Microhabitat and Landscape Drivers of Richness and Abundance of Freshwater Mussels (Unionida: Unionidae) in a Coastal Plain River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Sites Selection
2.3. Species Richness and Abundance
2.4. Microhabitat and Landscape Drivers
2.5. Statistical Analysis
3. Results
3.1. Mussel Survey
3.2. Microhabitat Characteristics
3.3. Land Use and Geology
3.4. Analyses of Mussel CPUE and Richness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haag, W.R.; Williams, J.D. Biodiversity on the brink: An assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 2014, 735, 45–60. [Google Scholar] [CrossRef]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Gido, K.B.; Spooner, D.B. Ecosystem processes performed by unionid mussels in stream mesocosms: Species roles and effects of abundance. Hydrobiologia 2004, 527, 35–47. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Nichols, S.J.; Spooner, D.E. Community and foodweb ecology of freshwater mussels. J. N. Am. Benthol. Soc. 2008, 27, 409–423. [Google Scholar] [CrossRef]
- Gutierrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Bohm, M.; Dewhurst-Richman, N.I.; Seddon, M.; Ledger, S.E.H.; Albrecht, C.; Allen, D.; Bogan, A.E.; Cordeiro, J.; Cummings, K.S.; Cuttelod, A.; et al. The conservation status of the world’s freshwater molluscs. Hydrobiologia 2021, 848, 3231–3254. [Google Scholar] [CrossRef]
- Williams, J.D.; Warren, M.L., Jr.; Cummings, K.S.; Harris, J.L.; Neves, R.J. Conservation status of freshwater mussels of the United States and Canada. Fisheries 1993, 18, 6–22. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Julian, J.P.; Vaughn, C.C. Scale-dependent longitudinal patterns in mussel communities. Freshw. Biol. 2012, 57, 2271–2284. [Google Scholar] [CrossRef]
- Daniel, W.M.; Cooper, A.R.; Badra, P.J.; Infante, D.M. Predicting habitat suitability for eleven imperiled fluvial freshwater mussels. Hydrobiologia 2018, 809, 265–283. [Google Scholar] [CrossRef]
- Brown, K.M.; George, G.; Daniel, W. Urbanization and a threatened freshwater mussel: Evidence from landscape scale studies. Hydrobiologia 2010, 655, 189–196. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, J.; Cummings, K.S. Modeling changes in freshwater mussel diversity in an agriculturally dominated landscape. Freshw. Sci. 2013, 32, 1205–1218. [Google Scholar] [CrossRef]
- Daniel, W.M.; Brown, K.M. Multifactorial model of habitat, host fish, and landscape effects on Louisiana freshwater mussels. Freshw. Sci. 2013, 32, 193–203. [Google Scholar] [CrossRef]
- Poole, K.E.; Downing, J.S. Relationship of declining mussel biodiversity to stream-reach and watershed characteristics in an agricultural landscape. J. N. Am. Benthol. Soc. 2004, 23, 114–125. [Google Scholar] [CrossRef]
- Arbuckle, K.E.; Downing, J.A. Freshwater mussel abundance and species richness: GIS relationships with watershed land use and geology. Can. J. Fish. Aquat. Sci. 2002, 59, 310–316. [Google Scholar] [CrossRef]
- McRae, S.E.; Allan, J.D.; Burch, J.B. Reach- and catchment-scale determinants of the distribution of freshwater mussels (Bivalvia: Unionidae) in south-eastern Michigan, U.S.A. Freshw. Biol. 2004, 49, 127–142. [Google Scholar] [CrossRef] [Green Version]
- Randklev, C.R.; Wang, H.-H.; Grant, W.E.; Groce, J.E.; Robertson, S.; Wilkins, R.N. Land use relationships for a rare freshwater mussel species endemic to central Texas. J. Fish Wildl. Manag. 2015, 6, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Ford, D.F.; Walters, A.S.; Williams, L.R.; Williams, M.G.; Ford, N.B. Mussel assemblages in streams of different sizes in the Neches River Basin of Texas. Southeast. Nat. 2016, 15, 26–40. [Google Scholar] [CrossRef]
- Hopkins, R.L., II. Use of landscape pattern metrics and multiscale data in aquatic species distribution models: A case study of a freshwater mussel. Landsc. Ecol. 2009, 24, 943–955. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A.; May, M.E.; Bennet, D.L.; Cook, M.J. Biogeography and conservation of freshwater mussels (Bivalvia: Unionidae) in Texas: Patterns of diversity and threats. Divers. Distrib. 2011, 17, 393–407. [Google Scholar] [CrossRef]
- Strayer, D.L. Macrohabitats of freshwater mussels (Bivalvia: Unionacea) in streams of the northern Atlantic Slope. J. N. Am. Benthol. Soc. 1993, 12, 236–246. [Google Scholar] [CrossRef]
- Morris, T.J.; Corkum, L.D. Assemblage structure of freshwater mussels (Bivalvia: Unionidae) in rivers with grassy and forested riparian zones. J. N. Am. Benthol. Soc. 1996, 15, 576–586. [Google Scholar] [CrossRef]
- Hastie, L.C.; Boon, P.J.; Young, M.R. Physical requirements of freshwater pearl mussels, Margaritifera margaritifera (L.). Hydrobiologia 2000, 429, 59–71. [Google Scholar] [CrossRef]
- Howard, J.K.; Cuffey, K.M. Freshwater mussels in a California north coast range river: Occurrence, distribution, and controls. J. N. Am. Benthol. Soc. 2003, 22, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.M.; Banks, P.D. The conservation of unionid mussels in Louisiana rivers: Diversity, assemblage composition and substrate use. Aquat. Conserv. 2001, 11, 189–198. [Google Scholar] [CrossRef]
- Allen, D.C.; Vaughn, C.C. Complex hydraulic and substrate parameters limit freshwater mussel species richness: A test of the substrate stability hypothesis. J. N. Am. Benthol. Soc. 2010, 29, 383–394. [Google Scholar] [CrossRef]
- Ma, L.; Beatty, S.J.; Morgan, D.L.; Lumbery, A.J. Population structure and microhabitat preference of a threatened freshwater mussel, Westralunio carteri, in south-western Australia. Hydrobiologia 2022, 849, 3227–3244. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Kwak, T.J.; Cope, W.G. Microhabitat suitability and niche breadth of common and imperiled Atlantic slope freshwater mussels. Freshw. Mollusk Biol. Conserv. 2016, 19, 27–50. [Google Scholar] [CrossRef]
- Bey, C.R.; Sullivan, S.M.P. Associations between stream hydrogeomorphology and co-dependent mussel-fish assemblages: Evidence from an Ohio, USA river system. Aquat. Conserv. 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Hardison, B.S.; Layzer, J.B. Relations between complex hydraulics and the localized distribution of mussels in three regulated rivers. Regul. Rivers Res. Manag. 2001, 17, 77–84. [Google Scholar] [CrossRef]
- Harriger, K.; Moerke, A. Freshwater mussel (Unionidae) distribution and demographics in relation to microhabitat in a first-order Michigan stream. Mich. Academ. 2009, 39, 149–162. [Google Scholar]
- Wang, N.; Ivey, C.D.; Ingersoll, C.G.; Brumbaugh, W.G.; Alvarez, D.; Hammer, E.J.; Bauer, C.R.; Augspurger, T.; Raimondo, S.; Barnhart, M.C. Acute sensitivity of a broad range of freshwater mussels to chemicals with different modes of toxic action. Environ. Toxicol. Chem. 2017, 3, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L.; Malcom, H.M. Causes of recruitment failure in freshwater mussel populations in southeastern New York. Ecol. Appl. 2012, 22, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Michener, W.K.; Freeman, M.C. Drought responses of freshwater mussels (Unionidae) in coastal plain tributaries of the Flint River basin, Georgia. J. Freshw. Ecol. 2011, 19, 667–679. [Google Scholar]
- Chen, L.-Y.; Heath, A.G.; Neves, R.J. Comparison of oxygen consumption in freshwater mussels (Unionidae) from different habitats during declining dissolved oxygen concentration. Hydrobiologia 2001, 450, 209–214. [Google Scholar] [CrossRef]
- Brim Box, J.; Mossa, J. Sediment, land use, and freshwater mussels: Prospects and problems. J. N. Am. Benthol. Soc. 1999, 18, 99–117. [Google Scholar]
- Brim Box, J.; Dorazio, R.M.; Liddell, W.D. Relationships between streambed substrate characteristics and freshwater mussels (Bivalvia: Unionidae) in Coastal Plain streams. J. N. Am. Benthol. Soc. 2002, 21, 253–260. [Google Scholar]
- Aldridge, D.W.; Payne, B.S.; Miller, A.C. The effects of intermittent exposure to suspended solids and turbulence on three species of freshwater mussels. Environ. Pollut. 1987, 45, 17–28. [Google Scholar] [CrossRef]
- Aldridge, D.W.; Payne, B.S.; Miller, A.C. Oxygen consumption, nitrogenous excretion, and filtration rates of Dreissena polymorpha at acclimation temperatures between 20 and 32 C. Can. J. Fish. Aquat. Sci. 1995, 52, 1761–1767. [Google Scholar] [CrossRef]
- Ganser, A.M.; Newton, T.J.; Haro, R.J. Effects of elevated water temperature on physiological responses in adult freshwater mussels. Freshw. Biol. 2015, 60, 1705–1716. [Google Scholar] [CrossRef]
- Kaller, M.D.; Kelso, W.E. Occurrence of freshwater bivalves in a chronically hypoxic coastal stream in Louisiana, USA. J. Freshw. Ecol. 2006, 21, 355–357. [Google Scholar] [CrossRef]
- Haag, W.R.; Warren, M.L., Jr. Role of ecological factors and reproductive strategies in structuring freshwater mussel communities. Can. J. Fish. Aquat. Sci. 1998, 55, 297–306. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Kwak, T.J.; Cope, W.G.; Heise, R.J.; Nichols, R.B.; Pacifici, K. Species traits and catchment-scale habitat factors influence the occurrence of freshwater mussel populations and assemblages. Freshw. Biol. 2016, 61, 1671–1684. [Google Scholar] [CrossRef]
- Johnson, P.D.; Brown, K.M. The importance of microhabitat factors and habitat stability to the threatened Louisiana pearl shell, Margaritifera hembeli (Conrad). Can. J. Zool. 2000, 78, 271–277. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.Y.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of freshwater bivalves at the global scale: Diversity, threats and research needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Williams, J.D. Reproductive biology of four freshwater mussels (Bivalvia: Unionidae) endemic to eastern Gulf Coastal Plain drainges of Alabama, Florida, and Georgia. Am. Malacol. Bull. 2002, 17, 147–158. [Google Scholar]
- Pilarczyk, M.M.; Stewart, P.M.; Shelton, D.N.; Blalock-Herod, H.N.; Williams, J.D. Current and recent historical freshwater mussel assemblages in the Gulf Coastal Plains. Southeast. Nat. 2006, 5, 205–226. [Google Scholar] [CrossRef]
- Rypel, A.L.; Haag, W.R.; Findlay, R.H. Pervasive hydrologic effects on freshwater mussels and riparian trees in southeastern floodplain ecosystems. Wetlands 2008, 29, 497–504. [Google Scholar] [CrossRef]
- Hagy, J.D., III; Lehrter, J.C.; Murrell, M.C. Effects of Hurricane Ivan on water quality in Pensacola Bay, Florida. Estuaries Coasts 2006, 29, 919–925. [Google Scholar] [CrossRef]
- Felley, J.D. Medium-low gradient streams of the Gulf Coastal Plain. In Biodiversity of the Southeastern United States: Aquatic Communities; Hackney, C.T., Adams, S.M., Martin, W.H., Eds.; John Wiley and Sons: New York, NY, USA, 1992; pp. 233–269. [Google Scholar]
- Holcomb, S.R.; Bass, A.A.; Reid, C.S.; Seymour, M.A.; Lorenz, N.F.; Gregory, B.B.; Javed, S.M.; Balkum, K.F. Louisiana Wildlife Action Plan; Louisiana Department of Wildlife and Fisheries: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Stewart, J.G.; Schieble, C.S.; Cashner, R.C.; Barko, V.A. Long-term trends in the Bogue Chitto River fish assemblage: A 27-year perspective. Southeast. Nat. 2005, 4, 261–272. [Google Scholar] [CrossRef]
- Geheber, A.D.; Piller, K.R. Spatio-temporal assessment of persistence and stability of a Gulf Coastal Plain assemblage: Appropriate scales reveal historical tales. Ecol. Freshw. Fish. 2012, 21, 627–639. [Google Scholar] [CrossRef]
- Gunning, G.E.; Suttkus, R.D. Reclamation of the Pearl River: A perspective of unpolluted versus polluted water. Fisheries 1985, 10, 14–16. [Google Scholar] [CrossRef]
- Piller, K.R.; Geheber, A.D. Black liquor and the hangover effect: Fish assemblage recovery dynamics following a pulse disturbance. Ecol. Evol. 2015, 5, 2433–2444. [Google Scholar] [CrossRef]
- Brown, K.M.; Daniel, W.; George, G. The effect of Hurricane Katrina on the mussel assemblage of the Pearl River, Louisiana. Aquat. Ecol. 2010, 44, 223–231. [Google Scholar] [CrossRef]
- Bambarger, A.R. Freshwater Mussel Communities of the Florida Parishes, Louisiana: The Importance of Spatial Scale. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2006. [Google Scholar]
- Vidrine, M.F. Louisiana Freshwater Mussels; MalcolmP F. Vidrine and Gail Q. Vidrine Collectables: Eunice, LA, USA, 2019. [Google Scholar]
- USFWS. Freshwater Mussels of the Pearl River Basin; Baton Rouge Fish and Wildlife Conservation Office, Southeast Region: Baton Rouge, LA, USA, 2014.
- Metcalfe-Smith, J.L.; Di Maio, J.; Slaton, S.K.; Mackie, G.L. Effect of sampling effort on the efficiency of the timed search method for sampling freshwater mussel communities. J. N. Am. Benthol. Soc. 2000, 19, 725–732. [Google Scholar] [CrossRef]
- Reid, S.M. Search effort and imperfect detection: Influence on timed-search mussel (Bivalvia: Unionidae) surveys in Canadian rivers. Knowl. Manag. Aquat. Ecol. 2016, 417, 17. [Google Scholar] [CrossRef]
- Stern, E.M. The Freshwater Mussels (Unionidae) off the Lake Maurepas-Ponchartrain-Borgne Drainage System, Louisiana and Mississippi. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 1976. [Google Scholar]
- Turgeon, D.D.; Quinn, J.F., Jr.; Bogan, A.E.; Coan, E.V.; Hochberg, F.G.; Lyons, W.G.; Mikkelsen, P.M.; Neves, R.J.; Roper, C.F.E.; Rosenberg, G.; et al. Common and Scientific Names of Aquatic Invertebrates from the United States and Canada: Mollusks, 2nd ed.; Special Publication 26; American Fisheries Society: Bethesda, MD, USA, 1998. [Google Scholar]
- Obermeyer, B.K. A comparison of quadrats versus timed snorkel searches for assessing freshwater mussels. Am. Midl. Nat. 1998, 139, 331–339. [Google Scholar] [CrossRef]
- Tiemann, J.S.; Cummings, K.S.; Mayer, C.A. Timed search technique used to evaluate freshwater mussel (Bivalvia: Unionidae) species richness in headwater streams: Is a single one-hour visit enough? J. Freshw. Ecol. 2009, 24, 85–92. [Google Scholar] [CrossRef]
- Cummins, K.W. An evaluation of some techniques for the collection and analysis of benthic samples with special emphasis on lotic waters. Am. Midl. Nat. 1962, 67, 477–504. [Google Scholar] [CrossRef]
- Shea, C.P.; Peterson, J.T.; Conroy, M.J.; Wisniewski, J.M. Evaluating the influence of land use, drought and reach isolation on the occurrence of freshwater mussel species in the lower Flint River Basin, Georgia (USA). Freshw. Biol. 2013, 58, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Dorman, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; García Marquéz, J.R.; Gruber, B.; Lafourcade, B.; Leitãno, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Palmer, M.W. Gradient analysis of ecological communities (ordination). In Handbook of Environmental and Ecological Statistics; Gelfand, A.E., Fuentes., M., Hoeting, J.A., Smith, R.L., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 241–274. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Harra, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package v. 2.6-2; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Jackson, D.A. Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology 1993, 74, 2204–2214. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Garcia-Martinez, M.Á.; Escobar-Sarria, D.; López-Barrera, F.; Castaño-Meneses, G.; Valenzuela-González, J.E. Value of riparian vegetation remnants for leaf-litter ants (Hymenoptera: Formicidae) in a human-dominated landscape in central Veracruz, Mexico. Environ. Entomol. 2016, 44, 1488–1497. [Google Scholar] [CrossRef]
- Brame, R.; Nagin, S.; Wasserman, L. Exploring some analytical characteristics of finite mixture models. J. Quant. Criminol. 2006, 22, 31–59. [Google Scholar] [CrossRef]
- McLachlan, G.J.; Lee, S.X.; Rathnayake, S.I. Finite mixture models. Ann. Rev. Stat. Appl. 2019, 6, 355–378. [Google Scholar] [CrossRef]
- Raim, A.M.; Neerchal, N.K.; Morel, J.G. An extension of generalized linear models to finite mixture outcomes. J. Comput. Graph. Stat. 2018, 27, 578–601. [Google Scholar] [CrossRef] [Green Version]
- Kéry, M.; Royle, J.A. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS; Elsevier: San Diego, CA, USA, 2016; Volume 1. [Google Scholar]
- Pituch, K.A.; Stevens, J.P. Applied Multivariate Statistics for the Social Science, 6th ed.; Routlege: New York, NY, USA, 2015. [Google Scholar]
- Lydeard, C.; Mayden, R.L. A diverse and endangered aquatic ecosystem of the southeast United States. Conserv. Biol. 1995, 9, 800–805. [Google Scholar] [CrossRef]
- Cushway, K.C.; Ring, N.S.; Patton, D.K.; Woolnough, D.A. Landscape associations with native and invasive freshwater mussels. Hydrobiologia 2022, 849, 2449–2462. [Google Scholar] [CrossRef]
- Daniel, W.M.; Brown, K.M. The role of life history and behavior in explaining unionid mussel distributions. Hydrobiologia 2014, 734, 57–68. [Google Scholar] [CrossRef]
- LDWF. Pearl River Fish Kill Post Incident Monitoring Report 2012–2014; Louisiana Department of Wildlife and Fisheries: Baton Rouge, LA, USA, 2014. [Google Scholar]
- Isphording, W.C.; Fitzpatrick, J.F. History of drainage systems in the southeastern United States. In Biodiversity of the Southeastern United States: Biological Communities; Hackney, C.T., Adams, S.M., Martins, W.H., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1992; pp. 19–56. [Google Scholar]
- Daniel, W.M.; Brown, K.M.; Kaller, M.D. A tiered aquatic life unit bioassessment model for Gulf of Mexico coastal streams. Fish. Manag. Ecol. 2014, 21, 491–502. [Google Scholar] [CrossRef]
- Markos, P.D.; Kaller, M.D.; Kelso, W.E. Channel stability and the temporal structure of coastal stream aquatic insect assemblages. Fund. Appl. Limnol. 2016, 188, 187–189. [Google Scholar] [CrossRef]
- Mallin, M.A.; Johnson, V.L.; Ensign, S.H.; MacPherson, T.A. Factors contributing to hypoxia in rivers, lakes, and streams. Limnol. Oceanogr. 2006, 51, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Justus, B.G.; Mize, S.V.; Kroes, D. Invertebrate and fish assemblage relations to dissolved oxygen minima in lowland streams of southwestern Louisiana. River Res. Appl. 2014, 30, 11–28. [Google Scholar] [CrossRef]
- Kaller, M.D.; Kelso, W.E. Association of macroinvertebrate assemblages with dissolved oxygen concentration and wood surface area in selected subtropical streams of the southeastern USA. Aquat. Ecol. 2007, 41, 95–110. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Cope, W.G.; Arellano, C.; Bringolf, R.B.; Barnhardt, M.C.; Hammer, E. Upper thermal tolerances of early life stages of freshwater mussels. J. N. Am. Benthol. Soc. 2010, 29, 959–969. [Google Scholar] [CrossRef]
- Galbraith, H.S.; Blakesee, C.J.; Lellis, W.A. Recent thermal history influences thermal tolerance in freshwater mussel species (Bivalvia: Unionidae). Freshw. Sci. 2012, 31, 83–92. [Google Scholar] [CrossRef]
- Jones, J.I.; Murphy, J.F.; Collins, A.L.; Sear, D.A.; Naden, P.S.; Armitage, P.D. The impact of fine sediment on macro-invertebrates. River Res. Appl. 2012, 28, 1055–1071. [Google Scholar] [CrossRef]
- Kaller, M.D.; Hartman, K. Evidence of a threshold level of fine sediment accumulation for altering benthic macroinvertebrate communities. Hydrobiologia 2004, 518, 95–104. [Google Scholar] [CrossRef]
- Keim, R.F.; Schoenholtz, S. Functions and effectiveness of silvicultural streamside management zones in loessial bluff forests. For. Ecol. Manag. 1999, 118, 197–209. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters, 2nd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- McWilliams, W.H.; Rosson, J.F., Jr. Composition and vulnerability of bottomland hardwood forests of the Coastal Plain Province of the southcentral United States. Forest Ecol. Manag. 1990, 33/34, 485–501. [Google Scholar] [CrossRef]
- Van Vranken, J.; O’Connell, M. Effects of Hurricane Katrina on freshwater fish assemblages in a small coastal tributary of Lake Ponchartrain, Louisiana. Trans. Am. Fish. Soc. 2010, 139, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Perret, A.J.; Kaller, M.D.; Kelso, W.E.; Rutherford, D.A. Effects of Hurricanes Katrina and Rita on sport fish community abundance in the eastern Atchafalaya River Basin, Louisiana. N. Am. J. Fish. Manag. 2010, 30, 511–517. [Google Scholar] [CrossRef]
- Schaefer, J.F.; Mickle, P.F.; Spaeth, J.; Viguera, P. Effects of Hurricane Katrina on the fish fauna of the Pascagoula River. Water Resour. 2006, 36, 62–68. [Google Scholar]
- French, S.K.; Ackerman, J.D. Responses of newly settled juvenile mussels to bed shear stress: Implications for dispersal. Freshw. Sci. 2014, 33, 46–55. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.H.; Marc, B. Habitat is more important than climate and animal richness at shaping latitudinal variation in plant diversity in China. Biodivers. Conserv. 2018, 27, 3679–3691. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.H. Climate stability is more important than water-energy variables in shaping the elevational variation in species richness. Ecol. Evol. 2018, 8, 6872–6879. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Luo, Z.; Lan, J.; Liu, Y. Elevational diversity gradients across seed plant taxonomic levels in the Lancang River Nature Reserve: Tole of temperature, water and the mid-domain effect. J. For. Res. 2018, 29, 1121–1127. [Google Scholar] [CrossRef]
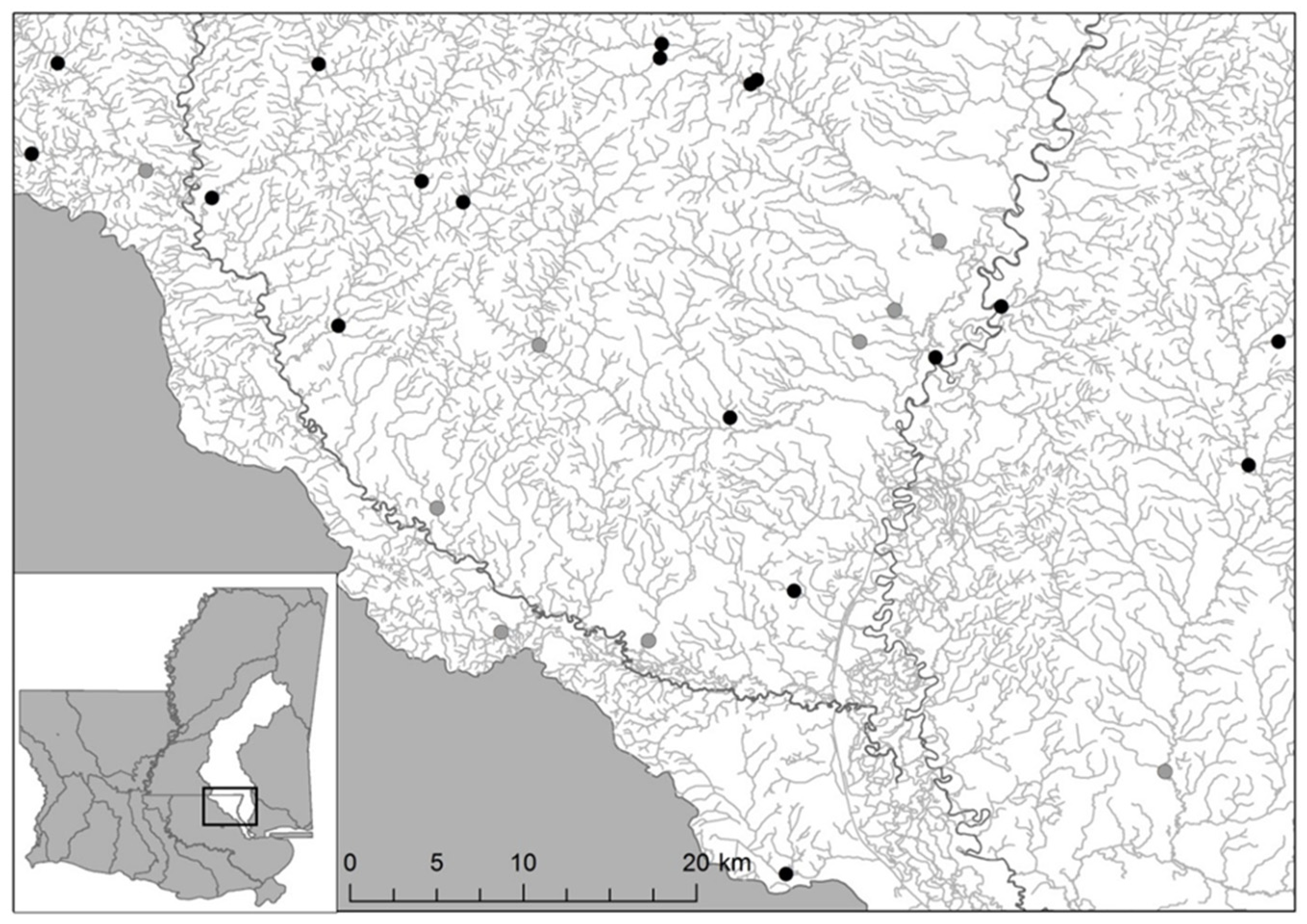
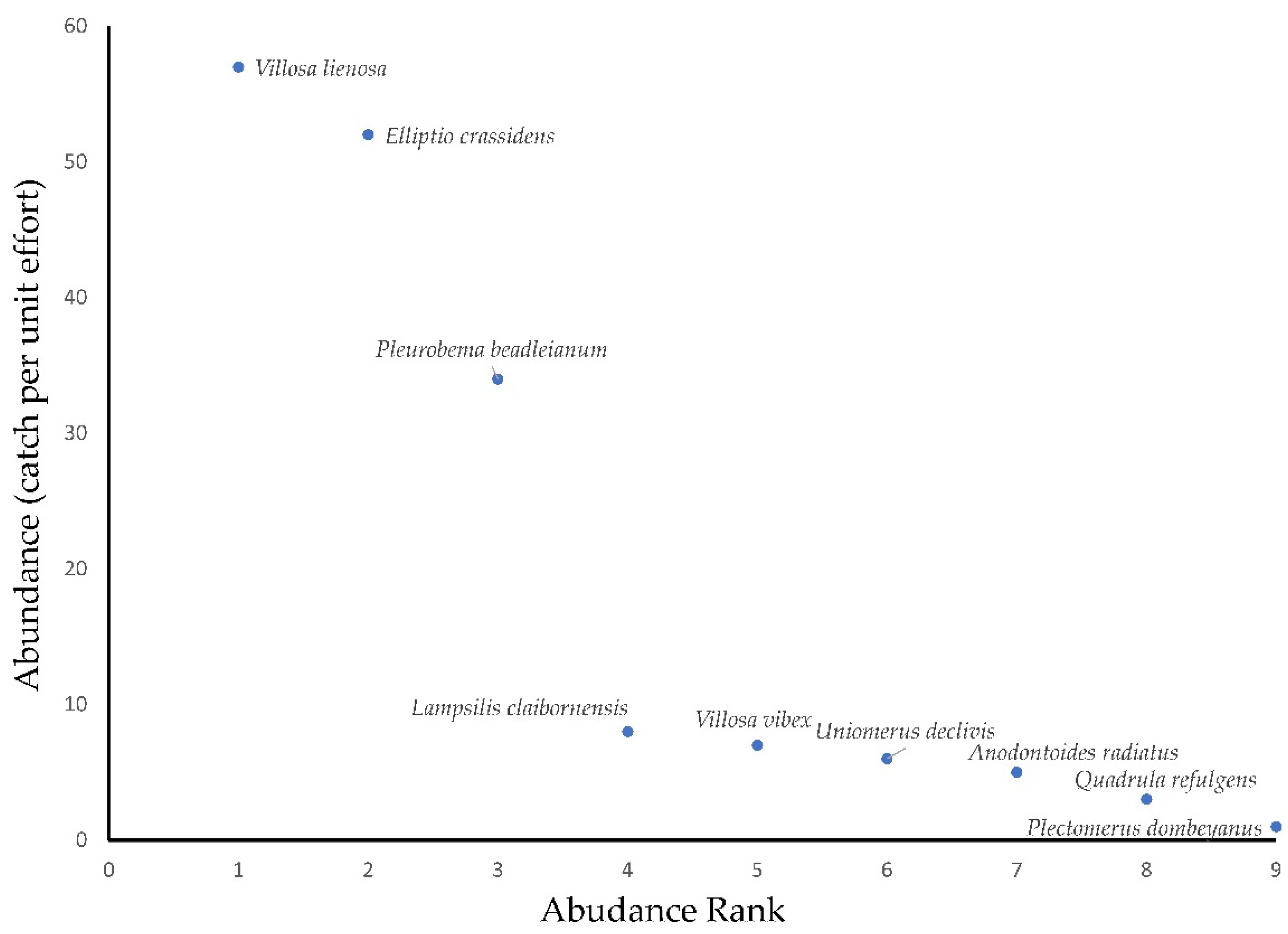

| Site | Anodontoides radiatus (Conrad, 1934) | Elliptio crassidens (Lamark, 1819) | Lampsilis claibornensis (Lee, 1838) | Plectomerus dombeyanus (Valenciennes, 1827) | Pleurobema beadleianum (Lea, 1961) |
|---|---|---|---|---|---|
| Adams Creek | 1 | ||||
| 1 Adams Creek | |||||
| Bogue Lusa Creek | 8 | ||||
| 1 Bogue Lusa Creek | 2 | ||||
| Crains Creek | 1 | ||||
| Deer Lick Creek | 1 | 1 | 2 | ||
| House Creek | |||||
| Mill Creek | |||||
| 1 Mill Creek | |||||
| Miller Creek | 1 | 1 | 6 | ||
| Peter’s Creek | 6 | ||||
| Pushepatapa Creek | 5 | ||||
| 1 Pushepatapa Creek | 1 | ||||
| Silver Creek | 3 | 52 | 3 | ||
| Silver Springs Creek | 1 | ||||
| Talley’s Creek | 3 | ||||
| 1 Talley’s Creek | 1 | ||||
| West Hobolochitto Ck. | 1 | 1 | |||
| 1 West Hobolochitto Ck. | |||||
| Relative abundance | 2.9% | 29.9% | 4.6% | 0.6% | 19.5% |
| Frequency of occurrence | 15.8% | 5.3% | 31.6% | 5.3% | 47.4% |
| Site | Quadrula refulgens (Lea, 1868) | Uniomerus declivis (Say, 1831) | Villosa lienosa (Conrad, 1834) | Villosa vibex (Conrad, 1834) | Estimated Sample Coverage |
| Adams Creek | 1 | 1 | 0.42 | ||
| 1 Adams Creek | 1 | 1 | 0.37 | ||
| Bogue Lusa Creek | 7 | 0.53 | |||
| 1 Bogue Lusa Creek | 1 | 0.53 | |||
| Crains Creek | 0.17 | ||||
| Deer Lick Creek | 2 | 1 | 0.64 | ||
| House Creek | 2 | 2 | 0.37 | ||
| Mill Creek | 4 | 0.33 | |||
| 1 Mill Creek | 1 | 0.33 | |||
| Miller Creek | 1 | 1 | 0.64 | ||
| Peter’s Creek | 2 | 0.53 | |||
| Pushepatapa Creek | 0.20 | ||||
| 1 Pushepatapa Creek | 0.20 | ||||
| Silver Creek | 7 | 1 | 0.90 | ||
| Silver Springs Creek | 0.05 | ||||
| Talley’s Creek | 3 | 7 | 0.41 | ||
| 1 Talley’s Creek | 3 | 21 | 0.41 | ||
| West Hobolochitto Ck. | 2 | 1 | 0.06 | ||
| 1 West Hobolochitto Ck. | 1 | 0.02 | |||
| Relative abundance | 1.7% | 3.4% | 33.3% | 4% | |
| Frequency of occurrence | 10.5% | 10.5% | 73.7% | 31.6% |
| Variable | Mean (SE) | Maximum | Minimum |
|---|---|---|---|
| Temperature (°C) | 24.4 (0.4) | 29.3 | 22.1 |
| Dissolved Oxygen (mg/L) | 7.2 (0.3) | 9.6 | 3.9 |
| Specific Conductance (µmhos/cm) | 0.04 (0.01) | 0.1 | 0.02 |
| pH | 7.4 (0.1) | 8.3 | 5.5 |
| Turbidity (NTU) | 10.1 (2.7) | 59.6 | 0.3 |
| Depth (cm) | 46.3 (1.3) | 121.0 | |
| Flow Velocity (cm/s) | 14.1 (0.7) | 83.8 | 0 |
| Bank Height (m) | 1.5 (0.04) | 5.0 | 0.2 |
| Bank Angle | 42.4 (1.4) | 90.0 | 2.7 |
| Stream Width (m) | 7.9 (0.3) | 21.5 | 0.4 |
| Distance to mainstem (km) | 12.4 (2.2) | 44.0 | 0.1 |
| Canopy Density (%) | 72.6 (2.4) | 100.0 | 6.3 |
| Sediment Diameter (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.12 |
| Big Trib | 99.9 | 99.9 | 99.3 | 98.1 | 90.4 | 72.3 | 14.2 | 0.6 |
| Adam’s Creek | 99.2 | 98.7 | 97.6 | 95.6 | 93.0 | 62.2 | 10.7 | 0.4 |
| Ben’s Creek | 99.5 | 99.1 | 98.1 | 96.1 | 92.3 | 44.9 | 8.1 | 0.2 |
| Bogue Lusa Creek | 99.8 | 99.7 | 99.5 | 98.7 | 95.4 | 35.3 | 4.9 | 0.2 |
| Crains Creek | 99.2 | 97.6 | 96.5 | 95.1 | 92.7 | 32.6 | 2.9 | <0.1 |
| Deer Lick Creek | 88.6 | 74.3 | 65.9 | 61.0 | 56.2 | 27.4 | 2.3 | 0.1 |
| Hays Creek | 81.7 | 57.9 | 41.7 | 30.7 | 25.5 | 14.7 | 4.1 | 0.2 |
| Hays Creek Site 2 | 91.5 | 86.3 | 83.6 | 81.8 | 78.1 | 18.4 | 1.7 | <0.1 |
| House Creek | 99.5 | 99.4 | 99.2 | 98.5 | 97.6 | 57.4 | 10.2 | 0.2 |
| Lawrence Creek | 77.0 | 65.0 | 55.8 | 50.0 | 46.1 | 14.4 | 2.4 | <0.1 |
| Lawrence Creek Site 2 | 100.0 | 99.9 | 99.9 | 99.9 | 99.7 | 20.4 | 2.0 | <0.1 |
| Mill Creek | 82.8 | 58.3 | 45.1 | 38.5 | 35.0 | 21.5 | 2.7 | <0.1 |
| Miller Creek | 99.2 | 99.1 | 98.8 | 98.3 | 97.2 | 29.9 | 3.1 | <0.1 |
| Peter’s Creek | 99.6 | 99.1 | 97.5 | 96.4 | 94.7 | 29.6 | 7.9 | 0.4 |
| Peter’s Cutoff | 98.8 | 98.1 | 95.6 | 91.4 | 87.4 | 52.0 | 18.4 | 0.7 |
| Pushepatapa Creek | 99.8 | 99.3 | 98.3 | 95.9 | 90.8 | 41.5 | 9.2 | 0.2 |
| Pushepatapa Site 2 | 90.1 | 73.7 | 61.5 | 54.3 | 43.9 | 9.3 | 1.7 | <0.1 |
| Sal’s Branch | 88.9 | 59.1 | 36.6 | 23.5 | 17.5 | 6.2 | 1.0 | <0.1 |
| Silver Creek | 72.7 | 61.8 | 57.5 | 52.4 | 35.6 | 4.7 | 1.0 | <0.1 |
| Silver Springs Creek | 94.6 | 87.2 | 83.8 | 82.1 | 73.0 | 41.8 | 12.8 | 0.3 |
| Stubbs Creek | 95.2 | 85.9 | 74.0 | 60.2 | 48.1 | 22.5 | 2.5 | 0.1 |
| Talisheek Creek | 99.6 | 99.5 | 99.4 | 99.0 | 96.4 | 48.9 | 5.0 | 0.1 |
| Talley’s Creek | 91.6 | 67.1 | 54.6 | 47.6 | 44.7 | 38.5 | 5.5 | 0.1 |
| Thomas Creek | 96.3 | 93.9 | 90.1 | 87.1 | 78.4 | 49.1 | 6.5 | 0.1 |
| West Hobolochitto Creek | 100.0 | 99.9 | 99.8 | 99.3 | 97.6 | 53.8 | 5.6 | 0.1 |
| West Hobolochitto Site 2 | 99.7 | 98.5 | 95.7 | 92.5 | 88.5 | 39.6 | 3.9 | 0.1 |
| White Sands Creek | 97.76 | 94.31 | 91.30 | 88.6 | 84.4 | 46.8 | 5.7 | 0.1 |
| Variable | Mean | Maximum | Minimum |
|---|---|---|---|
| Land Cover Types | |||
| Developed | 1.69% (0.00) | 3.44% | 0.58% |
| Agriculture | 50.21% (0.02) | 63.18% | 23.12% |
| Deciduous Forest | 0.64% (0.00) | 1.71% | 0.14% |
| Evergreen Forest | 20.98% (0.01) | 36.79% | 9.09% |
| Mixed Forest | 3.90% (0.00) | 8.07% | 0.79% |
| Wetland | 21.56% (0.02) | 53.40% | 10.02% |
| Barren | 1.02% (0.00) | 8.09% | 0.16% |
| Geology | |||
| % Alluvium | 20.55% (0.02) | 57.06% | 1.39% |
| % Prairie Terraces | 22.84% (0.07) | 98.61% | 0.00% |
| % High Terraces | 56.48% (0.07) | 95.74% | 0.00% |
| % Deweyville Terraces | 0.13% (0.00) | 2.02% | 0.00% |
| % Pascagoula Hattiesburg | 69.82% (0.06) | 85.32% | 58.61% |
| % Citronelle Formation | 19.83% (0.08) | 32.43% | 0.00% |
| % Coastal Deposits | 10.35% (0.10) | 41.39% | 0.00% |
| Variable | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 |
|---|---|---|---|---|---|---|---|
| Temperature | 0.59 | 0.17 | −0.31 | 0.43 | −0.41 | 0.07 | 0.22 |
| Dissolved oxygen | −0.55 | 0.12 | 0.23 | 0.28 | −0.20 | −0.10 | 0.52 |
| Specific conductance | 0.46 | 0.27 | −0.36 | 0.23 | 0.44 | 0.32 | 0.15 |
| pH | −0.09 | 0.59 | 0 | 0.24 | 0.50 | −0.08 | −0.09 |
| Turbidity | 0.27 | −0.29 | 0.39 | −0.27 | 0.44 | 0.23 | 0.08 |
| % Developed | −0.33 | −0.31 | −0.38 | 0.38 | 0.28 | 0.27 | 0.18 |
| % Agriculture | −0.60 | −0.24 | −0.69 | −0.13 | 0.03 | −0.02 | 0.10 |
| % Deciduous forest | −0.48 | 0.64 | −0.08 | −0.37 | 0.04 | 0.28 | −0.02 |
| % Evergreen forest | 0.56 | −0.45 | 0.20 | 0.06 | 0.15 | −0.56 | −0.04 |
| % Mixed forest | −0.36 | 0.60 | −0.08 | −0.62 | 0.18 | 0.05 | 0.06 |
| % Wetland | 0.24 | 0.54 | 0.61 | 0.21 | −0.13 | 0.36 | −0.08 |
| % Barren | 0.33 | −0.35 | 0 | −0.02 | −0.48 | 0.38 | −0.08 |
| % Prairie | 0.17 | −0.67 | −0.08 | −0.46 | 0.02 | 0.37 | 0.06 |
| % Alluvium | −0.54 | 0.01 | 0.53 | 0.48 | −0.13 | 0.35 | −0.10 |
| % High Terrace | −0.80 | 0.13 | 0.01 | 0.27 | 0 | −0.37 | −0.11 |
| % Deweyville | 0.08 | −0.38 | 0.17 | 0.37 | 0.73 | 0.11 | −0.01 |
| % Pascagoula | 0.80 | 0.48 | −0.19 | 0.02 | −0.01 | −0.10 | 0.09 |
| % Citronelle | 0.63 | 0.43 | −0.47 | 0.16 | 0.01 | 0.02 | −0.17 |
| % Coastal | 0.45 | 0.21 | 0.54 | −0.32 | 0.10 | −0.20 | 0.31 |
| Variance Explained | 23.5% | 16.6% | 12.3% | 10.3% | 9.4% | 7.2% | 5.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bird, C.T.; Kaller, M.D.; Pasco, T.E.; Kelso, W.E. Microhabitat and Landscape Drivers of Richness and Abundance of Freshwater Mussels (Unionida: Unionidae) in a Coastal Plain River. Appl. Sci. 2022, 12, 10300. https://doi.org/10.3390/app122010300
Bird CT, Kaller MD, Pasco TE, Kelso WE. Microhabitat and Landscape Drivers of Richness and Abundance of Freshwater Mussels (Unionida: Unionidae) in a Coastal Plain River. Applied Sciences. 2022; 12(20):10300. https://doi.org/10.3390/app122010300
Chicago/Turabian StyleBird, Corinne T., Michael D. Kaller, Tiffany E. Pasco, and William E. Kelso. 2022. "Microhabitat and Landscape Drivers of Richness and Abundance of Freshwater Mussels (Unionida: Unionidae) in a Coastal Plain River" Applied Sciences 12, no. 20: 10300. https://doi.org/10.3390/app122010300







