Study on the Removal of Iron and Manganese from Groundwater Using Modified Manganese Sand Based on Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Material and Equipment
2.2. Preparation of High-Efficiency Manganese Sand and Determination of Removal Rate
2.3. Characterization of Properties
2.4. Single-Factor Experimental Design
2.5. Response Surface Experimental Design
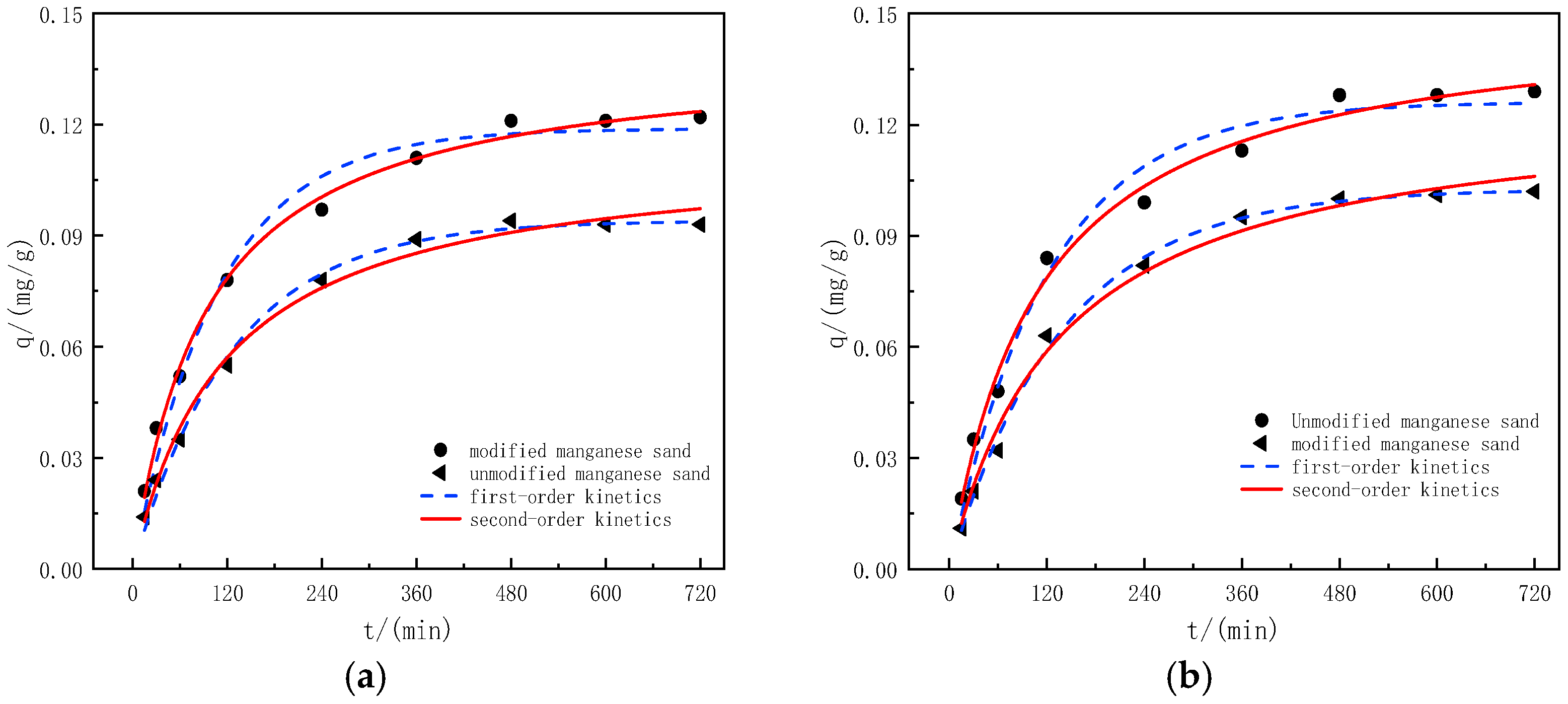
2.6. Adsorption Kinetic Experiment
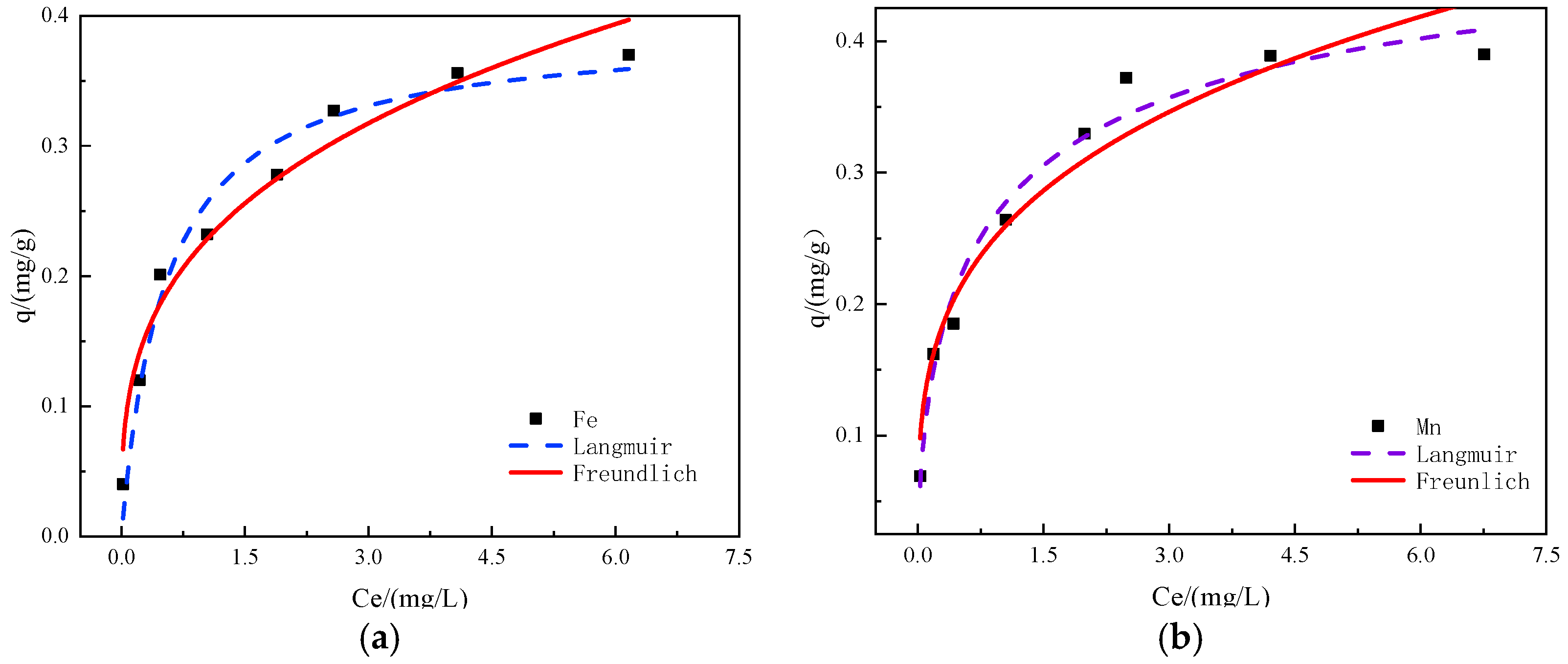
2.7. Adsorption Isotherm Experiment
3. Results and Discussion
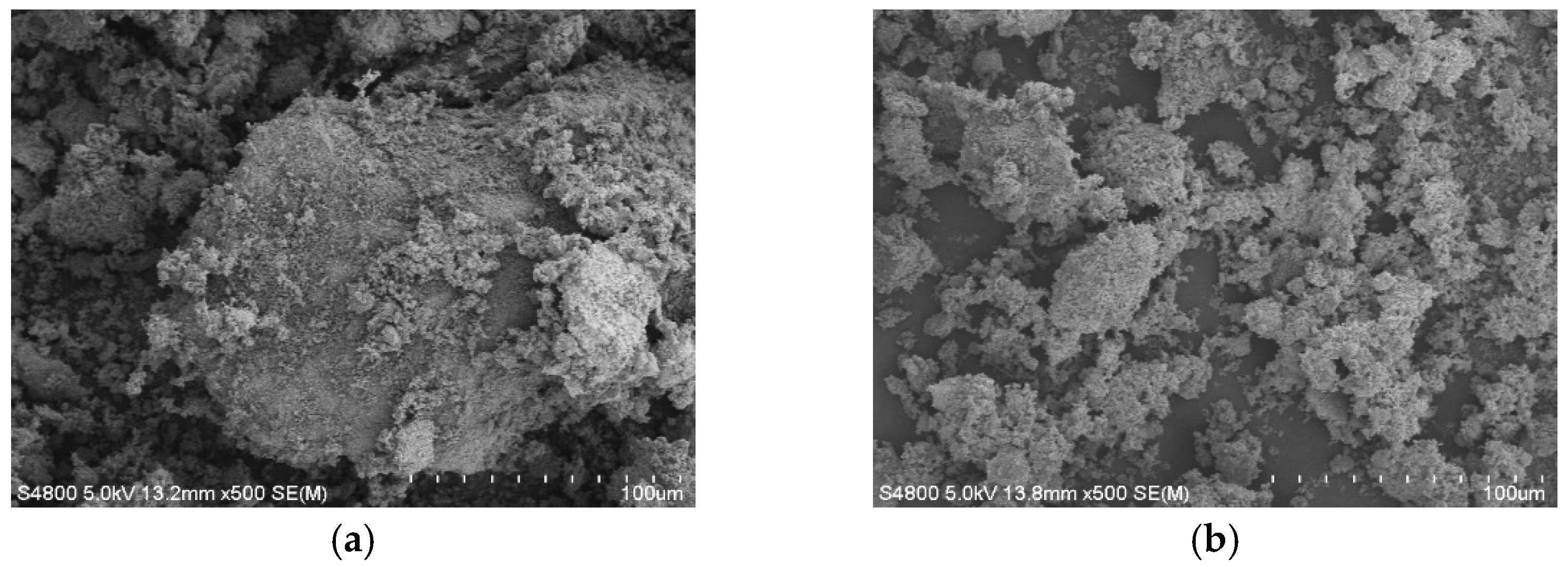
3.1. SEM Results of Manganese Sand
3.2. Surface Area and Porosity Analysis
3.3. Single-Factor Experimental Results and Analysis
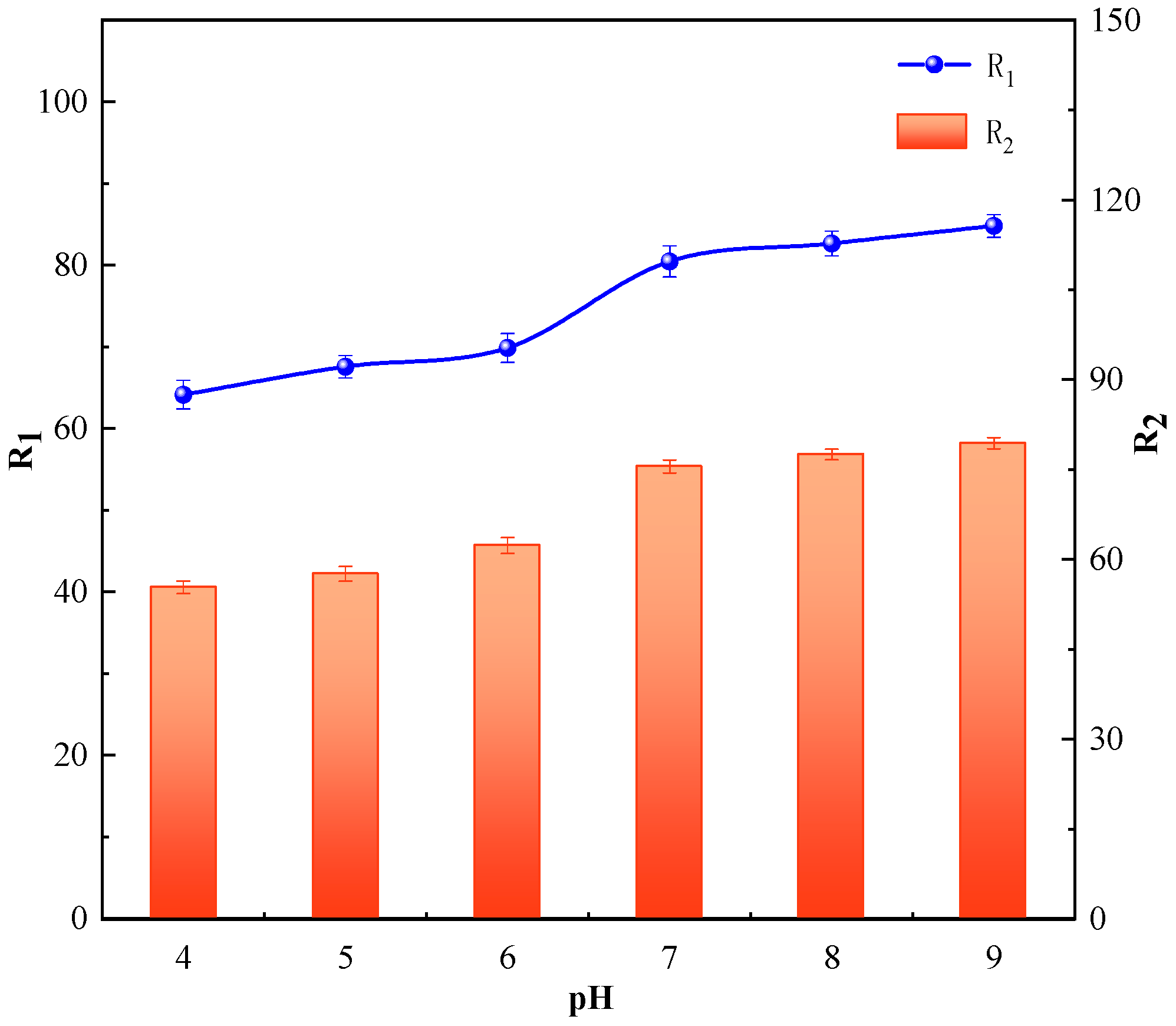
3.3.1. Effect of pH on the Iron and Manganese Ion Removal
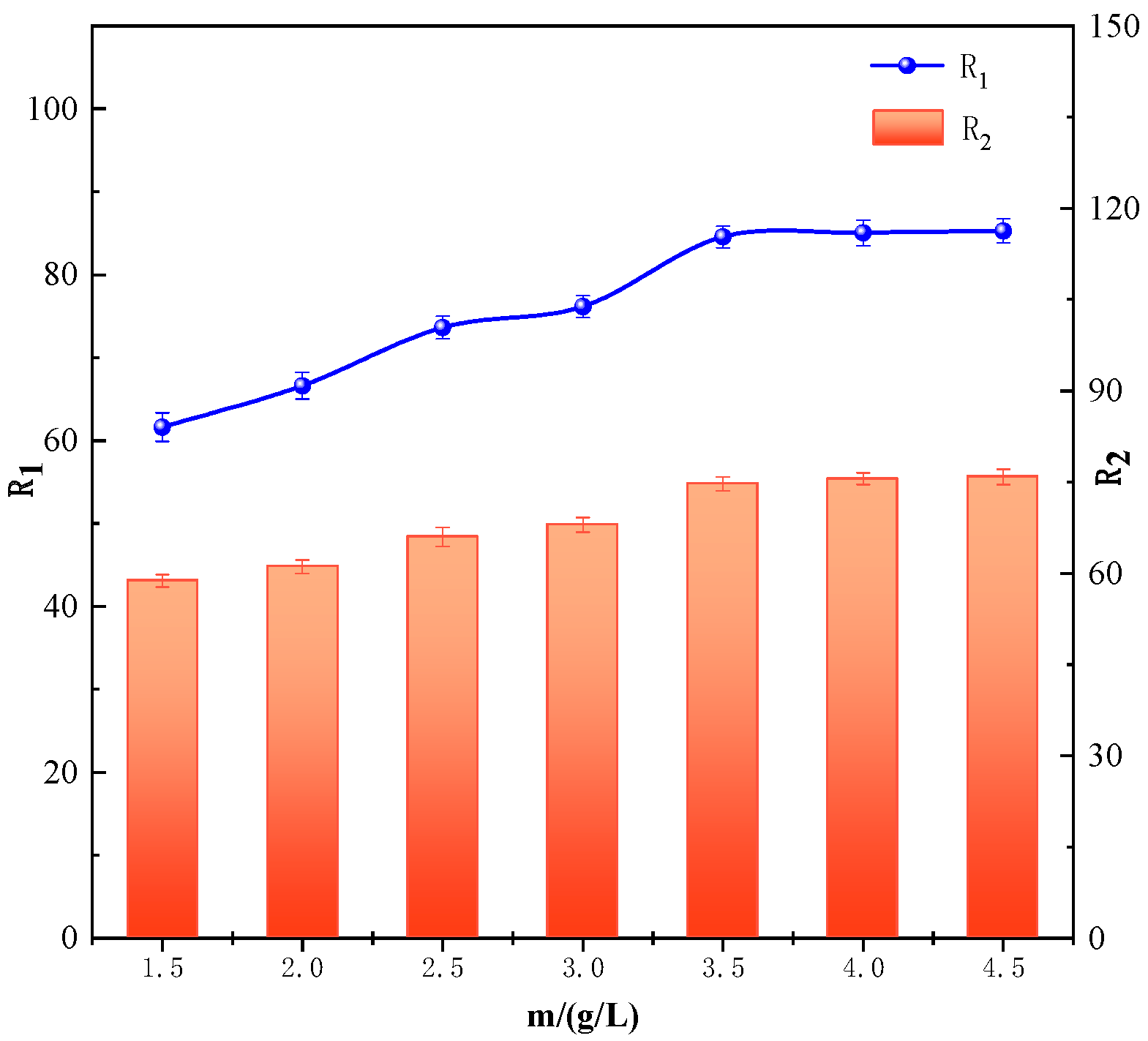
3.3.2. Effect of Manganese Sand Dosage on the Iron and Manganese Ion Removal
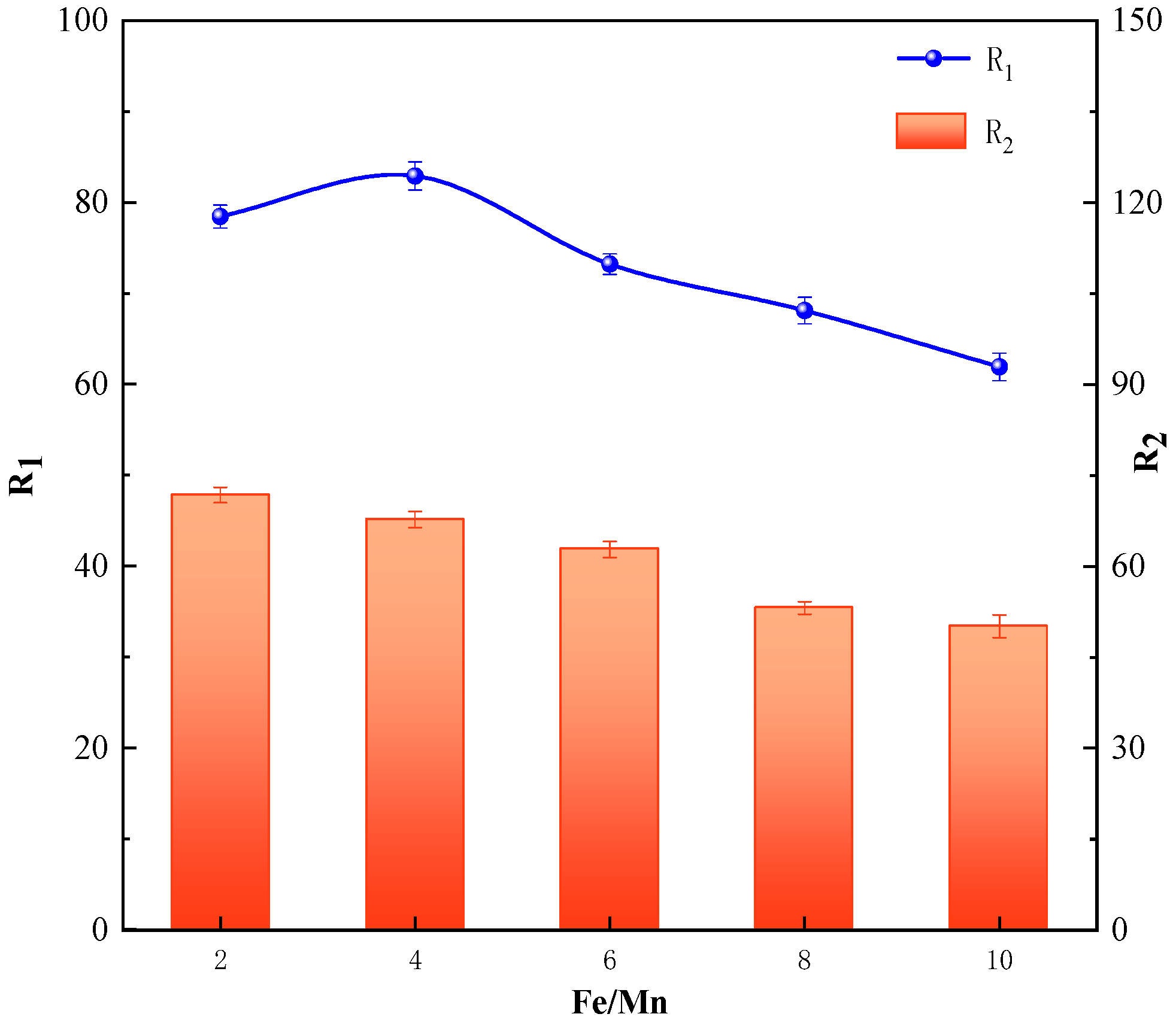
3.3.3. Effect of Initial Concentration of Fe/Mn on the Iron and Manganese Ion Removal
3.4. Analysis of Response Surface Experimental Results
Experimental Results
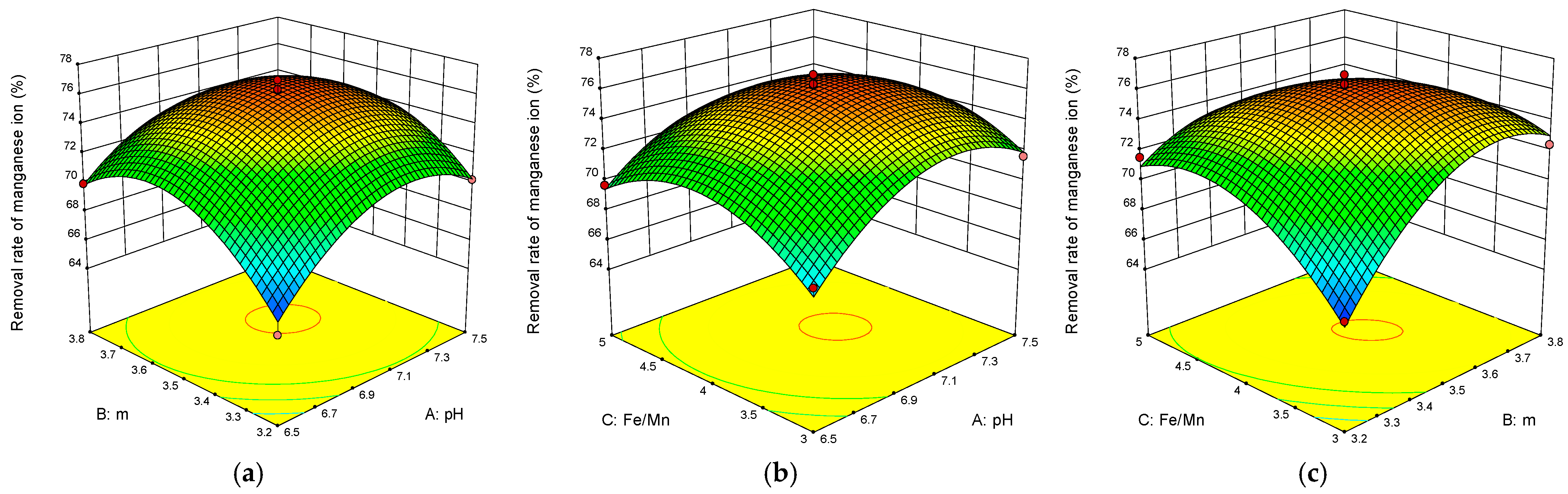
3.5. Analysis of Interaction between Factors
3.5.1. Interaction of Three Factors in Iron Ion Removal
3.5.2. Interaction of the Three Factors in Manganese Removal
3.6. Validation Experiment
3.7. Analysis of Adsorption Kinetic Model
3.8. Analysis of Adsorption Isotherm Model
4. Conclusions
- (i)
- The Box–Behnken experimental design results showed that the interaction between the pH value and the dosage was the most obvious for iron removal. The interaction between the dosage and the initial concentration ratio of iron to manganese was the most obvious for manganese removal;
- (ii)
- The results of response surface optimization showed that when pH was 7.20, the amount of adsorbent was 3.54 g/L, and when the initial concentration ratio of iron and manganese ions was 3.80, the adsorption rates of iron and manganese ions by manganese sand was higher, reaching 83.62% and 76.10%;
- (iii)
- The adsorption of iron and manganese ions by manganese sand followed the Langmuir isotherm adsorption model and the quasi-second-order kinetic model. The modified manganese sand had a remarkable adsorption effect on iron and manganese ions and thus can be used as a good material for a new type of ion adsorbent.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Zhou, L.; Li, G.; Zhang, F.; Li, T. Distribution and genetic analysis of iron and manganese microbial community in soil and groundwater of mining area. Environ. Chem. 2021, 40, 1464–1479. [Google Scholar]
- Zhang, W.; Zhu, J. Iron and Manganese Pollution in Groundwater and Its Treatment Methods. Guangdong Chem. Ind. 2018, 45, 163–164. [Google Scholar]
- Yu, D.; Zhou, J.; Chen, J. Spatial distribution characteristics and genesis of groundwater with high iron and manganese content in Kashi Prefecture, Xinjiang. Environ. Chem. 2020, 39, 3235–3245. [Google Scholar]
- Adeyeye, O.; Xiao, C.; Zhang, Z.; Liang, X. State, source and triggering mechanism of iron and manganese pollution in groundwater of Changchun, Northeastern China. Environ. Monit. Assess 2020, 192, 619. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, Y.; Yu, C.; Pan, Z. Treatment of groundwater with high iron and manganese by modified zeolite. Chin. J. Environ. Eng. 2013, 7, 2203–2207. [Google Scholar]
- Fleming, R.E.; PONK, A.P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Santra, S.; Agrawal, D.; Kumar, S.; Mishra, S.S. Incidence and prevalence of chronic iron poisoning and it’s management: A review. Int. J. Pharma Bio Sci. 2014, 5, 722–737. [Google Scholar]
- Ferreira, D.C.; Graziele, I.; Marques, R.C.; Gonçalves, J. Investment in drinking water and sanitation infrastructure and its impact on waterborne diseases dissemination: The Brazilian case. Sci. Total Environ. 2021, 779, 146–279. [Google Scholar] [CrossRef]
- Ghazi, M.M.; Qomi, M.H. Removal of manganese from an aqueous solution using micellar-enhanced ultrafiltration (MEUF) with SDS surfactants. Adv. Environ. Technol. 2015, 1, 17–23. [Google Scholar]
- Bright, K.A.; Baah, S.N.; Elizabeth, V.K.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. [Google Scholar]
- Bruins, J.H. Manganese Removal from Groundwater: Role of Biological and Physico-Chemical Autocatalytic Processes; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Li, G.; Liang, H.; Yu, H.; Du, X.; Yang, H. Research on manganese removal by chemical auto-catalytic oxidation mechanism involved in active manganese oxides film. Water Wastewater Eng. 2019, 45, 1–5. [Google Scholar]
- Diaz-Alarcón, J.A.; Alfonso-Pérez, M.P.; Vergara-Gómez, I.; Díaz-Lagos, M.; Martínez-Ovalle, S.A. Removal of iron and manganese in groundwater through magnetotacticbacteria. J. Environ. Manag. 2019, 249, 109381. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, M.A.; Guirguis, H.S.; Fathy, A. Removal of Iron and Manganese from Groundwater: A Study of Using Potassium Permanganate and Sedimentation. Mansoura Eng. J. 2017, 42, 7–12. [Google Scholar]
- Marsidi, N.; Hasan, H.A.; Abdullah, S.R.S. A review of biological aerated filters for iron and manganese ions removal in water treatment. J. Water Process Eng. 2018, 23, 1–12. [Google Scholar] [CrossRef]
- Shrestha, S.; Dhami, A.K.; Nyachhyon, A.R. Adsorptive Removal of Fe (II) By NaOH Treated Rice Husk: Adsorption Equilibrium And Kinetics. Sci. World 2021, 14, 75–82. [Google Scholar] [CrossRef]
- Arafat, M.; Marzouk, S.Y.; El Monayeri, O.D. Hybrid system for iron and manganese reduction from polluted water using adsorption and filtration. Ain Shams Eng. J. 2021, 12, 2465–2470. [Google Scholar] [CrossRef]
- Li, X.K.; Chu, Z.R.; Liu, Y.J.; Zhu, M.T.; Yang, L.; Zhang, J. Molecular characterization of microbial populations in full-scale biofilters treating iron, manganese and ammonia containing groundwater in Harbin. Bioresour. Technol. 2013, 147, 234–239. [Google Scholar] [CrossRef]
- Guo, F.; Li, X.; Yang, Y. Effect of Filter Material Characteristics on Iron and Manganese Removal Efficiency during Start-up Period of Manganese Sand Filter. China Water 2018, 34, 16–25. [Google Scholar]
- Ye, M.X.; Pan, J.; Chen, P. Study on optimum operation parameters of modified manganese sand filter for treatment of high iron and manganese water containing ammonia nitrogen. Jiangsu Water Resour. 2018, 9, 11–15. [Google Scholar]
- Liu, J.; Zhang, H.L.; You, K.; Yuan, Y.S. Different Filter Material on the Northeast Biological Iron Manganese Removal Effect of Groundwater in the Countryside. Appl. Mech. Mater. 2014, 522, 465–468. [Google Scholar] [CrossRef]
- Eri, I.R.; Hadi, W.; Slamet, A. Clarification of pharmaceutical wastewater with Moringa oleifera: Optimization through response surface methodology. J. Ecol. Eng. 2018, 19, 126–134. [Google Scholar] [CrossRef]
- Dong, X.; Jin, B.; Sun, Y.; Yu, L. Urban gas production from low H2/CO biogas using Re-promoted Ni catalysts supported on modified manganese sand. Fuel 2018, 220, 60–71. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Optimisation of orthophosphate and turbidity removal using an amphoteric chitosan-based flocculant-ferric chloride coagulant system. Environ. Chem. 2019, 16, 599–612. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lv, W.Y.; Zou, X.G.; Shu, R.J.; Huang, J.L.; Yao, K.; Liu, G.G. The study of adsorption mechanism of Cu(Ⅱ) from aqueous solutions by humin with response surface methodology. Acta Sci. Circumstantiae 2017, 37, 624–632. [Google Scholar]
- Chen, T.Y.; Chen, Z.H.; Jin, S.F.; Li, H.S.; Li, G.B.; Liang, H. Effect of pH Value on Treatment of Groundwater Containing High Concentrations of Iron, Manganese and Ammonia Nitrogen. China Water 2015, 31, 1–9. [Google Scholar]
- Wang, Y.Y.; Li, J.Y.; Lu, Y.W. Optimization of Cu2+ adsorption on corncob by response surface methodology. J. Shanghai Ocean Univ. 2020, 29, 355–363. [Google Scholar]
- Agarwal, M.; Patel, D.; Dinker, A. Optimization of Manganese Removal from Water Using Response Surface Methodology. Iran. Technol. 2016, 40, 63–73. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Flocculation optimization of orthophosphate with FeCl3 and alginate using the Box-Behnken response surface methodology. Ind. Eng. Chem. Res. 2017, 56, 3145–3155. [Google Scholar] [CrossRef]
- Yan, Y.P.; Wang, G.; Jiang, S.J.; Wang, L.L. Response surface methodology for optimizing Cu2+ removal by heavy metal flocculant DTAPAM. Acta Sci. Circumstantiae 2021, 41, 2156–2161. [Google Scholar]



| Factors | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| pH | A | 6.5 | 7 | 7.5 |
| Manganese sand dosage (g/L) | B | 3.2 | 3.5 | 3.8 |
| Initial concentration of Fe/Mn | C | 3 | 4 | 5 |
| Sample | BET Surface Area m2/g | Total Pore Volume cm3/g | Average Pore Size nm |
|---|---|---|---|
| Unmodified manganese sand | 19.281 | 0.060 | 12.451 |
| Modified manganese sand | 24.459 | 0.085 | 15.797 |
| No. | Independent Factors | Removal Rate % | No. | Independent Factors | Removal Rate % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Fe2+ | Mn2+ | A | B | C | Fe2+ | Mn2+ | ||
| 1 | −1 | −1 | 0 | 79.92 | 65.24 | 10 | 0 | 1 | −1 | 81.34 | 72.43 |
| 2 | 1 | −1 | 0 | 81.60 | 70.24 | 11 | 0 | −1 | 1 | 80.32 | 71.58 |
| 3 | −1 | 1 | 0 | 78.57 | 69.95 | 12 | 0 | 1 | 1 | 80.52 | 68.17 |
| 4 | 1 | 1 | 0 | 82.81 | 71.94 | 13 | 0 | 0 | 0 | 83.15 | 75.23 |
| 5 | −1 | 0 | −1 | 79.37 | 68.32 | 14 | 0 | 0 | 0 | 83.24 | 76.37 |
| 6 | 1 | 0 | −1 | 83.15 | 71.65 | 15 | 0 | 0 | 0 | 83.29 | 76.95 |
| 7 | −1 | 0 | 1 | 78.74 | 69.72 | 16 | 0 | 0 | 0 | 82.95 | 75.38 |
| 8 | 1 | 0 | 1 | 80.89 | 70.24 | 17 | 0 | 0 | 0 | 83.06 | 76.28 |
| 9 | 0 | −1 | −1 | 81.78 | 66.27 | ||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe2+ | Mn2+ | Fe2+ | Mn2+ | Fe2+ | Mn2+ | Fe2+ | Mn2+ | |||
| Model | 43.87 | 197.39 | 9 | 4.84 | 21.93 | 279.12 | 30.02 | <0.0001 | <0.0001 | significant |
| A-pH | 17.79 | 14.69 | 1 | 17.79 | 14.69 | 1018.64 | 20.01 | <0.0001 | 0.0029 | |
| B-Dosage | 0.026 | 10.49 | 1 | 0.026 | 10.49 | 1.51 | 14.35 | 0.2582 | 0.0068 | |
| C-Fe/Mn | 3.34 | 0.14 | 1 | 3.34 | 0.14 | 191.30 | 0.219 | <0.0001 | 0.6800 | |
| AB | 1.54 | 2.27 | 1 | 1.54 | 2.27 | 88.04 | 3.10 | <0.0001 | 0.1217 | |
| AC | 0.66 | 1.97 | 1 | 0.66 | 1.97 | 38.03 | 2.70 | 0.0005 | 0.1442 | |
| BC | 0.10 | 22.90 | 1 | 0.10 | 22.90 | 5.86 | 31.34 | 0.0460 | 0.0008 | |
| A2 | 8.52 | 42.09 | 1 | 8.52 | 42.09 | 488.01 | 57.61 | <0.0001 | 0.0001 | |
| B2 | 3.96 | 52.52 | 1 | 3.96 | 52.52 | 226.95 | 71.88 | <0.0001 | <0.0001 | |
| C2 | 5.84 | 35.21 | 1 | 5.84 | 35.21 | 334.41 | 48.19 | <0.0001 | 0.0002 | |
| Residual error | 0.12 | 5.11 | 7 | 0.017 | 0.73 | |||||
| Lack of fit | 0.047 | 3.05 | 3 | 0.016 | 1.02 | 1.07 | 1.97 | 0.5395 | 0.2613 | not significant |
| Pure error | 0.075 | 2.07 | 4 | 0.019 | 0.52 | |||||
| summation | 44 | 202.51 | 16 | |||||||
| C.V.% | 0.16 | Adeq Precision | 45.006 | R2 | 0.9972 | |||||
| 1.20 | 15.392 | 0.9747 | ||||||||
| First-Order Kinetic Equation | Second-Order Kinetic Equation | |||||
|---|---|---|---|---|---|---|
| Qe | k1 | R2 | Qe | k1 | R2 | |
| Unmodified manganese sand | 0.0941 | 0.4701 | 0.9839 | 0.110 | 5.059 | 0.995 |
| Modified manganese sand | 0.1189 | 0.5564 | 0.9772 | 0.139 | 4.828 | 0.998 |
| First-Order Kinetic Equation | Second-Order Kinetic Equation | |||||
|---|---|---|---|---|---|---|
| Qe | k1 | R2 | Qe | k1 | R2 | |
| Unmodified manganese sand | 0.1026 | 0.4298 | 0.9819 | 0.126 | 3.307 | 0.992 |
| Modified manganese sand | 0.1261 | 0.4961 | 0.9790 | 0.150 | 3.687 | 0.996 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| KL | qm | R2 | KF | 1/n | R2 | |
| Fe | 1.97 | 0.395 | 0.9908 | 0.2148 | 0.395 | 0.9670 |
| Mn | 2.05 | 0.444 | 0.9918 | 0.2505 | 0.337 | 0.9759 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Liu, Y.; Li, D.; Xu, L. Study on the Removal of Iron and Manganese from Groundwater Using Modified Manganese Sand Based on Response Surface Methodology. Appl. Sci. 2022, 12, 11798. https://doi.org/10.3390/app122211798
Kang H, Liu Y, Li D, Xu L. Study on the Removal of Iron and Manganese from Groundwater Using Modified Manganese Sand Based on Response Surface Methodology. Applied Sciences. 2022; 12(22):11798. https://doi.org/10.3390/app122211798
Chicago/Turabian StyleKang, Han, Yan Liu, Dan Li, and Li Xu. 2022. "Study on the Removal of Iron and Manganese from Groundwater Using Modified Manganese Sand Based on Response Surface Methodology" Applied Sciences 12, no. 22: 11798. https://doi.org/10.3390/app122211798





