A Review on Carbon Quantum Dots Modified g-C3N4-Based Photocatalysts and Potential Application in Wastewater Treatment
Abstract
:1. Introduction
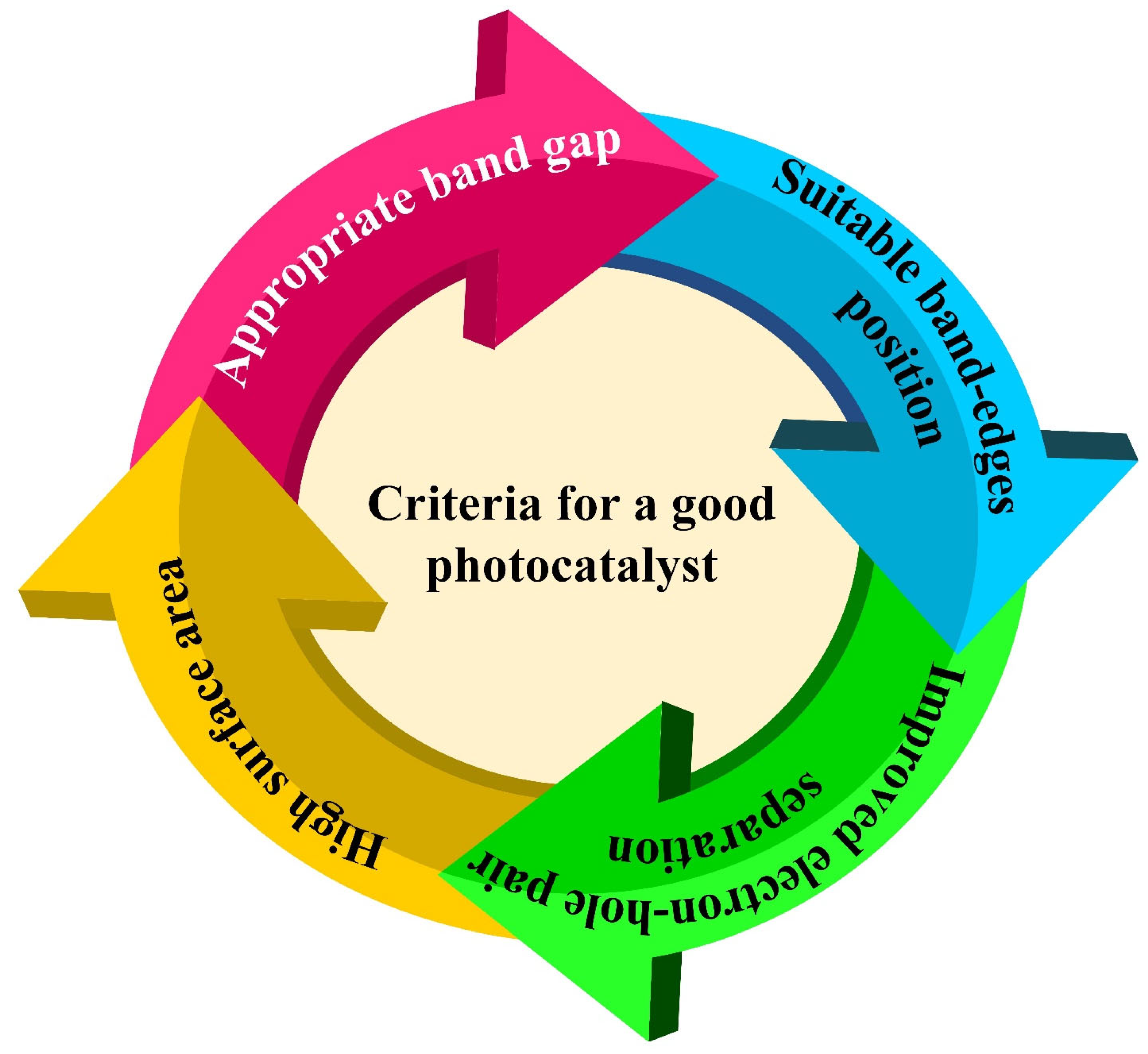
2. g-C3N4 as a Potential Photocatalyst
3. Design Consideration for g-C3N4/CQDs-Based Nanocomposites
3.1. g-C3N4/CQDs-Based Nanocomposites for Pollutant Degradation
3.2. g-C3N4/CQDs-Based Nanocomposites for Different Antibiotic Degradation
3.3. g-C3N4/CQDs-Based Nanocomposites for CO2 Reduction
4. Conclusions
- i.
- Even though there is prodigious development in the modified g-C3N4-based composite, the visible-light utilization is still limited to near blue-violet spectrum range, and subsequently, the efficacy of light-harvesting and solar-light employment is not high. However, the g-C3N4/CQDs nanocomposites are only limited to laboratory scale due to photocatalyst dissolution during photoreaction and corrosion of the photocatalyst. Hence, future research should focus more on developing modified g-C3N4 by constructing more stable structural framework and stronger bonds between the components. For instance, in thermal polymerization approach followed through the conversion of monomers into polymeric precursors under a high-temperature environment, forming strong hydrogen bonds amid precursors, resulting into enhanced connection between g-C3N4 and CQDs.
- ii.
- The green fabrication approaches of the g-C3N4/CQDs hybrid composite with excellent photochemical stability, intrinsic electronic structure, efficient photoactivity, and reusability with potential scalability are still in their infancy stage. Thus, further research must be devoted to employing more efficient synthesis processes without affecting any of the above-mentioned parameters.
- iii.
- The charge transport pathway in distinct g-C3N4/CQDs-based nanocomposites, as well as photocatalytic mechanism, need to be explored. Basically, the photo-assisted performance of g-C3N4 was optimized with QDs, which effectively captured the photo-excited electrons in the g-C3N4. In addition, a deep understanding of the synergistic effect between g-C3N4 and CQDs interfaces is still lacking. In light of this, in situ test techniques and theoretical calculations and their experimental results are predictable to help researchers to understand the mystery of the charge migrating route.
- iv.
- Although there are an abundance of reports on g-C3N4/CQDs-based nanocomposites for different types of pollutant degradation, they are still in their infancy, specifically concerning CO2 conversion with some indefinite reduction product challenges, imprecise number of generated products, and inappropriate charge migration route. The future research should focus on CO2 mitigation using this composite and also on innovative experiments and computation techniques to have enough knowledge of the reaction mechanism and migration route from thermodynamic and kinetic view point.
Author Contributions
Funding
Conflicts of Interest
References
- Liras, M.; Barawi, M.; Víctor, A. Hybrid materials based on conjugated polymers and inorganic semiconductors as photocatalysts: From environmental to energy applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini review on the structure and properties (photocatalysis), and preparation techniques of graphitic carbon nitride nano-based particle, and its applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanroman, A. Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Appl. Sci. 2022, 12, 8240. [Google Scholar] [CrossRef]
- Vukšić, M.; Kocijan, M.; Ćurković, L.; Radošević, T.; Vengust, D.; Podlogar, M. Photocatalytic Properties of Immobilised Graphitic Carbon Nitride on the Alumina Substrate. Appl. Sci. 2022, 12, 9704. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Martinez-Treinta, R.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Heterogeneous Electro-Fenton-like Designs for the Disposal of 2-Phenylphenol from Water. Appl. Sci. 2021, 11, 12103. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A. Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review. Appl. Sci. 2021, 11, 12032. [Google Scholar] [CrossRef]
- Shtepa, V.; Balintova, M.; Chernysh, Y.; Chubur, V.; Demcak, S.; Gautier, M. Rationale for the combined use of biological processes and AOPs in wastewater treatment tasks. Appl. Sci. 2021, 11, 7551. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from water by adsorption and advanced oxidation processes: State of the art and trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Oswald, K.A. Photocatalytic applications of heterostructure graphitic carbon nitride: Pollutant degradation, hydrogen gas production (water splitting), and CO2 reduction. Nanoscale Res. Lett. 2019, 14, 234. [Google Scholar] [CrossRef]
- Ding, F.; Yang, D.; Tong, Z.; Nan, Y.; Wang, Y.; Zou, X.; Jiang, Z. Graphitic carbon nitride-based nanocomposites as visible-light driven photocatalysts for environmental purification. Environ. Sci. Nano. 2017, 4, 1455–1469. [Google Scholar] [CrossRef]
- Huang, D.; Yan, X.; Yan, M.; Zeng, G.; Zhou, C.; Wan, J.; Cheng, M.; Xue, W. Graphitic carbon nitride-based heterojunction photoactive nanocomposites: Applications and mechanism insight. ACS Appl. Mater. Interfaces 2018, 10, 21035–21055. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Wu, Y. A mini-review on the synthesis and structural modification of gC 3 N 4-based materials, and their applications in solar energy conversion and environmental remediation. Sustain. Energy Fuels. 2019, 3, 2907–2925. [Google Scholar] [CrossRef]
- Akira, F.; Kenichi, H. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Krakowiak, R.; Frankowski, R.; Mylkie, K.; Mlynarczyk, D.T.; Ziegler-Borowska, M.; Zgoła-Grześkowiak, A.; Goslinski, T. TiO2–Fe3O4 Composite Systems—Preparation, Physicochemical Characterization, and an Attempt to Explain the Limitations That Arise in Catalytic Applications. Appl. Sci. 2022, 12, 8826. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Khan, M.; Luque, R.; Jalalah, M.; Alsaiari, M.A. Microwave Assisted Preparation of Barium Doped Titania (Ba/TiO2) as Photoanode in Dye Sensitized Solar Cells. Appl. Sci. 2022, 12, 9280. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Patial, S.; Hasija, V.; Raizada, P.; Singh, P.; Singh, A.A.P.K.; Asiri, A.M. Tunable photocatalytic activity of SrTiO3 for water splitting: Strategies and future scenario. J. Environ. Chem. Eng. 2020, 8, 103791. [Google Scholar] [CrossRef]
- Alhokbany, N.; Ahamad, T.; Alshehri, S.M. Fabrication of highly porous ZnO/Ag2O nanoparticles embedded in N-doped graphitic carbon for photocatalytic degradation of tetracycline. J. Environ. Chem. Eng. 2022, 10, 107681. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Q.; Tong, Y.; Wang, J. Light-induced ambient degradation of few-layer black phosphorus: Mechanism and protection. Angew. Chem. Int. Ed. 2016, 55, 11437–11441. [Google Scholar] [CrossRef] [PubMed]
- Pare, B.; Jonnalagadda, S.; Tomar, H.; Singh, P.; Bhagwat, V.J.D. ZnO assisted photocatalytic degradation of acridine orange in aqueous solution using visible irradiation. Desalination 2008, 232, 80–90. [Google Scholar] [CrossRef]
- Patial, S.; Kumar, R.; Raizada, P.; Singh, P.; Van Le, Q.; Lichtfouse, E.; Nguyen, D.L.T.; Nguyen, V.-H. Boosting light-driven CO2 reduction into solar fuels: Mainstream avenues for engineering ZnO-based photocatalysts. Environ. Res. 2021, 197, 111134. [Google Scholar] [CrossRef] [PubMed]
- Chankhanittha, T.; Somaudon, V.; Watcharakitti, J.; Piyavarakorn, V.; Nanan, S. Performance of solvothermally grown Bi2MoO6 photocatalyst toward degradation of organic azo dyes and fluoroquinolone antibiotics. Mater. Lett. 2020, 258, 126764. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation-A review. J. Environ. Manage. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.P.; Aust, S.D. Pollutant degradation by white rot fungi. Rev. Environ. Contam. Toxicol. 1994, 49–72. [Google Scholar]
- Shen, R.; Ren, D.; Ding, Y.; Guan, Y.; Ng, Y.H.; Zhang, P.; Li, X. Nanostructured CdS for efficient photocatalytic H2 evolution: A review. Sci. China Mater. 2020, 63, 2153–2188. [Google Scholar] [CrossRef]
- Sahara, G.; Ishitani, O. Efficient photocatalysts for CO2 reduction. Inorg. Chem. 2015, 54, 5096–5104. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Li, L.; Sun, Y.; Xie, Y. Progress and perspective for in situ studies of CO2 reduction. J. Am. Chem. Soc. 2020, 142, 9567–9581. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Alarcón, D.C.; Maldonado, M.I.; Fernandez-Ibanez, P.; Gernjak, W. Photocatalytic decontamination and disinfection of water with solar collectors. Catal. Today 2007, 122, 137–149. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Yeong Wu, T.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Hernández-Ramírez, A.; Medina-Ramírez, I. Photocatalytic Semiconductors; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Duan, Y.; Zhou, S.; Deng, L.; Shi, Z.; Jiang, H.; Zhou, S. Enhanced photocatalytic degradation of sulfadiazine via g-C3N4/carbon dots nanosheets under nanoconfinement: Synthesis, Biocompatibility and Mechanism. J. Environ. Chem. Eng. 2020, 8, 104612. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.; Rohit, G.; Holkar, C.; Pinjari, D.; Bhanvase, B.; Pandit, A. Investigation of TiO2 photocatalyst performance for decolorization in the presence of hydrodynamic cavitation as hybrid AOP. Ultrason. Sonochem. 2016, 28, 150–160. [Google Scholar] [CrossRef]
- Kim, S.-J.; Gwak, G.-d.; Won, C.-H. The study on the phenol removal characteristics by using AOP processes. J. Korean Soc. Mar. Environ. 2010, 26, 303–310. [Google Scholar]
- Lincho, J.; Gomes, J.; Martins, R.C. Paraben Compounds—Part II: An Overview of Advanced Oxidation Processes for Their Degradation. Appl. Sci. 2021, 11, 3556. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Torretta, V. Innovative Approaches for Drinking-and Waste-Water Treatment: An Editorial Review Summarizing and Assessing the Findings of the Special Issue. Appl. Sci. 2021, 11, 2063. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Shen, T.; Wang, Q.; Wang, X.; Xu, P.; Zheng, Q.; Zhang, G. The application of microwaves in sulfate radical-based advanced oxidation processes for environmental remediation: A review. Sci. Total Environ. 2020, 722, 137831. [Google Scholar] [CrossRef]
- Sohail, M.; Anwar, U.; Taha, T.; Qazi, H.; Al-Sehemi, A.G.; Ullah, S.; Gharni, H.; Ahmed, I.; Amin, M.A.; Palamanit, A. Nanostructured materials based on g-C3N4 for enhanced photocatalytic activity and potentials application: A review. Arab. J. Chem. 2022, 15, 104070. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Yuvakkumar, R.; Velauthapillai, D.; Ahamad, T.; Khan, M.M.; Mohammed, M.K.; Vijayaprabhu, N.; Ravi, G. The bifunctional performance analysis of synthesized Ce doped SnO2/g-C3N4 composites for asymmetric supercapacitor and visible light photocatalytic applications. J. Alloy. Compd. 2021, 866, 158807. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, X.; Liu, J.; Zhang, Q.; Ji, X.; Tian, G. Mesoporous magnetic g-C3N4 nanocomposites for photocatalytic environmental remediation under visible light. Ecotoxicol. Environ. Saf. 2020, 205, 111147. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Guan, G.; Ye, E.; You, M.; Li, Z. Hybridized 2D nanomaterials toward highly efficient photocatalysis for degrading pollutants: Current status and future perspectives. Small 2020, 16, 1907087. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef]
- Noor, S.; Haider, R.S.; Noor, S.; Sajjad, S.; Leghari, S.A.K.; Mehboob, M.; Long, M. Role of conductive channels via CQDs on NiO/g-C3N4 Z-scheme composite as a bi-functional photocatalyst. Int. J. Hydrog. Energy. 2022, 47, 36517–36529. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Lu, J.; Guo, Z.; Zhang, M.; Li, D.; Zhao, Z.; Song, S.; Liu, Y.; Qin, L. A CQD/CdS/g-C3N4 photocatalyst for dye and antibiotic degradation: Dual carrier driving force and tunable electron transfer pathway. Sep. Purif. Technol. 2022, 122333. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Vinesh, V.; Sabarikirishwaran, P.; Rajkamal, A.; Ashokkumar, M.; Neppolian, B. Investigating the role of ultrasound in improving the photocatalytic ability of CQD decorated boron-doped g-C3N4 for tetracycline degradation and first-principles study of nitrogen-vacancy formation. Carbon 2022, 192, 405–417. [Google Scholar] [CrossRef]
- Smith, A.M.; Lim, S.J. Brightness-equalized quantum dots: Engineering strategies derived from spectral trends. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications X, San Francisco, CA, USA, 7–12 February 2015; p. 933810. [Google Scholar]
- Li, H.; Gao, X.; Niu, X.; Zhang, D.; Fan, H.; Wang, K. Preparation of g-C3N4/CQDs/Ag2S Composite Material and Its Antibacterial Properties. J. Biomater. Tissue Eng. 2022, 12, 1683–1691. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Moharramnezhad, M. A novel cathodic electrochemiluminescent sensor based on CuS/carbon quantum dots/g-C3N4 nanosheets and boron nitride quantum dots for the sensitive detection of organophosphate pesticide. Microchem. J. 2022, 179, 107421. [Google Scholar] [CrossRef]
- Wong, K.T.; Jang, S.B.; Saravanan, P.; Nah, I.W.; Park, S.; Choi, J.; Park, C.; Kim, Y.; Yoon, Y.; Jang, M.J.A.S.S. Critical insight on the hydrothermal effects toward exfoliation of g-C3N4 and simultaneous in-situ deposition of carbon quantum dots. Appl. Surf. Sci. 2019, 471, 703–713. [Google Scholar] [CrossRef]
- Lewis, N.S.J.S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef] [Green Version]
- Medintz, I.L.; Stewart, M.H.; Trammell, S.A.; Susumu, K.; Delehanty, J.B.; Mei, B.C.; Melinger, J.S.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H.J.N.m. Quantum-dot/dopamine bioconjugates function as redox coupled assemblies for in vitro and intracellular pH sensing. Nat. Mater. 2010, 9, 676–684. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, L.; Su, R.; Hu, B.; Wang, Z.; Jin, Y.; Wang, Y.; Besenbacher, F.; Dong, M. Nanostructured heterogeneous photo-catalysts for hydrogen production and water splitting: A comprehensive insight. Appl. Mater. Today. 2019, 17, 159–182. [Google Scholar] [CrossRef]
- Qiu, L.F.; Zhou, Z.W.; Qiu, X.B.; Duo, S.W. Synthesis and photocatalytic degradation performance of g-C3N4/CQDs/SAPO-5 ternary composite. Key Eng. Mater. 2018, 768, 201–205. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B. 2016, 185, 225–232. [Google Scholar] [CrossRef]
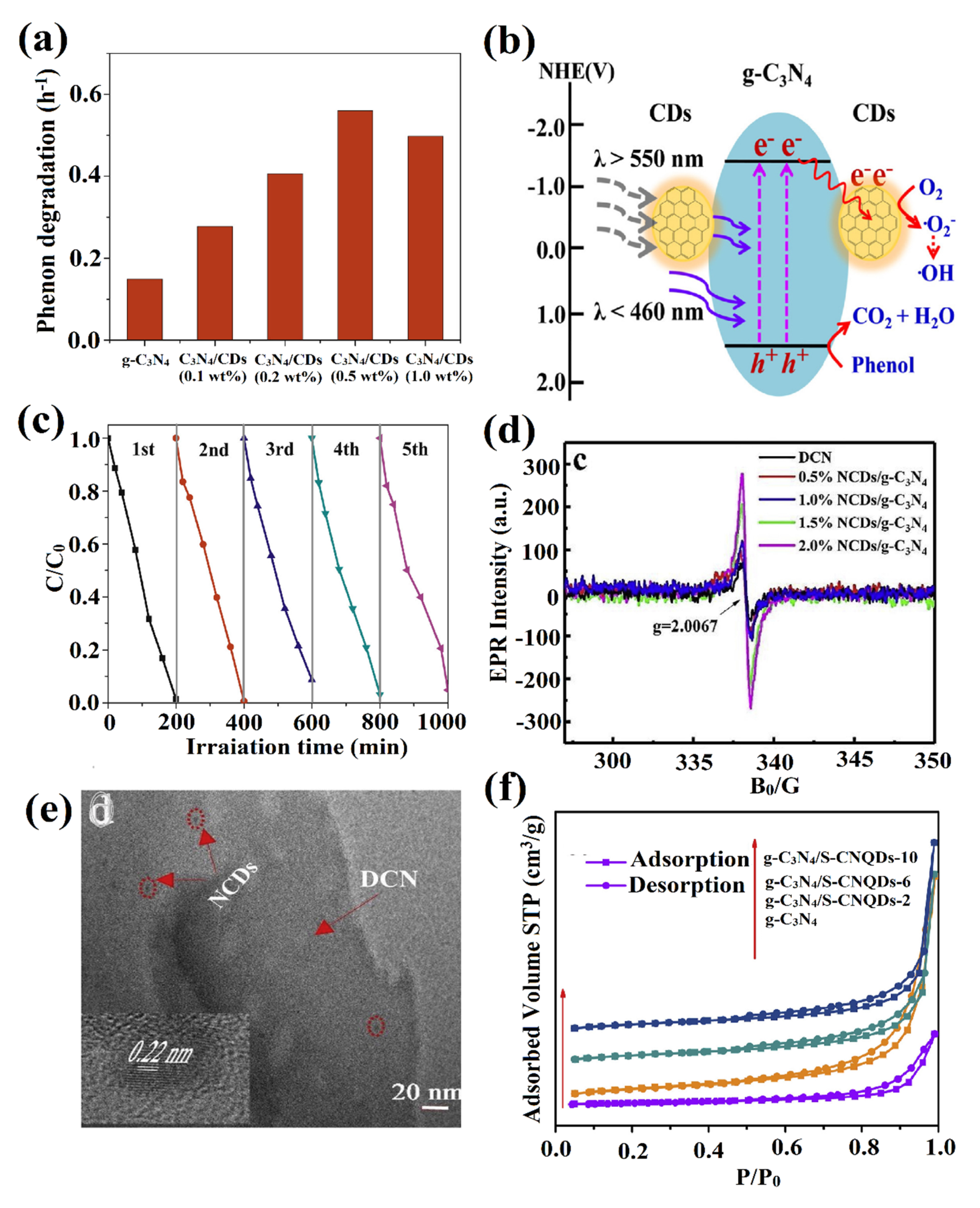
- Zhang, H.; Zhao, L.; Geng, F.; Guo, L.-H.; Wan, B.; Yang, Y. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B. 2016, 180, 656–662. [Google Scholar] [CrossRef]
- Tai, J.Y.; Leong, K.H.; Saravanan, P.; Sim, L.C. Bioinspired Synthesis of Carbon Dots/g-C3N4 Nanocomposites for Photocatalytic Application. In Proceedings of the E3S Web of Conferences, International Conference on Civil and Environmental Engineering (ICCEE), Kuala Lumpur, Malaysia, 2–5 October 2018; p. 05015. [Google Scholar]
- Liu, H.; Liang, J.; Fu, S.; Li, L.; Cui, J.; Gao, P.; Zhao, F.; Zhou, J. N doped carbon quantum dots modified defect-rich g-C3N4 for enhanced photocatalytic combined pollutions degradation and hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124552. [Google Scholar] [CrossRef]
- Li, H.; Huang, G.; Xu, H.; Yang, Z.; Xu, X.; Li, J.; Qu, A.; Chen, Y. Enhancing photodegradation activity of g-C3N4 via decorating with S-doped carbon nitride quantum dots by in situ polymerization. J. Solid State Chem. 2020, 292, 121705. [Google Scholar] [CrossRef]
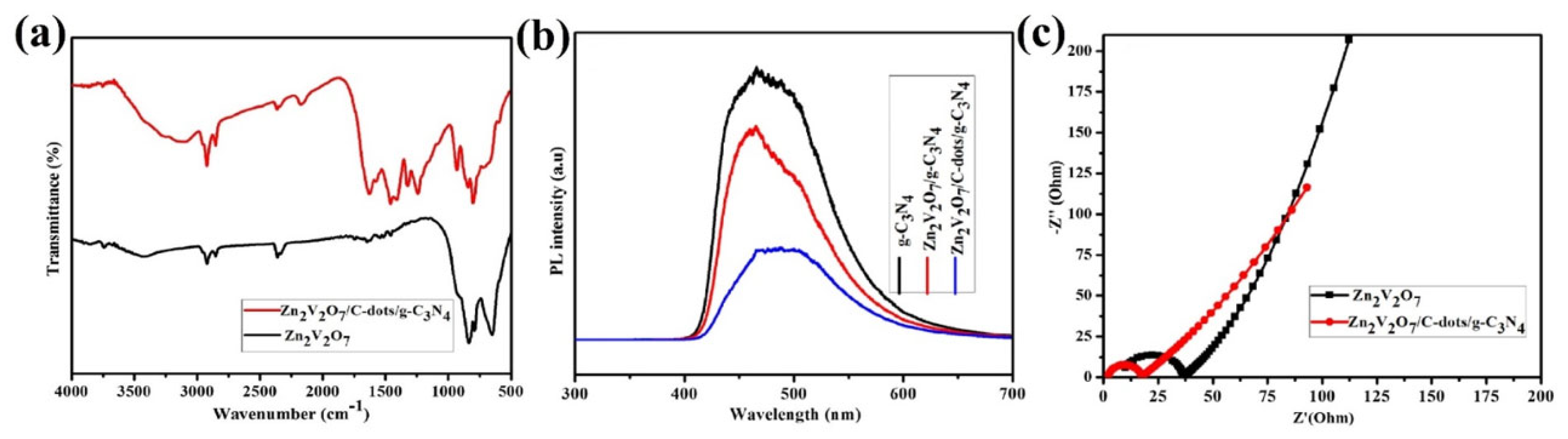
- Wu, P.; Feng, L.; Liu, J.; Zhang, X.; Tian, S.; Hu, H.; Xu, C.; Yin, G. Enhanced photocatalytic activity of Jamun-like Zn2V2O7/C-dots/g-C3N4 nanocomposites for Rhodamine B degradation under the visible light radiation. J. Mater. Sci. Mater. 2020, 31, 1879–1890. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, S.; Zhang, L.; Yang, R. Ultrasound-assisted synthesis of BiVO4/C-dots/gC3N4 Z-scheme heterojunction photocatalysts for degradation of minocycline hydrochloride and Rhodamine B: Optimization and mechanism investigation. New J. Chem. 2020, 44, 17641–17653. [Google Scholar] [CrossRef]
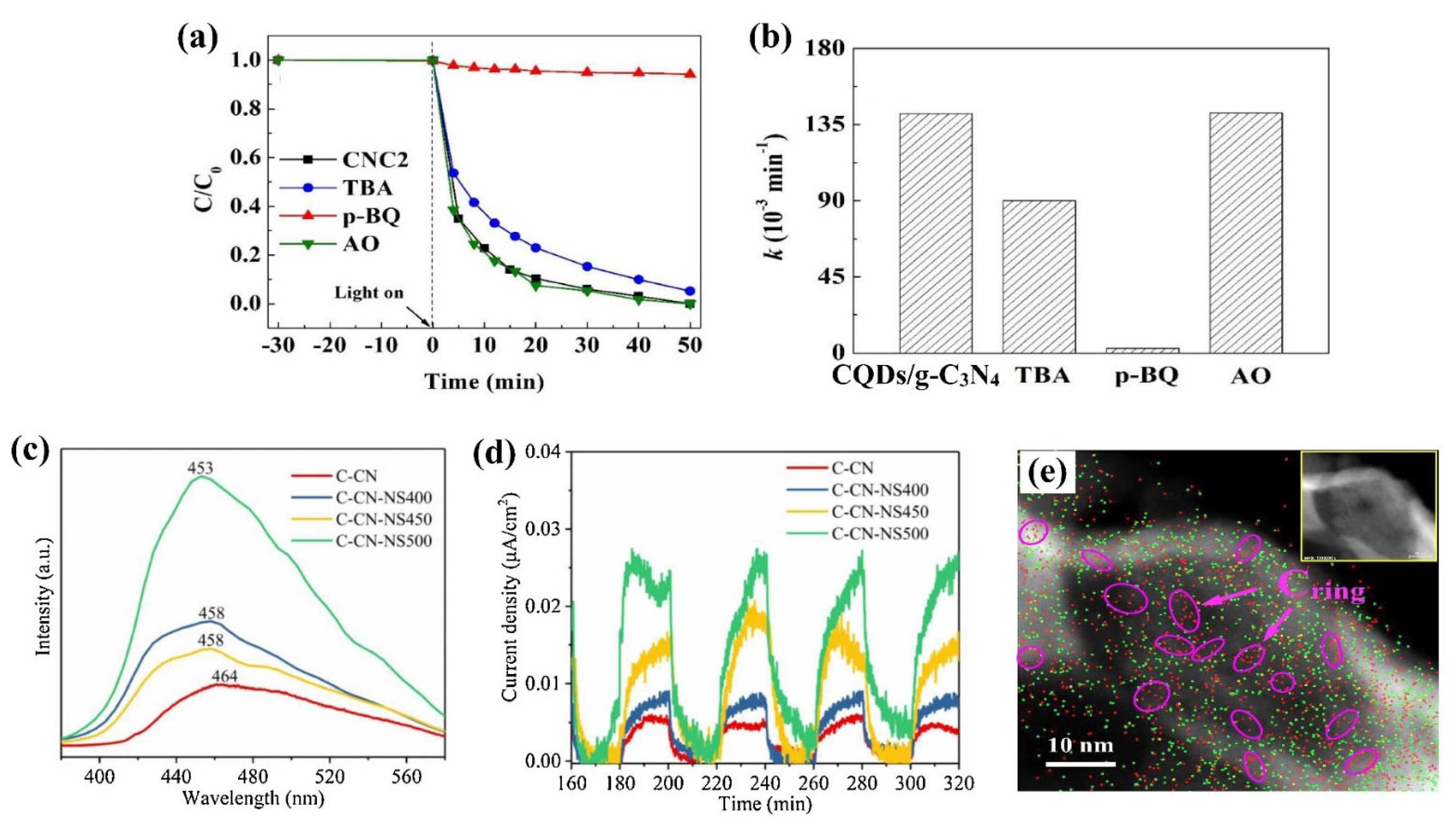
- Xu, S.; Zhao, Y.; Sun, X.; Zheng, X.; Ni, S.; Yue, Q.; Gao, B. Introduction of porous structure via facile carbon-dot modulation: A feasible and promising approach for improving the photocatalytic capability of sulfur doped g-C3N4. Catal. Today 2019, 335, 502–510. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef]
- Duan, Y.; Deng, L.; Shi, Z.; Liu, X.; Zeng, H.; Zhang, H.; Crittenden, J. Efficient sulfadiazine degradation via in-situ epitaxial grow of Graphitic Carbon Nitride (g-C3N4) on carbon dots heterostructures under visible light irradiation: Synthesis, mechanisms and toxicity evaluation. J. Colloid Interface Sci. 2020, 561, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liao, Z.; Liu, W.; Liu, F.; Ye, J.; Liang, J.; Li, Y. Carbon quantum dots modified tubular g-C3N4 with enhanced photocatalytic activity for carbamazepine elimination: Mechanisms, degradation pathway and DFT calculation. J. Hazard. Mater. 2020, 381, 120957. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yang, S.; Sun, H.; Wang, J.; Lin, X.; Guo, F.; Shi, J. Carbon dots anchored high-crystalline g-C3N4 as a metal-free composite photocatalyst for boosted photocatalytic degradation of tetracycline under visible light. J. Mater. Sci. 2021, 56, 2226–2240. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, Z.; Long, R.; Wang, Y.; Yu, Y.; Pei, Y. Design of a Z-scheme g-C3N4/CQDs/CdIn2S4 composite for efficient visible-light-driven photocatalytic degradation of ibuprofen. Environ. Pollut. 2020, 259, 113770. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumari, A.; Sharma, G.; Du, B.; Naushad, M.; Stadler, F.J. Carbon quantum dots and reduced graphene oxide modified self-assembled S@ C3N4/B@ C3N4 metal-free nano-photocatalyst for high performance degradation of chloramphenicol. J. Mol. Liq. 2020, 300, 112356. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Amo-Duodu, G.; Rathilal, S. Exploring CO2 Bio-Mitigation via a Biophotocatalytic/Biomagnetic System for Wastewater Treatment and Biogas Production. Appl. Sci. 2022, 12, 6840. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.; Kadhum, A.A.H.; Hasan, H.A.; Hasan, M.R. Advances in photocatalytic CO2 reduction with water: A review. Materials 2017, 10, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today. 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Son, H.-J.; Pac, C.; Kang, S.O. Inorganometallic Photocatalyst for CO2 Reduction. Acc. Chem. Res. 2021, 54, 4530–4544. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, X.; Chen, S.; Wang, H.; Huo, P. g-C3N4 quantum dots-modified mesoporous CeO2 composite photocatalyst for enhanced CO2 photoreduction. J. Mater. Sci. Mater. 2020, 31, 20495–20512. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.K.; Sharma, G.; Naushad, M.; Stadler, F.J. CeO2/g-C3N4/V2O5 ternary nano hetero-structures decorated with CQDs for enhanced photo-reduction capabilities under different light sources: Dual Z-scheme mechanism. J. Alloy. Compd. 2020, 838, 155692. [Google Scholar] [CrossRef]


| Type of Composite | Target Pollutant | Source of Illumination Light | Degradation Efficiency | Ref. |
|---|---|---|---|---|
| Carbon-dots/g-C3N4 | Rhodamine B (RhB) dye | 3 W LED lamp (365 ± 5 nm) | RhB degradation: 0.013 mol L−1 min−1 within 60 min | [60] |
| g-C3N4/Carbon dots (0.5 wt%) | Phenol | 300 W Xe lamp (λ < 400 nm) | Phenol degradation: 100% within 200 min | [61] |
| Carbon-dots/g-C3N4 | 2,4-dichlorophenol (2,4-DCP) | Solar light | 2,4-DCP degradation: 71.53% within 2 h | [62] |
| N-doped Carbon-dots/defect-rich g-C3N4 | RhB dye | 300 W Xe lamp (λ > 420 nm) | RhB degradation: 100% within 4 h | [63] |
| g-C3N4/S-doped carbon nitride quantum dots (S-CNQDs) | Methyl Orange (MO) | 300 W Xe lamp (λ > 350 nm) | MO degradation: 100% within 2 h | [64] |
| Zn2V2O7/Carbon dots/g-C3N4 | RhB dye | 300 W Xe lamp (λ > 420 nm) | RhB degradation: 97% within 30 min | [65] |
| BiVO4/Carbon dots/g-C3N4 | Minocycline hydrochloride (Mn-HCL) and RhB dye | 350 W LED lamp (460 nm) | Mn-HCL degradation: 65.3 within 140 min RhB degradation: 85% within 30 min | [66] |
| Carbon quantum dots/g-C3N4 | Diclofenac (DCF) | 300 W Xe lamp (λ > 420 nm) | Diclofenac degradation: 100% within 60 min | [68] |
| g-C3N4/Carbon-dots nanosheet | Sulfadiazine (SDZ) | 500 W Xe lamp (λ < 420 nm) | SDZ degradation: 97.82% within 60 min | [34] |
| Carbon-dots/g-C3N4 | SDZ | Visible-light | SDZ degradation: 92.8% within 80 min | [69] |
| Carbon quantum dots/g-C3N4 | Carbamazepine (CBZ) | UV-light with cutoff filter (λ < 400 nm) | CBZ degraded with first-order rate constant of 5.73 × 10−2 min−1 | [70] |
| High-crystalline g-C3N4/Carbon-dots | Tetracycline (TC) | 300 W Xe lamp (λ > 420 nm) | TC degradation: 86% within 120 min | [71] |
| Acidified g-C3N4/Carbon quantum dots-3 wt%/CdIn2S4 | Ibuprofen (IBU) | 300 W Xe lamp (λ > 420 nm) | IBU degradation: 91.0% within 60 min | [72] |
| S@g-C3N4/B@B-g-C3N4 | Chloramphenicol (CMP) | 300 W Xe lamp with cutoff filters (380–780 nm) | CMP degradation: 99.1% within 1 h | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patial, S.; Sonu; Sudhaik, A.; Chandel, N.; Ahamad, T.; Raizada, P.; Singh, P.; Chaukura, N.; Selvasembian, R. A Review on Carbon Quantum Dots Modified g-C3N4-Based Photocatalysts and Potential Application in Wastewater Treatment. Appl. Sci. 2022, 12, 11286. https://doi.org/10.3390/app122111286
Patial S, Sonu, Sudhaik A, Chandel N, Ahamad T, Raizada P, Singh P, Chaukura N, Selvasembian R. A Review on Carbon Quantum Dots Modified g-C3N4-Based Photocatalysts and Potential Application in Wastewater Treatment. Applied Sciences. 2022; 12(21):11286. https://doi.org/10.3390/app122111286
Chicago/Turabian StylePatial, Shilpa, Sonu, Anita Sudhaik, Naresh Chandel, Tansir Ahamad, Pankaj Raizada, Pardeep Singh, Nhamo Chaukura, and Rangabhashiyam Selvasembian. 2022. "A Review on Carbon Quantum Dots Modified g-C3N4-Based Photocatalysts and Potential Application in Wastewater Treatment" Applied Sciences 12, no. 21: 11286. https://doi.org/10.3390/app122111286






