
| Period of gratings (μm) | pg (p0 = p1 = p2) | 50 |
| Height of gratings (μm) | h0 | 100 (Gadox) |
| h1 | 34.39 (Silicon) | |
| h2 | 20 (Gadox) | |
| Duty cycle of gratings | d0 | 0.75 |
| d1 | 0.5 | |
| d2 | 0.5 |
| Milk Sample | ||||
|---|---|---|---|---|
| 5 wt% | 0.2805 | 0.0937 | 0.9975 | 0.9965 |
| 10 wt% | 0.4299 | 0.1393 | 0.9841 | 0.9778 |
| 25 wt% | 0.9454 | 0.1812 | 0.9156 | 0.8818 |
| 50 wt% | 1.4360 | 0.1647 | 0.5422 | 0.3591 |
| Milk Sample | |||||||
|---|---|---|---|---|---|---|---|
| 5 wt% | 0.2974 | −16.7194 | 1.4899 | 0.0550 | 0.1668 | 0.9996 | 0.9993 |
| 10 wt% | 0.4845 | −15.7739 | 1.4156 | 0.1411 | 0.3843 | 0.9999 | 0.9998 |
| 25 wt% | 1.0627 | −15.3192 | 1.0670 | 0.2223 | 0.3928 | 0.9980 | 0.9966 |
| 50 wt% | 1.5560 | −15.4524 | 0.8750 | 0.1946 | 0.2853 | 0.9731 | 0.9530 |
| Gel Sample | ||||||
|---|---|---|---|---|---|---|
| GA Skim | 0.3931 | 0.2170 | −0.1201 | 2.7598 | 0.9672 | 0.9425 |
| GP Skim | 0.3214 | 0.3545 | −0.2287 | 2.5427 | 0.8677 | 0.7684 |
| Gel Sample | |||||||
|---|---|---|---|---|---|---|---|
| GA Fat | 0.4141 | −14.5536 | 0.9906 | 0.4781 | 0.7808 | 0.9898 | 0.9822 |
| GP Fat | 0.9128 | −14.9336 | 1.1449 | 0.3269 | 0.6300 | 0.9973 | 0.9953 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Valsecchi, J.; Oh, O.; Kim, J.; Lee, S.W.; Boue, F.; Lutton, E.; Busi, M.; Garvey, C.; Strobl, M. Quantitative Neutron Dark-Field Imaging of Milk: A Feasibility Study. Appl. Sci. 2022, 12, 833. https://doi.org/10.3390/app12020833
Kim Y, Valsecchi J, Oh O, Kim J, Lee SW, Boue F, Lutton E, Busi M, Garvey C, Strobl M. Quantitative Neutron Dark-Field Imaging of Milk: A Feasibility Study. Applied Sciences. 2022; 12(2):833. https://doi.org/10.3390/app12020833
Chicago/Turabian StyleKim, Youngju, Jacopo Valsecchi, Ohsung Oh, Jongyul Kim, Seung Wook Lee, Francois Boue, Evelyne Lutton, Matteo Busi, Christopher Garvey, and Markus Strobl. 2022. "Quantitative Neutron Dark-Field Imaging of Milk: A Feasibility Study" Applied Sciences 12, no. 2: 833. https://doi.org/10.3390/app12020833






