Effects of Wearing a Swimming Cap and Goggles on Phoria and Fusional Vergence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Swimming Equipment
2.3. Clinical Refraction
2.4. Measurement of Phoria and Fusional Vergence
2.5. Statistical Analysis
3. Results
3.1. Participant Demographics and Vision Correction
3.2. Changes in Phoria and Fusional Vergence
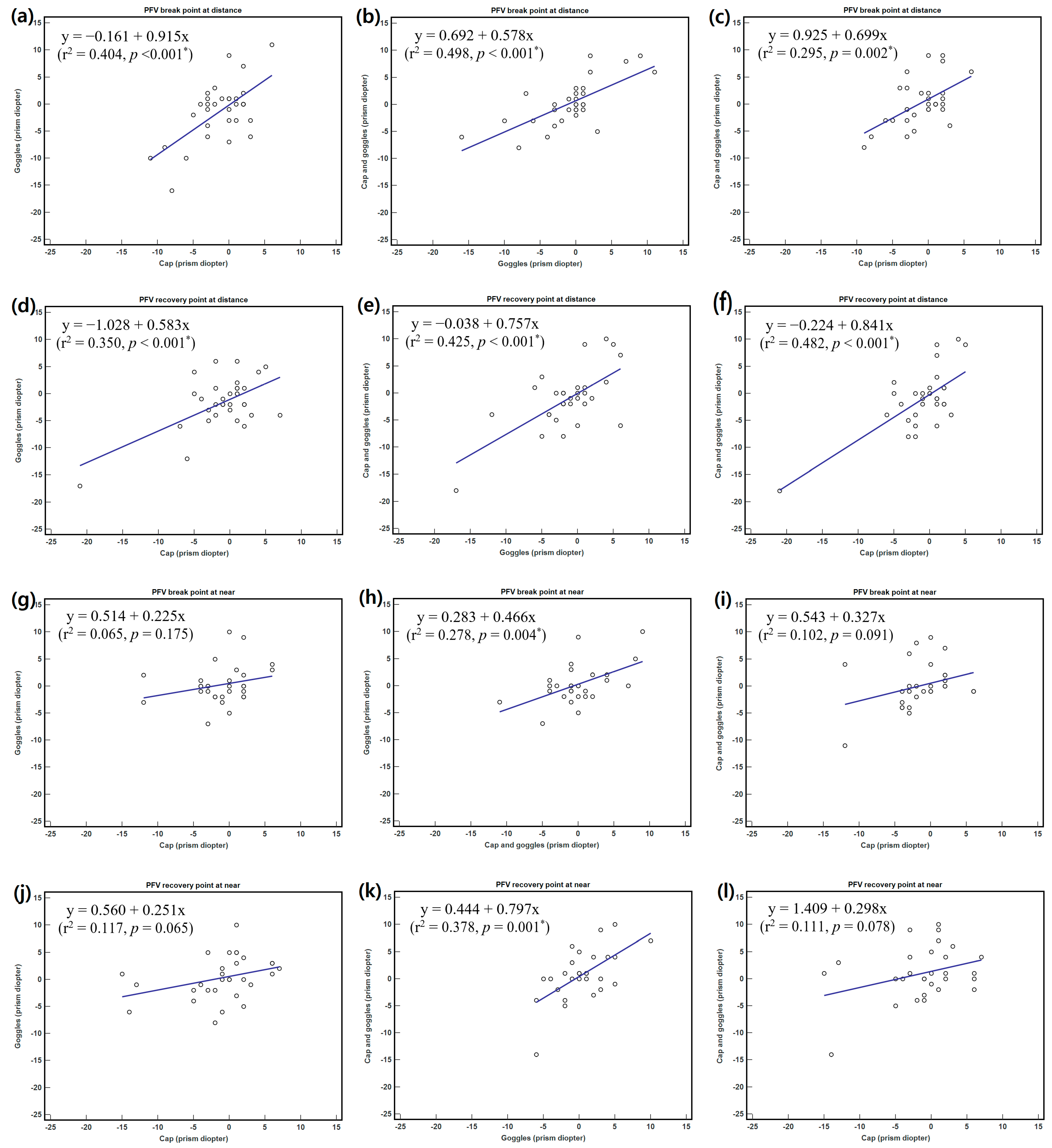
3.3. Relationships between Changes in Phoria and Fusional Vergence
4. Discussion
4.1. Changes in Phoria and Fusional Vergence
4.2. Changes in Prism Prescription
4.3. Relationship between Swimming Equipment and Visual Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asaoka, R.; Crabb, D.P.; Yamashita, T.; Russell, R.A.; Wang, Y.X.; Garway-Heath, D.F. Patients have two eyes!: Binocular versus better eye visual field indices. Invest. Ophthalmol. Vis. Sci. 2011, 52, 7007–7011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yibekal, B.T.; Alemu, D.S.; Anbesse, D.H.; Alemayehu, A.M.; Alimaw, Y.A. Vision-related quality of life among adult patients with visual impairment at University of Gondar, Northwest Ethiopia. J. Ophthalmol. 2020, 2020, 9056097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatt, S.R.; Leske, D.A.; Kirgis, P.A.; Bradley, E.A.; Holmes, J.M. The effects of strabismus on quality of life in adults. Am. J. Ophthalmol. 2007, 144, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, T.M.; Hsu, W.M.; Chou, C.K.; Tsai, S.Y.; Chou, P. Impact of stereopsis on quality of life. Eye (Lond). 2005, 19, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Akon, M. Non-strabismic binocular vision abnormalities. J. Ophthalmol. Vis. Sci. 2016, 1, 1006. [Google Scholar]
- Hu, J.; Wang, G.; Zhou, Z.; Sun, Y.; Zhang, Q.; Wu, J.; Gao, Y. Evaluation of a novel quality of life scale for schoolchildren with nonstrabismic binocular vision anomalies. Biomed. Res. Int. 2020, 2020, 4723402. [Google Scholar] [CrossRef]
- Cacho Martínez, P.; García Muñoz, A.; Ruiz-Cantero, M.T. Treatment of accommodative and nonstrabismic binocular dysfunctions: A systematic review. Optometry 2009, 80, 702–716. [Google Scholar] [CrossRef]
- Dain, S.J. Materials for occupational eye protectors. Clin. Exp. Optom. 2012, 95, 129–139. [Google Scholar] [CrossRef]
- Eppig, T.; Speck, A.; Zelzer, B.; Langenbucher, A. Protective glasses. Personal eye protection for professional use. Ophthalmologe 2014, 111, 681–691. [Google Scholar] [CrossRef]
- Hoskin, A.K.; Mackey, D.A.; Keay, L.; Agrawal, R.; Watson, S. Eye Injuries across history and the evolution of eye protection. Acta Ophthalmol. 2019, 97, 637–643. [Google Scholar] [CrossRef]
- Miller, B.A.; Miller, S.J. Visual fields with protective eyewear. J. Orthop. Sports Phys. Ther. 1993, 18, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.K.; Kaur, M.; Choity, M. Effect of helmet use on visual and auditory reaction time and peripheral field of vision. Natl. J. Physiol. Pharm. Pharmacol. 2019, 9, 307–311. [Google Scholar] [CrossRef]
- Kramer, M.R.; Teel, E.F.; Wasserman, E.B.; Mihalik, J.P. Effect of protective helmets on vision and sensory performance in healthy men. Athletic Train. Sports Health Care 2021, 13, 130–135. [Google Scholar] [CrossRef]
- Kramer, M.R.; Wasserman, E.B.; Teel, E.F.; Mihalik, J.P. Effect of protective helmets on vision and sensory performance. Br. J. Sports Med. 2017, 51, A65. [Google Scholar] [CrossRef]
- Ma, K.T.; Chung, W.S.; Seo, K.Y.; Seong, G.J.; Kim, C.Y. The effect of swimming goggles on intraocular pressure and blood flow within the optic nerve head. Yonsei Med. J. 2007, 48, 807–809. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, H.; Nie, Y.; Li, W. Short-Term Effects of Two Types of Goggles on Intraocular Pressure and Anterior Eye Segment Biometrics. BMC Ophthalmol. 2021, 22, 73. [Google Scholar] [CrossRef]
- Jiménez, R.; Molina, R.; García, J.A.; Redondo, B.; Vera, J. Wearing swimming goggles reduces central corneal thickness and anterior chamber angle, and increases intraocular pressure. Curr. Eye Res. 2020, 45, 535–541. [Google Scholar] [CrossRef]
- Vera, J.; Redondo, B.; Molina, R.; Jiménez, R. Effects of wearing swimming goggles on non-invasive tear break-up time in a laboratory setting. J. Optom. 2022, 15, 535–541. [Google Scholar] [CrossRef]
- Chase, N.L.; Sui, X.; Blair, S.N. Comparison of the health aspects of swimming with other types of physical activity and sedentary lifestyle habits. Int. J. Aquatic Res. Educ. 2008, 2, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.K.C.; Lee, A.S.Y.; Hamilton, K. Descriptive epidemiology and correlates of children’s swimming competence. J. Sports Sci. 2020, 38, 2253–2263. [Google Scholar] [CrossRef]
- Sheard, C. Zones of ocular comfort. Am. J. Optom. 1930, 7, 9–25. [Google Scholar] [CrossRef]
- Michaels, D.D. Indications for prescribing spectacles. Surv. Ophthalmol. 1981, 26, 55–74. [Google Scholar] [CrossRef]
- Shaunak, S.; O’Sullivan, E.; Kennard, C. Eye movements. J. Neurol. Neurosurg. Psychiatry 1995, 59, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demer, J.L.; Clark, R.A.; Crane, B.T.; Tian, J.R.; Narasimhan, A.; Karim, S. Functional anatomy of the extraocular muscles during vergence. Prog. Brain Res. 2008, 171, 21–28. [Google Scholar] [PubMed] [Green Version]
- Gupta, S.K.; Aparna, S. Effect of yoga ocular exercises on binocular vision functions. Optom. Vis. Perf. 2019, 7, 229–237. [Google Scholar]
- Prajapati, B.; Dunne, M.C.M.; Armstrong, R.A. Sample size estimation and statistical power analyses. Optometry Today 2010, 50, 1–9. Available online: https://www.researchgate.net/publication/265399772_Sample_size_estimation_and_statistical_power_analyses (accessed on 25 January 2022).
- Scheiman, M.; Herzberg, H.; Frantz, K.; Margolies, M. A normative study of step vergence in elementary schoolchildren. J. Am. Optom. Assoc. 1989, 60, 276–780. [Google Scholar]
- Worrell, B.E., Jr.; Hirsch, M.J.; Morgan, M.W. An evaluation of prism prescribed by Sheard’s criterion. Am. J. Optom. Arch. Am. Acad. Optom. 1971, 48, 373–376. [Google Scholar] [CrossRef]
- Penisten, D.K.; Hofstetter, H.W.; Goss, D.A. Reliability of rotary prism fusional vergence ranges. Optometry 2001, 72, 117–122. [Google Scholar]
- Anstice, N.S.; Davidson, B.; Field, B.; Mathan, J.; Collins, A.V.; Black, J.M. The repeatability and reproducibility of four techniques for measuring horizontal heterophoria: Implications for clinical practice. J. Optom. 2021, 14, 275–281. [Google Scholar] [CrossRef]
- Goss, D.A.; Becker, E. Comparison of near fusional vergence ranges with rotary prisms and with prism bars. Optometry 2011, 82, 104–107. [Google Scholar] [CrossRef]
- Jiménez, R.; Martínez-Almeida, L.; Salas, C.; Ortíz, C. Contact lenses vs spectacles in myopes: Is there any difference in accommodative and binocular function? Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.Y.; Kim, K.H.; Lee, D.H. Clinical evaluation on variation of face form angle of eyewear. J. Korean Ophthalmic Opt. Soc. 2015, 20, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, M.L.; Gizzi, M.S. The effect of lid elevation on the cross-cover test. J. Neuroophthalmol. 2001, 21, 87–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.M. Understanding and misunderstanding extraocular muscle pulleys. J. Vis. 2007, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bour, L.J.; Aramideh, M.; de Visser, B.W. Neurophysiological aspects of eye and eyelid movements during blinking in humans. J. Neurophysiol. 2000, 83, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kumar, Y.S.; Yoo, J.; Kwon, S. Change of blink rate in viewing virtual reality with HMD. Symmetry 2018, 10, 400. [Google Scholar] [CrossRef] [Green Version]
- Morgan, W.H.; Cunneen, T.S.; Balaratnasingam, C.; Yu, D.Y. Wearing swimming goggles can elevate intraocular pressure. Br. J. Ophthalmol. 2008, 92, 1218–1221. [Google Scholar] [CrossRef] [Green Version]
- Plaut, G.S. Diplopia in a swimmer due to badly fitting goggles. Postgrad. Med. J. 1998, 74, 607. [Google Scholar] [CrossRef] [Green Version]
- Roh, H. Change in visual perception and balance caused by different types of hat. J. Phys. Ther. Sci. 2014, 26, 199–201. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.C., Jr. Swimmer’s headache, or supraorbital neuralgia. Proc. Bayl. Univ. Med. Cent. 2004, 17, 418–419. [Google Scholar] [CrossRef] [Green Version]
- Krymchantowski, A.V. Headaches due to external compression. Curr. Pain Headache Rep. 2010, 14, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Wakely, L.A.; Reeves, G.; Ashraff, N.; Wells, A.P. Swimming goggles suck. Br. J. Ophthalmol. 2004, 88, 1600–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, K.A. Prescribing relieving prism for patients with binocular vision disorders. J. Optometr. Vision Dev. 1997, 28, 54–67. [Google Scholar]
- Percival, A. The Prescribing of Spectacles, 3rd ed.; John Wright & Sons: Bristol, UK, 1928; pp. 102–109. Available online: https://archive.org/details/b21287405/page/102 (accessed on 21 January 2022).
- Antona, B.; Barrio, A.; Barra, F.; Gonzalez, E.; Sanchez, I. Repeatability and agreement in the measurement of horizontal fusional vergences. Ophthalmic Physiol. Opt. 2008, 28, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Eskridge, J.B. An investigation of voluntary vergence. Am. J. Optom. Arch. Am. Acad. Optom. 1971, 48, 741–746. [Google Scholar] [CrossRef]
- Stoller, S.H.; Meyer, D.R. Quantitating the change in upper eyelid position during downgaze. Ophthalmology 1994, 101, 1604–1607. [Google Scholar] [CrossRef]
- Read, S.A.; Collins, M.J.; Carney, L.G.; Iskander, D.R. The morphology of the palpebral fissure in different directions of vertical gaze. Optom. Vis. Sci. 2006, 83, 715–722. [Google Scholar] [CrossRef]
- Chu, B.S. Assessment of visual satisfaction and visual function with prescription swimming goggles in-air and underwater. J. Korean. Oph. Opt. Soc. 2013, 18, 357–363. [Google Scholar] [CrossRef]


| Mean ± SD | Range | |
|---|---|---|
| All participants (n) | 35 | |
| Age (years) | 22.31 ± 1.71 | 20–25 |
| Male/female (n) | 24/11 | |
| Manifest refraction (n) | 26 | |
| Spherical power | −4.29 ± 2.91 D | −9.75–0.50 D |
| Cylindrical power | −1.38 ± 0.81 D | −3.50–−0.25 D |
| Spherical equivalent | −4.91 ± 3.07 D | −11.00–−0.50 D |
| Refractive correction with soft contact lenses | 26 | |
| Spherical equivalent | −4.50 ± 2.72 D | −9.50–−0.50 D |
| Visual acuity (decimal) | 0.8 (0.7–1.0) 1 | 0.5–1.2 |
| Visual acuity (logMAR) | 0.09 ± 0.09 | −0.0–0.30 |
| No refractive error (n) | 9 | |
| Visual acuity (decimal) | 1.1 (1.0–1.2) 1 | 0.8–1.2 |
| Visual acuity (logMAR) | −0.04 ± 0.05 | −0.08–0.10 |
| Myopia after wearing soft contact lenses (n) | 26 | |
| Phoria at distance (∆) 2 | −1.46 ± 3.07 | −8.33–6.33 |
| Phoria at near (∆) | −5.08 ± 6.32 | −16.00–10.00 |
| Emmetropia after non-wearing of soft contact lenses (n) | 9 | |
| Phoria at distance (∆) | −1.33 ± 2.06 | −6.00–0.67 |
| Phoria at near (∆) | −5.71 ± 5.72 | −13.00–1.00 |
| Soft Contact Lenses | p-Value | ||
|---|---|---|---|
| Wearing (Myopia) | Non-Wearing (Emmetropia) | ||
| Non-wearing of swimming equipment | |||
| Distance | −1.46 ± 3.07 | −1.33 ± 2.06 | 0.913 |
| Near | −5.08 ± 6.32 | −5.71 ± 5.72 | 0.794 |
| Wearing cap | |||
| Distance | −1.13 ± 3.50 | −1.19 ± 2.36 | 0.964 |
| Near | −4.35 ± 6.50 | −4.63 ± 6.28 | 0.913 |
| Wearing goggles | |||
| Distance | −1.49 ± 3.09 | −1.41 ± 2.38 | 0.940 |
| Near | −4.43 ± 5.34 | −5.00 ± 5.95 | 0.789 |
| Wearing cap and goggles | |||
| Distance | −1.45 ± 3.09 | −1.17 ± 2.49 | 0.807 |
| Near | −4.66 ± 5.37 | −5.52 ± 6.89 | 0.703 |
| a. Non-wearing (n) | b. Cap (n: +, −, 0) 1 | c. Goggles (n: +, −, 0) | d. Cap + Goggles (n: +, −, 0) | p-Value | |
|---|---|---|---|---|---|
| Phoria 2 | |||||
| Distance | −1.42 ± 2.82 (35) | −1.14 ± 3.21 (14, 13, 8) | −1.47 ± 2.89 (12, 18, 5) | −1.38 ± 2.91 (14, 18, 3) | 0.204 |
| Near | −5.24 ± 6.10 (35) | −4.42 ± 6.35 (22, 9, 4) | −4.58 ± 5.42 (21, 11, 3) | −4.88 ± 5.70 (16, 15, 4) | 0.084 |
| NFV at distance | |||||
| Blur (n, blur vs. no blur) | (9:26) | (6:29) | (13:22) | (6:29) | |
| Break | 10.26 ± 3.22 (35) | 10.63 ± 4.65 (16, 14, 5) | 11.00 ± 4.41 (16, 11, 8) | 11.03 ± 4.85 (14, 13, 8) | 0.435 |
| Recovery | 6.40 ± 3.22 (35) | 6.09 ± 2.78 (13, 18, 4) | 6.26 ± 3.21 (13, 13, 9) | 7.03 ± 3.44 (16, 12, 7) | 0.190 |
| NFV at near | Suppression (n = 2) | Suppression (n = 2) | |||
| Blur (n, blur vs. no blur) | (11:24) | (7:28) | (5:30) | (8:27) | |
| Break | 17.54 ± 6.38 (35) | 17.51 ± 6.12 (16, 16, 3) | 16.30 ± 5.38 (8, 20, 5) | 17.09 ± 4.64 (10, 17, 6) | 0.052 |
| Recovery | 12.91 ± 5.55 (35) | 12.63 ± 5.35 (14, 15, 6) | 11.67 ± 4.84 (9, 19, 5) | 12.79 ± 4.40 (11, 17, 5) | 0.072 |
| PFV at distance | Suppression (n = 1) | Suppression (n = 2) | Suppression (n = 4) | ||
| Blur (n, blur vs. no blur) | (21:14) | (15:20) | (11:24) | (9:26) | |
| Break | 14.00 ± 6.33 (35) | 12.53 ± 6.20 (12, 16, 6) | 12.55 ± 6.47 (11, 14, 8) | 13.61 ± 5.55 (12, 14, 5) | 0.263 |
| Recovery | 9.57 ± 5.55 (35) | 8.62 ± 5.46 (15, 15, 4) | 8.03 ± 5.15 (10, 19, 4) | 8.26 ± 4.75 (9, 17, 5) | 0.378 |
| PFV at near | Suppression (n = 5) | Suppression (n = 6) | |||
| Blur (n, blur vs. no blur) | (6:29) | (5:30) | (4:31) | (5:30) | |
| Break | 14.77 ± 5.75 (35) | 13.31 ± 5.51 (10, 20, 5) | 14.77 ± 6.36 (10, 13, 7) | 15.10 ± 6.85 (10, 14, 5) | 0.286 |
| Recovery | 9.77 ± 5.83 (35) | 8.54 ± 5.51 (13, 18, 4) | 9.97 ± 6.35 (14, 12, 4) | 11.00 ± 6.19 (15, 8, 6) | 0.220 |
| a. Cap | b. Goggles | c. Cap + Goggles | Subtotal Average | p-Value | |||
|---|---|---|---|---|---|---|---|
| a vs. b | b vs. c | a vs. c | |||||
| Phoria | |||||||
| Distance | 6 (17.1%) | 7 (20.0%) | 9 (25.7%) | 22/105 (21.05%) | 0.010 * | 0.009 * | 0.326 |
| Near | 9 (25.7%) | 10 (28.6%) | 16 (45.7%) | 35/105 (33.3%) | 0.099 | 0.003 * | 0.009 * |
| NFV at distance | |||||||
| Break | 10 (28.6%) | 13 (37.1%) | 10 (28.6%) | 33/105 (31.4%) | 0.167 | 0.034 * | 0.003 * |
| Recovery | 10 (28.6%) | 9 (25.7%) | 14 (40.0%) | 33/105 (31.4%) | 0.427 | 0.134 | 0.056 |
| NFV at near | |||||||
| Break | 3 (8.6%) | 7 (20.0%) | 7 (20.0%) | 17/105 (16.2%) | 0.880 | 0.027 * | 0.174 |
| Recovery | 13 (37.1%) | 22 (62.9%) | 17 (48.6%) | 52/105 (49.5%) | 0.336 | 0.204 | 0.897 |
| PFV at distance | |||||||
| Break | 7 (20.0%) | 12 (34.3%) | 12 (34.3%) | 31/105 (29.5%) | 0.006 * | 0.001 * | 0.006 * |
| Recovery | 5 (14.3%) | 8 (22.9%) | 13 (45.7%) | 26/105 (24.8%) | 0.007 * | 0.035 * | 0.101 |
| PFV at near | |||||||
| Break | 5 (14.3%) | 10 (28.6%) | 12 (34.3%) | 27/105 (25.7%) | 0.939 | 0.001 * | 0.827 |
| Recovery | 7 (20.0%) | 9 (25.7%) | 12 (34.3%) | 28/105 (26.7%) | 0.772 | <0.001 * | 0.423 |
| Subtotal average | 75/350 (21.4%) | 107/350 (30.6%) | 122/350 (34.9) | 304/1050 (29.0%) 1 | <0.001 * | <0.001 * | <0.001 * |
| Distance | Near | |
|---|---|---|
| Non-wearing | 6 (17.1%) | 15 (42.9%) |
| a. Cap | 7 (20.0%) | 16 (45.7%) |
| b. Goggles | 8 (22.9%) | 15 (42.9%) |
| c. Cap + goggles | 7 (20.0%) | 18 (51.4%) |
| p-value | ||
| Non-wearing vs. cap | 0.010 * | <0.001 * |
| Non-wearing vs. goggles | 0.023 * | 0.001 * |
| Non-wearing vs. cap + goggles | 0.010 * | 0.001 * |
| Cap vs. goggles | <0.001 * | <0.001 * |
| Goggles vs. cap + goggles | <0.001 * | <0.001 * |
| Cap vs. cap + goggles | <0.001 * | <0.001 * |
| Relative Weight | r (p-Value) | ||
|---|---|---|---|
| Mean ± SD (g) | Phoria at Distance | Phoria at Near | |
| Cap | 400 ± 95 | 0.163 (0.350) | 0.023 (0.896) |
| Goggles | 464 ± 108 | 0.314 (0.066) | 0.345 (0.042 *) |
| Cap + goggles | 653 ± 90 | 0.426 (0.011 *) | 0.225 (0.194) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-C.; Cho, H.-G.; Moon, B.-Y.; Kim, S.-Y.; Yu, D.-S. Effects of Wearing a Swimming Cap and Goggles on Phoria and Fusional Vergence. Appl. Sci. 2022, 12, 10797. https://doi.org/10.3390/app122110797
Park S-C, Cho H-G, Moon B-Y, Kim S-Y, Yu D-S. Effects of Wearing a Swimming Cap and Goggles on Phoria and Fusional Vergence. Applied Sciences. 2022; 12(21):10797. https://doi.org/10.3390/app122110797
Chicago/Turabian StylePark, Seung-Chul, Hyun-Gug Cho, Byeong-Yeon Moon, Sang-Yeob Kim, and Dong-Sik Yu. 2022. "Effects of Wearing a Swimming Cap and Goggles on Phoria and Fusional Vergence" Applied Sciences 12, no. 21: 10797. https://doi.org/10.3390/app122110797





