Bio-Sourced and Biodegradable Membranes
Abstract
:1. Introduction
2. Green Membrane Materials
2.1. Bio-Sourced Polymers for Membrane Fabrication
2.1.1. Cellulose and Its Derivatives
2.1.2. Chitin and Chitosan
2.1.3. Carrageenan
2.1.4. Other Polysaccharides and Biopolymers
2.2. Synthetic Biodegradable Polymers
2.2.1. Polymers Synthesized from Renewable Sources
Polylactic Acid
Polybutylene Succinate
2.2.2. Petrochemical Polymers
Poly-ε-caprolactone
Poly-propylene Fumarate
Poly-ethylene Glycol
Poly-vinyl Alcohol
Polyurethane and Polyurethane Urea
3. Biodegradable Antifouling Membrane Properties
3.1. Bio-Based Materials
3.2. Biodegradable Membranes for Oil Fouling
3.3. Polyvinyl Alcohol (PVA) Membranes
3.4. Cellulose Acetate (CA) Membranes
3.5. Polylactic Acid (PLA) Membranes
4. Green Membrane Technology Applications
4.1. Wastewater Treatment
4.2. Gas Separation
4.3. Pervaporation
4.4. Biomedical Applications
4.5. Food Packaging
4.6. Fuel Cell
| Polymer | Application | Advantage | Disadvantage | Ref |
|---|---|---|---|---|
| PLA/PBS/SNPs PLA/PPC/SNPs PLA/PHB/SNPs | Oil/water separation | Favourable thermal stability, high separation efficiency and turbidity removal (>98% and >89%, respectively) | Low metal ion and dissolved solid removal | [1] |
| CS | Oil/water separation | Excellent underwater superoleophobicity, stable wettability in various pH, >99% oil separation efficiency | Low diesel removal in saline solutions | [113] |
| CAF/CS/CF | Oil/water separation | Superhydrophilicity and underwater superoleophobicity property, >99% separation efficiency, good reusability, high recyclability | [116] | |
| PLA | Oil/water separation | Superhydrophobic properties, excellent oil absorption capacity, antifouling property, not complicated preparation method | [179] | |
| PLA | Oil/water separation | Superoleophilic and superhydrophobic property, excellent reusability | Low oil adsorption capacity | [189] |
| PLA/TiO2 | Oil/water separation | Superhydrophilicity and underwater superoleophobicity, long-term behavior, excellent separation efficiency (>99%), high permeate flux, antifouling property | Low BSA adsorption capacity | [190] |
| PLA | Oil/water separation | Robust recyclability, excellent adsorption capacity | [191] | |
| CA/PVP | Oil, dye, metal removal | Excellent removal efficiency for oil, dye and metal ions (>99%), long-term reusability, super hydrophilicity and underwater superoleophobicity | [192] | |
| CA | Oil/water separation | Robust recyclability, antifouling property, high infuse flux, enhanced adsorption capacity | lower infused flux for solutions, containing crude oil | [193] |
| CA/MOFDPC | Dye removal | Excellent reusability, High MB removal, long term behaviour, high water flux | Lower tensile strength and flexibility compared to CA membrane | [176] |
| CA/Cotton | Dye removal | MB rejection of 98% over 8 filtration cycle, high durability, low fouling, high mechanical stability | Tested for low volume wastewater (5–10 mL, dead end filtration setup) | [186] |
| CS/PVA | Dye removal | Strong interaction between CS and PVA, higher tensile strength, stability in DI, acidic and alkaline medium | Lower thermal stability compared to pristine membranes | [8] |
| CS/GO | Dye removal | At least 95% of cationic MB removal | GO size affected the anionic MB removal such that the maximum removal efficiency was 64% for nanoscale GO | [187] |
| PLA/CS | Dye removal | Large porous framework though a nanofibrous structure, High removal capacity of 86.43 and 82.37 mg/g for rhodamine B and MB | Adsorption capacity reduction after fourth cycle | [255] |
| PCL/MXene | Dye removal | High hydrophilicity and water permeability by adding only 4 wt% of MXene, more than 99% or crystal violet removal | Lower CV rejection after adding MXene nanoparticles | [78] |
| CS/PVA/MMT | Heavy metal removal | High hydrophilicity and water flux, antibiofouling property, 84–88.34% of Chromium removal efficiency | Low removal efficacy after 1 h and at high pH (pH of 9) | [138] |
| PLA/PBS | Heavy metal removal | Heat resistance improvement, enhanced hydrophilicity and mechanical stability, about 83% of ion removal efficiency (cobalt and nickel), antifouling property | [182] | |
| CS/PVA | Heavy metal removal | Improved chemical stability in acidic medium, enhanced hydrophilicity and anti-swelling, good adsorption recovery | Adsorption capacity decay by increasing the Cd (II) concentration (about 95% at 40 mg/L to 75% at 50 mg/L) | [195] |
| CS/Cellulose | Heavy metal removal | High removal efficiency of 85% and 94% for Pb and Cd, respectively, Excellent adsorption capacity for Pb, Cd | Low removal efficacy and adsorption capacity for Cr. | [256] |
| SCS/PVA | Heavy metal removal | Up to 90% of CU ion removal after 3 h, improved areal swelling | Low Ni ion removal | [196] |
| PLA-HAp/PDA | NOM removal | Antifouling properties, Flux improvement, thermal stability, more resistance to damage in harsh environment | Reversible fouling increased from 3.6% to 10.5 % | [7] |
| PLA | NOM removal | Enhanced BSA removal up to 92%, improved antifouling property, increased FRR from 57% to 93% | Lower water flux by increasing the PLA concentration | [180] |
| PCL/PBS | Wastewater treatment | Enhanced hydrophilicity and biodegradability, improved water flux and FRR, and pollution rejection | Lower mechanical properties compared to neat PCL | [197] |
| PLLA/PDLA/AlCl3 | Pathogen removal | Excellent filtration efficiency, high porosity, small pore size, low pressure drops | [198] | |
| PBS/CA/DEX | Dairy wastewater treatment | Enhanced porosity and hydrophilicity, robust permeate flux, antifouling property, >99% turbidity removal | Foulant rejection decreased, decreased mechanical property, low TDS removal | [199] |
| PU/PVA | Gas separation | Enhanced CO2 solubility, high CO2/N2 and CO2/CH4 selectivity | Low permeability for pure gases such as N2 and O2 | [200] |
| CS/DS-PVA | Gas separation | Robust self-healing efficiency, enhanced CO2/N2 selectivity, amino groups increase the CO2 permeance | Lower CO2 and N2 permeability by increasing the PVA concentration | [201] |
| PU | Gas separation | Improved mechanical and chemical stability, high CO2 separation | Low CO2/H2 selectivity | [203] |
| PLA/PBS/MWCNT | Gas separation | Improved hydrophilicity, tensile strength, porosity, and crystallinity, enhanced pure gas permeability (Ar, CO2, H2, and N2), H2/N2 selectivity improvement | Low selectivity and no improvement for CO2/N2, Ar/N2, and CO2/Ar | [204] |
| PLA/layer silicate | Gas separation | Improved thermal and mechanical stability, enhanced pure gas permeability by increasing silicate concentration | Decreased CO2, N2 and O2 permeability by increasing clay content | [205] |
| CS/PVA | Gas separation | Improved specific surface area, high CO2 adsorption capacity | [206] | |
| PVA/CNC | Gas separation | Increased CO2 permeability and CO2/N2 separation factor | [257] | |
| CMS | Gas separation | High specific area, pore volume, and CO2 absorption capacity, improved N2 absorption, | [258] | |
| CS/PHB/MWCNT | Pervaporation (1,4-dioxane/water) | Low swelling degree compared to pristine CS, improved mechanical properties, improved water selectivity by increasing the 1,4-dioxane concentration in feed | Not significant water selectivity improvement for mixed matrix membrane compared to pristine CS | [207] |
| SA/PCL/GO | Pervaporation (Alcohol/water) | Excellent membrane hydrophilicity and dehydration performance for alcohol-water, water flux and separation factor improvement | Increased swelling degree by increasing GO content | [208] |
| CS/FGS | Pervaporation (isopropanol/water and ethanol-water) | Good isopropanol and ethanol barrier, good water selectivity | Low water permeability | [209] |
| PLA | Pervaporation (MeOH/MTBE) | Good mechanical properties, good methanol selectivity | Enrichment factor dropped drastically by 75% as the methanol concentration changed from 1 wt% to 10 wt% | [210] |
| PUU | Pervaporation (phenol/water) | Low swelling degree, good separation factor and flux for phenolic components | Separation factor dropped by 60% as the phenol concentration changed from 0.1% to 0.4% | [212] |
| PHA/PHBHV | Pervaporation (MeOH/MTBE) | Good mechanical properties, favourable methanol selectivity | Reduced methanol selectivity by adding the additives (PEG, EBO) to the polymer solution | [215] |
| CS/PVA/NH2-MWCNT | Pervaporation (Isopropyl alcohol/water) | Improved mechanical property and separation factor and reduced swelling degree by adding NH2-MWCNT, excellent water flux and PSI | Lower separation factor compared to similar studies | [259] |
| PLA | Pervaporation (MeOH/MTBE) | Good mechanical and chemical stability, good methanol selectivity | Low permeate flux compared to similar researches | [260] |
| PVA/APS/MBA | Pervaporation (Ethanol/water) | Reduced swelling rate, high separation efficiency (95%), water flux, and membrane durability | Reduced selectivity by increasing the feed temperature (from 40 °C to 70 °C) | [261] |
| PGS APS | Pervaporation (Organic solvents/water) | Higher separation factor and PSI compared to commercial membrane (PDMS), good stability | [262] | |
| PLA/Fe-MOF | Pervaporation (MeOH/MTBE) | Improved methanol selectivity by adding Fe-MO and pressure increase (from 0 to 7.5 mbar) | Lower flexibility and mechanical strength by adding 0.5 wt% of Fe-MO, reduced selectivity by increasing the feed temperature (from 25 °C to 45 °C) | [263] |
| Agarose | Pervaporation (Organic solvents/water) | High water flux and permselectivity | [264] | |
| CS/Agarose | Artificial skin | Nontoxicity, high exudate absorption capacity, high elastic deformations, extracellular matrix similarity, support reproduction of skin fibroblast | [225] | |
| ALG/GC | Hepatocyte attachment | Increase spheroid formation, higher viability and mechanical property rather than alginate sponge | [265] | |
| PLLA/CS | Periodontitis treatment | Higher hydrophilicity, biocompatibility and bioactivity rather than electrospun PLLA membrane, higher cell reproduction, fibroblast barrier (mitigate the destructive effect of fibroblast on tissue recovery) | Lower degradation rate due to the presence of electrospun PLLA | [266] |
| CS/PEEK | Culturing the liver cells | Favourable microenvironment for liver cells, higher cell proliferation in 8–11 days, higher level of specific functions for longer time (compared to former substrates like collagen and PSCD) | [267] | |
| PCE | Biomedical implants | Elastomeric behavior, antibacterial activity, biomimetic mechanical property, photoluminescent capacity (favourable for real-time monitoring), high cytocompatibility and hemocompatibility, low inflammatory response | [268] | |
| GEL | Tissue regeneration | High tensile strength, improved water resistance, good biocompatibility | Low elastic and ductile characteristic in dry state | [269] |
| PU/PGSAP | Nerve tissue | Increasing the Schwann cells’ (SCs) myelin gene expressions, higher neurotrophin secretion, inducing neurite growth and elongation of PC12 cells, reducing the intracellular Ca+2 level | [270] | |
| PCL | Drug delivery | Constrained diffusion for drug release control, no negative effect on cells’ growth, cellular compatibility | Low diffusivity of water-soluble components like FS and FITC-BSA due to the hydrophobic nature of PCL | [226] |
| CA/Phospholipid | Blood purification | Good water and solute permeability, lower protein adsorption (compared to CA and commercial PES membranes), sharp molecule weight cut-off, permselectivity and antifouling property over a long-term filtration, good hemocompatibility | Lower dye and protein retention (compare to pristine CA membrane) | [178] |
| PLA (blended with three different copolymers: PA, PD, and PH) | Hemodiafiltration | Desirable protein and urea retention, improved hydrophilicity and permeate flux (compared to PLA), antifouling property, hemocompatibility | Relatively low lysozyme retention (17.4%) for PLA/PA | [184] |
| PLA/DA/HEP | Hemodialysis | Improved hemocompatibility, low platelet adhesion and hemolysis ratio, reasonable urea and BSA separation | Relatively low lysozyme retention (18%), it was bioincompatible with human blood. | [227] |
| PLA/ DA/GOC | Hemodialysis | High hydrophilicity and electronegativity, improved hemocompatibility, low platelet adhesion and hemolysis ratio, longer plasma recalcification time, high BSA separation | Lower lysozyme retention compared to pristine PLA, the hemocompatibility was not proved for human serum. | [228] |
| CS/PVA | Food packaging | Good antimicrobial property, molecular miscibility between PVA and CS, improved crystallinity | [238] | |
| PLA/limonene | Food packaging | Improved water barrier ability, excellent flexibility | Reduced oxygen barrier (still acceptable for food packaging) | [240] |
| PLA/TP | Food packaging | Improved antioxidant and antimicrobial activity | Reduced tensile strength and breakage elongation | [241] |
| PLA/PHB/limonene | Food packaging | Robust elongation at break, improved oxygen barrier, increased hydrophobicity | [242] | |
| PLA/ATBC/CS | Food packaging | Improved thermal and mechanical properties, reduced brittleness, increased transparency, good antifungal and antibacterial activity | Low water vapour barrier | [243] |
| PVA/CS | Food packaging | Good antimicrobial activity, improved thermal property | Reduced film stretchability, reduced water vapour barrier | [244] |
| PVA/CS/Silica | Food packaging | Improved tensile strength, increased oxygen and moisture barrier | Deferred resolving time | [245] |
| PVA/CS | Microbial fuel cell | Compared to Nafion: Reduced O2 permeability, lower cost, higher power generation, higher water uptake capacity environmentally friendly | Low proton conductivity | [251] |
| CSS | Methanol fuel cell | Much lower methanol cross over compared to Nafion 112, improved mechanical strength | Low breaking elongation compared to Nafion112 | [30] |
| PVA/CS/SA | Methanol fuel cell | Low methanol permeability, high ion exchange capacity, excellent thermal and mechanical stability | Low proton conductivity compared to Nafion117 | [253] |
| CS/SA | Methanol fuel cell | Low methanol permeability, excellent mechanical property, low cost | Low proton conductivity compared to Nafion117 | [254] |
| Graphene-PVA/CS | Methanol fuel cell | Improved methanol barrier, conductivity and ion selectivity compared to Nafion117 | Lower elongation break by adding graphene | [271] |
| CCS | Direct borohydride fuel cell | Higher ionic conductivity and power performance compared to Nafion212, cost effective, stable performance for >100 h | Higher borohydride crossover compared to Nafion212 | [272] |
| CS/PEO | Fuel cell | Improved conductivity, reduced swelling, low production cost | [273] | |
| PVA/GO | Ethanol fuel cell | Reduced water uptake and ethanol permeability, and improved proton conductivity by adding GO, higher power density compared to Nafion117 | Reduced elongation at break ratio | [274] |
| CS/PMC | Microbial fuel cell | Lower bioelectricity start-up time compared to Nafion117 and Agar salt bridge, acceptable power density, improved retention time | Antifouling property was not investigated | [275] |
5. Green Membranes Challenges and Limitations
6. Future Trends
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ALG | Alginate |
| APS | Poly(1,3-diamino-2-hydroxypropane-co-polyol sebacinate) |
| ATBC | Tributyl o-acetyl citrate |
| BMA | Butyl methacrylate |
| BSA | Bovine serum albumin |
| CA | Cellulose acetate |
| CAF | Caffeic acid |
| CCS | Cross-linked chitosan |
| CD | Cyclodextrin |
| CDA | Cellulose diacetate |
| CF | Cotton Fiber |
| CMS | Carboxymethyl starch |
| CNC | Cellulose nanocrystals |
| CNT | Carbon nano-tubes |
| CS | Chitosan |
| CSS | Chitosan sulfate |
| DA | Dopamine |
| DEX | Dextran |
| DS-PVA | Dialdehyde starch-polyvinyl alcohol |
| FC | Fuel cell |
| Fe-MOF | Iron metal-organic Framework |
| FGS | Functionalized graphene sheets |
| FITC-BSA | Fluorescein isothiocyanate-labelled bovine serum albumin |
| FO | Forward osmosis |
| GC | Galactosylated chitosan |
| GO | Graphene oxide |
| GOC | Carboxylated graphene oxide |
| GEL | Gelatin |
| GO | Graphene oxide |
| HA | Humic acid |
| HAp | Hydroxyapatite |
| HEP | Heparin |
| MB | Methylene Blue |
| MBA | N,N’-methylene bisacrylamide |
| MeOH | Methanol |
| MFC | Microbial fuel cell |
| MMT | Montmorillonite |
| MOFDPC | Metal-organic framework derived porous carbon |
| MPC | Poly(2-methacryloyloxyethyl phosphorylcholine) |
| MTBE | Methyl tert-butyl ether |
| MWCNT | Multiwalled carbon nano tube |
| SCS | Sulfated chitosan |
| SFC | Sodium fluorescein |
| SNPs | Silica nanoparticles |
| PA | Poly(methyl methacrylate)-b-poly(2-acryloamido-2-methyl-1-propanesulfonic acid) |
| PBS | Polybutylene Succinate |
| PCE | Polycitrate-(ε-polypeptide) |
| PCL | Poly(caprolactone) |
| PD | Poly(methyl methacrylate)-b-poly(2-dimethylamino ethyl methacrylate) |
| PDA | Polydopamine |
| PDLA | Poly(D-lactic acid) |
| PDMS | Polydimethyl-siloxane |
| PEEK | Polyetheretherketone |
| PEO | Poly(ethylene oxide) |
| PES | Polyethersulfone |
| PEG | Polyethylene glycol |
| PGS | Poly(glycerol sebacate) |
| PGSAP | Poly(glycerol sebacate)-co-aniline pentamer |
| PH | Poly(methyl methacrylate)-b-poly(2-hydroxyethyl methacrylate) |
| PHB | Polyhydroxy butyrate |
| PHBHV | Poly(hydroxybutyrate-co- hydroxyvalerate) |
| PLA | Polylactic acid |
| PLLA | Poly(L-lactic acid) |
| PMC | Poly(malic acid-citric acid) |
| PP | Polypropylene |
| PPC | Polypropylene carbonate |
| PPF | Polypropylene fumarate |
| PSI | Pervaporation separation index |
| PTFE | Polytetraflouroethylene |
| PVA | Polyvinyl alcohol |
| PVDF | Polyvinylidene fluoride |
| PVP | Polyvinyl pyrrolidone |
| PU | Polyurethan |
| PUU | Polyurethan urea |
| SA | Sodium alginate |
| TDS | Total dissolved solid |
| TP | Tea polyphenol |
References
- Ghorbani, M.; Vakili, M.H.; Ameri, E. Fabrication and evaluation of a biopolymer-based nanocomposite membrane for oily wastewater treatment. Mater. Today Commun. 2021, 28, 102560. [Google Scholar] [CrossRef]
- Bandehali, S.; Sanaeepur, H.; Amooghin, A.E.; Shirazian, S.; Ramakrishna, S. Biodegradable polymers for membrane separation. Sep. Purif. Technol. 2021, 269, 118731. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Abokwiek, R.; Al-Othman, A.; Tawalbeh, M.; Karaman, C.; Orooji, Y.; Karimi, F. Biodegradable polymers and their nano-composites for the removal of endocrine-disrupting chemicals (EDCs) from wastewater: A review. Environ. Res. 2021, 202, 111694. [Google Scholar] [CrossRef]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef] [Green Version]
- Ouda, M.; Ibrahim, Y.; Kallem, P.; Govindan, B.; Banat, F.; Hasan, S.W. Highly permeable, environmentally-friendly, antifouling polylactic ultrafiltration membranes. J. Clean. Prod. 2022, 330, 129871. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe(III) and Cr(VI) ions. Carbohydr. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Dutta, K. Poly(vinyl alcohol)-based membranes for fuel cell and water treatment applications: A review on recent advancements. Polym. Adv. Technol. 2021, 32, 4175–4203. [Google Scholar] [CrossRef]
- European Bioplastics, Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market-update-2020-bioplastics-continue-to-become-mainstream-as-the-global-bioplastics-market-is-set-to-grow-by-36-percent-over-the-next-5-years/ (accessed on 23 July 2021).
- Bioplastics & Biopolymers Market by Type (Non-Biodegradable/Bio-Based, Biodegradable), End-Use Industry (Packaging, Consumer Goods, Automotive & Transportation, Textiles, Agriculture & Horticulture), Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/market-reports/biopolymersbioplastics-market-88795240.html (accessed on 23 July 2021).
- Rohrbach, K.; Li, Y.; Zhu, H.; Liu, Z.; Dai, J.; Andreasen, J.; Hu, L. A cellulose based hydrophilic, oleophobic hydrated filter for water/oil separation. Chem. Commun. 2014, 50, 13296–13299. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shan, H.; Zhang, W.; Li, B. 3D printed robust superhydrophilic and underwater superoleophobic composite membrane for high efficient oil/water separation. Sep. Purif. Technol. 2020, 237, 116324. [Google Scholar] [CrossRef]
- Xu, X.; Long, Y.; Li, Q.; Li, D.; Mao, D.; Chen, X.; Chen, Y. Modified cellulose membrane with good durability for effective oil-in-water emulsion treatment. J. Clean. Prod. 2019, 211, 1463–1470. [Google Scholar] [CrossRef]
- Yao, A.; Yan, Y.; Tan, L.; Shi, Y.; Zhou, M.; Zhang, Y.; Zhu, P.; Huang, S. Improvement of filtration and antifouling performance of cellulose acetate membrane reinforced by dopamine modified cellulose nanocrystals. J. Membr. Sci. 2021, 637, 119621. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Németh, Z.; Schrantz, K.; Németh, K.; Schabikowski, M.; Traber, J.; Pronk, W.; Hernádi, K.; Graule, T. Copper-Coated Cellulose-Based Water Filters for Virus Retention. ACS Omega 2018, 3, 446–454. [Google Scholar] [CrossRef]
- Pei, X.; Gan, L.; Tong, Z.; Gao, H.; Meng, S.; Zhang, W.; Wang, P.; Chen, Y. Robust cellulose-based composite adsorption membrane for heavy metal removal. J. Hazard. Mater. 2021, 406, 124746. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Heydaripour, S.; Abdouss, M. Novel carboxymethyl cellulose based nanocomposite membrane: Synthesis, characterization and application in water treatment. J. Environ. Manag. 2016, 166, 457–465. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Xiao, H.; Zhang, H.; Cao, S.; Chen, L.; Ni, Y.; Huang, L. Ultra-low pressure cellulose-based nanofiltration membrane fabricated on layer-by-layer assembly for efficient sodium chloride removal. Carbohydr. Polym. 2021, 255, 117352. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, S.; Cheng, Z.; Huang, G. Thin-film composite forward osmosis membrane prepared from polyvinyl chloride/cellulose carbamate substrate and its potential application in brackish water desalination. J. Appl. Polym. Sci. 2021, 138, 49939. [Google Scholar] [CrossRef]
- Elkony, Y.; Mansour, E.; Elhusseiny, A.; Ebrahim, S. Effect of cellulose acetate/cellulose triacetate ratio on reverse osmosis blend membrane performance. Polym. Eng. Sci. 2020, 60, 2852–2863. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Ghaee, A.; Vatanpour, V.; Ghaemi, N. Surface modification of PES membrane via aminolysis and immobilization of carboxymethylcellulose and sulphated carboxymethylcellulose for hemodialysis. Carbohydr. Polym. 2018, 188, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, M.; Dumee, L.F.; Fong, Y.Y.; Jusoh, N.; Lukose, J.; Chai, W.S.; Show, P.L. Cellulose acetate-based membranes by interfacial engineering and integration of ZIF-62 glass nanoparticles for CO2 separation. J. Hazard. Mater. 2021, 415, 125639. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Vankelecom, I.F.J. The performance of affordable and stable cellulosebased poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Liu, L.; Doherty, C.M.; Ricci, E.; Chen, G.Q.; De Angelis, M.G.; Kentish, S.E. The influence of propane and n-butane on the structure and separation performance of cellulose acetate membranes. J. Membr. Sci. 2021, 638, 119677. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Ali, Q.; Taweepreda, W.; Techato, K. Preparation and characterization of polymer electrolyte membrane from chloroacetate chitosan/chitosan blended with epoxidized natural rubber. Polym. Test. 2020, 82, 106294. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Gandhi, N.S.; Gu, Y.; Zhang, Y.; Tang, Y. Chitosan/graphene complex membrane for polymer electrolyte membrane fuel cell: A molecular dynamics simulation study. Int. J. Hydrogen Energy 2020, 45, 25960–25969. [Google Scholar] [CrossRef]
- Tang, C.C.H.; Zhang, L. Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem. Eng. J. 2011, 173, 689–697. [Google Scholar] [CrossRef]
- Shen, S.S.; Yang, J.J.; Liu, C.X.; Bai, R.B. Immobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption. RSC Adv. 2017, 7, 10424–10431. [Google Scholar] [CrossRef]
- Wu, J.X.; Zhang, J.; Kang, Y.L.; Wu, G.; Chen, S.C.; Wang, Y.Z. Reusable and recyclable superhydrophilic electrospun nanofibrous membranes with in situ cocross-linked polymer—Chitin nanowhisker network for obust oil-in-water emulsion separation. ACS Sustain. Chem. Eng. 2017, 6, 1753–1762. [Google Scholar] [CrossRef]
- Zhijiang, C.; Ping, X.; Cong, Z.; Tingting, Z.; Jie, G.; Kongyin, Z. Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 2018, 25, 5123–5137. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. RETRACTED: Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, A.; Udomsin, J.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Robust underwater superoleophobic membranes with bio-inspired carrageenan/laponite multilayers for the effective removal of emulsions, metal ions, and organic dyes from wastewater. Chem. Eng. J. 2020, 391, 123585. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Zarei, V.G.A. Preparation and characterization of biodegradable cellulose acetate-starch membrane. Polym.-Plast. Technol. Eng. 2013, 52, 387–392. [Google Scholar] [CrossRef]
- Woranuch, S.; Pangon, A.; Puagsuntia, K.; Subjalearndee, N.; Intasanta, V. Starch-based and multi-purpose nanofibrous membrane for high efficiency nanofiltration. RSC Adv. 2017, 7, 35368–35375. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, M.M.K.; Ghaffarian, V.; Zabihi, E. Polyacrylonitrile/starch semibiodegradable blend membrane: Preparation, morphology and performance. Desalin. Water Treat. 2016, 57, 495–504. [Google Scholar] [CrossRef]
- Wu, B.T.H.; Wu, P. Preparation and characterization of anti-fouling β-cyclodextrin/polyester thin film nanofiltration composite membrane. J. Membr. Sci. 2013, 428, 301–308. [Google Scholar] [CrossRef]
- Adams, E.N.F.; Krause, R.; Hoek, E.; Mamba, B. Application of polysulfone/cyclodextrin mixed-matrix membranes in the removal of natural organic matter from wate. Phys. Chem. Earth Parts A/B/C 2014, 67, 71–78. [Google Scholar] [CrossRef]
- Smidsrød, G.S.O. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.L.Y.S.; Kim, J.-H. Dye adsorption characteristics of alginate/polyaspartate hydrogels. J. Ind. Eng. Chem. 2008, 14, 726–731. [Google Scholar] [CrossRef]
- Abdel-Halim, S.S.A.-D.E. Removal of heavy metals from their aqueous solutions through adsorption onto natural polymers. Carbohydr. Polym. 2011, 84, 454–458. [Google Scholar] [CrossRef]
- Lim, J.P.C.S. Synthesis of an innovative calcium-alginate magnetic sorbent for removal of multiple contaminants. Appl. Surf. Sci. 2007, 253, 5772–5775. [Google Scholar] [CrossRef]
- Chen, J.H.; Xing, H.T.; Guo, H.X.; Li, G.P.; Weng, W.; Hu, S.R. Preparation, characterization and adsorption properties of a novel 3-aminopropyltriethoxysilane functionalized sodium alginate porous membrane adsorbent for Cr(III) ions. J. Hazard. Mater. 2013, 248–249, 285–294. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef]
- Costa, R.S.F.; Boccaccini, A. Fibrous Protein-Based Biomaterials (Silk, Keratin, Elastin, and Resilin Proteins) for Tissue Regeneration and Repair Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–204. [Google Scholar]
- Khosa, S.S.S.M.A.; Feng, X. Metal sericin complexation and ultrafiltration of heavy metals from aqueous solution. Chem. Eng. J. 2014, 244, 446–456. [Google Scholar] [CrossRef]
- Gao, A.; Xie, K.; Song, X.; Zhang, K.; Hou, A. Removal of the heavy metal ions from aqueous solution using modified natural biomaterial membrane based on silk fibroin. Ecol. Eng. 2017, 99, 343–348. [Google Scholar] [CrossRef]
- Shoulders, R.T.R.M.D. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Wang, Y.; Hao, B.; Cao, Y.; Cui, Y.; Bi Shi, X. Collagen Fiber-Based Advanced Separation Materials: Recent Developments and Future Perspectives. Adv. Mater. 2022, 34, 2107891. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.K.F.; Shibue, T. Formation having a tanning gradient structure of collagen membrane by the pervaporation technique. J. Membr. Sci. 2000, 165, 169–175. [Google Scholar] [CrossRef]
- Maser, F.; Ströher-Glowienka, C.; Kimmerle, K.; Gudernatsch, W. Collagen film as a new pervaporation membrane. J. Membr. Sci. 1991, 61, 269–278. [Google Scholar] [CrossRef]
- Ackermann, J.U.; Müller, S.; Lösche, A.; Bley, T. Wolfgang Babel Methylobacterium rhodesianum cells tend to double the DNA content under growth limitations and accumulate PHB. J. Biotechnol. 1995, 39, 9–20. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Wang, Y.; Yang, X.; Jung, J.; Li, Z.; Ren, X.; Sun, Y. Fabrication of pH-responsive hydrophobic/hydrophilic antibacterial polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes for biomedical application. Mater. Chem. Phys. 2021, 260, 124087. [Google Scholar] [CrossRef]
- Lin, X.; Yin, M.; Liu, Y.; Li, L.; Ren, X.; Sun, Y.; Huang, T.-S. Biodegradable polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes modified by silica composite hydrol for super hydrophobic and outstanding antibacterial application. J. Ind. Eng. Chem. 2018, 63, 303–311. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Sep. Purif. Technol. 2016, 161, 69–79. [Google Scholar] [CrossRef]
- Tomietto, P.; Carré, M.; Loulergue, P.; Paugam, L.; Audic, J.-L. Polyhydroxyalkanoate (PHA) based microfiltration membranes: Tailoring the structure by the non-solvent induced phase separation (NIPS) process. Polymer 2020, 204, 122813. [Google Scholar] [CrossRef]
- Leja, G.L.K. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Ummartyotin, C.P.S. Strategies for development and implementation of bio-based materials as effective renewable resources of energy: A comprehensive review on adsorbent technology. Renew. Sustain. Energy Rev. 2016, 62, 654–664. [Google Scholar] [CrossRef]
- Singhvi, D.G.M. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558–13568. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, L.; Huang, R.; Bai, D.; Bai, H.; Zhang, Q.; Fu, Q. Ultrahigh-performance electrospun polylactide membranes with excellent oil/water separation ability via interfacial stereocomplex crystallization. J. Mater. Chem. A 2017, 5, 19729–19737. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Leejarkpai, T.; Suwanmanee, U.; Rudeekit, Y.; Mungcharoen, T. Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag. 2011, 31, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M. Properties and application of aliphatic polyester products. Biopolymers 2005, 20. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Lin, C.-J.; Lee, W.-C.; Teng, H.-Y.; Chuang, M.-H. Production of succinic acid through the fermentation of Actinobacillus succinogenes on the hydrolysate of Napier grass. Biotechnol. Biofuels Bioprod. 2022, 15, 9. [Google Scholar] [CrossRef]
- Le, S.D.; Nishimura, S. Highly Selective Synthesis of 1,4-Butanediol via Hydrogenation of Succinic Acid with Supported Cu–Pd Alloy Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 18483–18492. [Google Scholar] [CrossRef]
- Guo, J.X.A.B.-H. Plastics from Bacteria: Natural Functions and Applications. Microbiol. Monogr. 2010, 14, 347–385. [Google Scholar]
- Mizuno, S.; Maeda, T.; Kanemura, C.; Hotta, A. Biodegradability, reprocessability, and mechanical properties of polybutylene succinate (PBS) photografted by hydrophilic or hydrophobic membranes. Polym. Degrad. Stab. 2015, 117, 58–65. [Google Scholar] [CrossRef]
- Almwli, H.H.A.; Mousavi, S.M.; Kiani, S. Preparation of poly(butylene succinate)/polyvinylpyrrolidone blend membrane for pervaporation dehydration of acetone. Chem. Eng. Res. Des. 2021, 165, 361–373. [Google Scholar] [CrossRef]
- Ghaffarian, V.; Mousavi, S.M.; Bahreini, M.; Jalaei, H. Polyethersulfone/poly (butylene succinate) membrane: Effect of preparation conditions on properties and performance. J. Ind. Eng. Chem. 2014, 20, 1359–1366. [Google Scholar] [CrossRef]
- Nelson, T.F.; Baumgartner, R.; Jaggi, M.; Bernasconi, S.M.; Battagliarin, G.; Sinkel, C.; Künkel, A.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of poly(butylene succinate) in soil laboratory incubations assessed by stable carbon isotope labelling. Nat. Commun. 2022, 13, 5691. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Geravand, M.H.A.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. Biodegradable polycaprolactone/MXene nanocomposite nanofiltration membranes for the treatment of dye solutions. J. Taiwan Inst. Chem. Eng. 2021, 128, 124–139. [Google Scholar] [CrossRef]
- Ho, T.T.-P.; Doan, V.K.; Tran, N.M.-P.; Nguyen, L.K.-K.; Le, A.N.-M.; Ho, M.H.; Trinh, N.-T.; Van Vo, T.; Tran, L.D.; Nguyen, T.-H. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater. Sci. Eng. C 2021, 120, 111724. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zong, Y.; Liu, Q.; Sun, Y.; Li, Z.; Wang, H.; Li, Z. A highly stretchable and biodegradable superamphiphobic fluorinated polycaprolactone nanofibrous membrane for antifouling. Prog. Org. Coat. 2020, 147, 105776. [Google Scholar] [CrossRef]
- Zafari, M.; Aghajani, S.; Boroujeni, M.M.; Nosrati, H. Vancomycin-loaded electrospun polycaprolactone/nano-hydroxyapatite membrane for the treatment of blood infections. Med. Hypotheses 2020, 144, 109992. [Google Scholar] [CrossRef]
- Nowwarote, N.; Chanjavanakul, P.; Kongdecha, P.; Clayhan, P.; Chumprasert, S.; Manokawinchoke, J.; Egusa, H.; Pavasant, P.; Osathanon, T. Characterization of a bioactive Jagged1-coated polycaprolactone-based membrane for guided tissue regeneration. Arch. Oral Biol. 2018, 88, 24–33. [Google Scholar] [CrossRef]
- Abdel-Motaal, F.F.; El-Sayed, M.A.; El-Zayat, S.A.; Ito, S.I. Biodegradation of poly (epsilon-caprolactone) (PCL) film and foam plastic by Pseudozyma japonica sp. nov., a novel cutinolytic ustilaginomycetous yeast species. 3 Biotech 2014, 4, 507–512. [Google Scholar] [CrossRef] [Green Version]
- MShung, A.K.; Timmer, M.D.; Jo, S.; Engel, P.S.; Mikos, A.G. Kinetics of poly(propylene fumarate) synthesis by step polymerization of diethyl fumarate and propylene glycol using zinc chloride as a catalyst. J. Biomater. Sci. Polym. Ed. 2002, 13, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Frazier, V.K.L.D.D.; Gerhart, T.N.; Altobelli, D.E.; Hayes, W.C. In-Vivo Degradation Of A Poly(Propylene-Fumarate) Biodegradable, Particulate Composite Bone Cement. MRS Online Proc. Library 1995, 394, 15–19. [Google Scholar] [CrossRef]
- Diez-Pascual, M.A. Mechanical properties of poly(propylene fumarate)/nanotube composites: Experimental results and theoretical predictions. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bangkok, Thailand, 24–26 February 2018; Volume 383. [Google Scholar]
- Díez-Pascual, A.L.D.-V.A.M. Multifunctional poly (glycolic acid-copropylene fumarate) electrospun fibers reinforced with graphene oxide and hydroxyapatite nanorods. J. Mater. Chem. B 2017, 5, 4084–4096. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, R.; Lv, R.; Na, B.; Liu, Q. Water-permeable polylactide blend membranes for hydrophilicity-based separation. Chem. Eng. J. 2015, 269, 180–185. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Dean, D.; Chen, J.E.; Fisher, J.P.; Han, S.; Rimnac, C.M.; Mikos, A.G. In vitro degradation and fracture toughness of multilayered porous poly(propylene fumarate)/beta-tricalcium phosphate scaffolds. J. Biomed. Mater. Res. 2002, 61, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Ohta, S.; Ito, T. Analysis of model drug permeation through highly crosslinked and biodegradable polyethylene glycol membranes. J. Membr. Sci. 2022, 645, 120218. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked poly(ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: Swelling and permeation studies. J. Appl. Polym. Sci. 2017, 134, 2. [Google Scholar] [CrossRef]
- He, X.C.H.; Lee, L.J. Design of a novel hydrogel-based intelligent system for controlled drug release. J. Control. Release 2004, 95, 391–402. [Google Scholar] [CrossRef]
- Tokuyama, Y.N.H.; Ban, T. Diffusion coefficient of solute in heterogeneous and macroporous hydrogels and its correlation with the effective crosslinking density. J. Membr. Sci. 2020, 595, 117533. [Google Scholar] [CrossRef]
- Asai, H.; bin Miswan, M.H.; Shimada, N.; Nakane, K.; Sakai, T.; Ogata, N. Preparation and characterization of a nanofiber mat consisting of Tetra-PEG prepolymers. J. Appl. Polym. Sci. 2014, 132, 41353. [Google Scholar] [CrossRef]
- Ulbricht, J.; Jordan, R.; Luxenhofer, R. On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 2014, 35, 4848–4861. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F. Microbial degradation of polyethers. Appl. Microbiol. Biotechnol. 2002, 58, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, F.K.a.H. Biodegradation of polyethylene glycol by symbiotic mixed culture (obligate mutualism). Arch. Microbiol. 1986, 146, 125–129. [Google Scholar]
- Ulrike Kohlweyer, B.T.; Schrader, T.; Andreesen, J.R. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 2000, 186, 301–306. [Google Scholar] [CrossRef]
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly(vinyl alcohol)-hyaluronic Acid Membranes for Wound Dressing Applications: Synthesis and in vitro Bio-Evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474. [Google Scholar] [CrossRef]
- Yang, C.C.H.J.M. Preparation and characterizaton of poly(dimethyl amino ethyl methacrylate) modified poly(vinyl alcohol) membrane by UV radiation for the permeation of 5-fluorouracil. J. Appl. Polym. Sci. 2012, 123, 3182–3188. [Google Scholar] [CrossRef]
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Market Volume of Polyurethane Worldwide from 2015 to 2025, with a Forecast for 2022 to 2029, Polyurethane Global Market Volume 2015–2029. Statista. Available online: https://www.statista.com/statistics/720341/global-polyurethane-market-size-forecast/ (accessed on 23 July 2021).
- Cooper, J.G.S.L. Advances in Polyurethane Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791. [Google Scholar] [CrossRef]
- Pavlova, M.; Draganova, M. Biocompatible and biodegradable polyurethane polymers. Biomaterials 1993, 14, 1024–1029. [Google Scholar] [CrossRef]
- Wong, C.S.; Liu, X.; Xu, Z.; Lin, T.; Wang, X. Elastin and collagen enhances electrospun aligned polyurethane as scaffolds for vascular graft. J. Mater. Sci. Mater. Med. 2013, 24, 1865–1874. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Y.; Wu, J.; Tang, L.; Hong, Y. Triggerable Degradation of Polyurethanes for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2015, 7, 20377–20388. [Google Scholar] [CrossRef] [PubMed]
- Howard, G. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Braunack, M.V.; Zaja, A.; Tam, K.; Filipović, L.; Filipović, V.; Wang, Y.; Bristow, K.L. A Sprayable Biodegradable Polymer Membrane (SBPM) technology: Effect of band width and application rate on water conservation and seedling emergence. Agric. Water Manag. 2020, 230, 105900. [Google Scholar] [CrossRef]
- Xu, C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 2021, 15, 250–271. [Google Scholar] [CrossRef]
- Fuwad, A.; Ryu, H.; Malmstadt, N.; Kim, S.M.; Jeon, T.J. Biomimetic membranes as potential tools for water purification: Preceding and future avenues. Desalination 2019, 458, 97–115. [Google Scholar] [CrossRef]
- Ehsani, M.; Doan, H.; Lohi, A. A comprehensive review of membrane fouling and cleaning methods with emphasis on ultrasound-assisted fouling control processes. Korean J. Chem. Eng. 2021, 38, 1531–1555. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, F.; Tao, L.; Liu, N.; Gao, C.; Feng, L.; Wei, Y. Bio-Inspired Anti-Oil-Fouling Chitosan-Coated Mesh for Oil/Water Separation Suitable for Broad pH Range and Hyper-Saline Environments. ACS Appl. Mater. Interfaces 2013, 5, 11971–11976. [Google Scholar] [CrossRef]
- Hou, D.; Wang, Z.; Wang, K.; Wang, J.; Lin, S. Composite membrane with electrospun multiscale-textured surface for robust oil-fouling resistance in membrane distillation. J. Membr. Sci. 2018, 546, 179–187. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Zhang, G.; Qian, P.Y. Environmentally Friendly Antifouling Coatings Based on Biodegradable Polymer and Natural Antifoulant. ACS Sustain. Chem. Eng. 2017, 5, 6304–6309. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Anbazhagan, R.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Biodegradable, superwettable caffeic acid/chitosan polymer coated cotton fibers for the simultaneous removal of oils, dyes, and metal ions from water. Chem. Eng. J. 2021, 427, 131920. [Google Scholar] [CrossRef]
- Shen, Y.x.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Dendritic Saccharide Surfactant Polymers as Antifouling Interface Materials to Reduce Platelet Adhesion. Biomacromolecules 2006, 7, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, C.M.; Brennan, A.B. Bio-inspired antifouling strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Messersmith, P.B. Bioinspired antifouling polymers. Mater. Today 2005, 8, 38–46. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coatings 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Tian, M.; Xu, H.; Yao, L.; Wang, R. A biomimetic antimicrobial surface for membrane fouling control in reverse osmosis for seawater desalination. Desalination 2021, 503, 114954. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Wicaksana, F.; Tang, C.; Torres, J.; Fane, A.G. Preparation of high performance nanofiltration (NF) membranes incorporated with aquaporin Z. J. Membr. Sci. 2014, 450, 181–188. [Google Scholar] [CrossRef]
- Chun, Y.; Qing, L.; Sun, G.; Bilad, M.R.; Fane, A.G.; Chong, T.H. Prototype aquaporin-based forward osmosis membrane: Filtration properties and fouling resistance. Desalination 2018, 445, 75–84. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Y.; Wang, R.; Hélix-Nielsen, C.; Fane, A. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Huo, P.; Gu, J. Exploration of zwitterionic cellulose acetate antifouling ultrafiltration membrane for bovine serum albumin (BSA) separation. Carbohydr. Polym. 2017, 165, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, J.; Liu, X.; Wei, D.; Zhou, H.; Xiao, H.; Zhang, Z.; Yu, H.; Chen, S. Bactericidal and antifouling electrospun PVA nanofibers modified with a quaternary ammonium salt and zwitterionic sulfopropylbetaine. Mater. Sci. Eng. C 2020, 111, 110855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Xie, M.; Zhang, B.; Li, G.; Luo, W. Resource recovery from digested manure centrate: Comparison between conventional and aquaporin thin-film composite forward osmosis membranes. J. Membr. Sci. 2020, 593, 117436. [Google Scholar] [CrossRef]
- Valverde-Pérez, B.; Pape, M.L.; Kjeldgaard, A.F.; Zachariae, A.A.; Schneider, C.; Hélix-Nielsen, C.; Zarebska, A.; Smets, B.F. Dewatering methanotrophic enrichments intended for single cell protein production using biomimetic aquaporin forward osmosis membranes. Sep. Purif. Technol. 2020, 235, 116133. [Google Scholar] [CrossRef]
- Liu, K.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Hegab, H.M.; Wimalasiri, Y.; Ginic-Markovic, M.; Zou, L. Improving the fouling resistance of brackish water membranes via surface modification with graphene oxide functionalized chitosan. Desalination 2015, 365, 99–107. [Google Scholar] [CrossRef]
- Su, C.; Yang, H.; Zhao, H.; Liu, Y.; Chen, R. Recyclable and biodegradable superhydrophobic and superoleophilic chitosan sponge for the effective removal of oily pollutants from water. Chem. Eng. J. 2017, 330, 423–432. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-Based Aerogel Membrane for Robust Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Zhang, Y.; Wang, L.; Lv, L.; Ma, X.; Zeng, S.; Wang, H. Biodegradable functional chitosan membrane for enhancement of artemisinin purification. Carbohydr. Polym. 2020, 246, 116590. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, K.; Sudha, P.N.; Sukumaran, A. Novel chitosan based thin sheet nanofiltration membrane for rejection of heavy metal chromium. Int. J. Biol. Macromol. 2019, 132, 939–953. [Google Scholar]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, J.; Huang, J.; Zhang, H.; Lin, S.; Chen, L.; Ni, Y.; Huang, L. A chitosan/dopamine-TiO2 composite nanofiltration membrane for antifouling in water purification. Cellulose 2021, 28, 4959–4973. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Meng, F.; Zhang, H.; Xue, Y.; Fu, G. High flux and antifouling filtration membrane based on non-woven fabric with chitosan coating for membrane bioreactors. Bioresour. Technol. 2010, 101, 5469–5474. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Liu, R.; Xu, X.; Zhuang, X.; Cheng, B. Solution blowing of chitosan/PVA hydrogel nanofiber mats. Carbohydr. Polym. 2014, 101, 1116–1121. [Google Scholar] [CrossRef]
- Rafique, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef]
- Cao, N.; Lyu, Q.; Li, J.; Wang, Y.; Yang, B.; Szunerits, S.; Boukherroub, R. Facile synthesis of fluorinated polydopamine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem. Eng. J. 2017, 326, 17–28. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Gholap, S.G.; Badiger, M.V.; Gopinath, C.S. Molecular Origins of Wettability of Hydrophobic Poly(vinylidene fluoride) Microporous Membranes on Poly(vinyl alcohol) Adsorption: Surface and Interface Analysis by XPS. J. Phys. Chem. B 2005, 109, 13941–13947. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.F.; Lalia, B.S.; Hashaikeh, R. Controlling swelling behavior of poly (vinyl) alcohol via networked cellulose and its application as a reverse osmosis membrane. Desalination 2014, 336, 138–145. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous Polyvinyl Alcohol Membranes: Preparation Methods and Applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Na, L.; Zhongzhou, L.; Shuguang, X. Dynamically formed poly (vinyl alcohol) ultrafiltration membranes with good anti-fouling characteristics. J. Membr. Sci. 2000, 169, 17–28. [Google Scholar] [CrossRef]
- Razmgar, M.N. Polyvinyl alcohol-based membranes for filtration of aqueous solutions: A comprehensive review. Polym. Eng. Sci. 2021, 62, 25–43. [Google Scholar] [CrossRef]
- Amanda, A.; Mallapragada, S. Comparison of Protein Fouling on Heat-Treated Poly(vinyl alcohol), Poly(ether sulfone) and Regenerated Cellulose Membranes Using Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Biotechnol. Prog. 2001, 17, 917–923. [Google Scholar] [CrossRef]
- Wu, C.; Li, A.; Li, L.; Zhang, L.; Wang, H.; Qi, X.; Zhang, Q. Treatment of oily water by a poly(vinyl alcohol) ultrafiltration membrane. Desalination 2008, 225, 312–321. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Yoon, K.; Hsiao, B.S.; Chu, B. High performance ultrafiltration composite membranes based on poly(vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly(vinyl alcohol) scaffold. J. Membr. Sci. 2006, 278, 261–268. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Highly improved reverse osmosis performance of novel PVA/DGEBA cross-linked membranes by incorporation of Pluronic F-127 and MWCNTs for water desalination. Desalination 2016, 397, 53–66. [Google Scholar] [CrossRef]
- An, Q.; Li, F.; Ji, Y.; Chen, H. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Membr. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Akther, N.; Ali, S.M.; Phuntsho, S.; Shon, H. Surface modification of thin-film composite forward osmosis membranes with polyvinyl alcohol–graphene oxide composite hydrogels for antifouling properties. Desalination 2020, 491, 114591. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Pervaiz, E.; Hussain, A.; Mehran, M.T. Enhancing the Thermal, Mechanical and Swelling Properties of PVA/Starch Nanocomposite Membranes Incorporating g-C3N4. J. Polym. Environ. 2020, 28, 100–115. [Google Scholar] [CrossRef]
- Qing, W.; Li, X.; Wu, Y.; Shao, S.; Guo, H.; Yao, Z.; Chen, Y.; Zhang, W.; Tang, C.Y. In situ silica growth for superhydrophilic-underwater superoleophobic Silica/PVA nanofibrous membrane for gravity-driven oil-in-water emulsion separation. J. Membr. Sci. 2020, 612, 118476. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P. Fabrication of cellulose acetate-zirconia hybrid membranes for ultrafiltration applications: Performance, structure and fouling analysis. Sep. Purif. Technol. 2010, 74, 230–235. [Google Scholar] [CrossRef]
- Vetrivel, S.; Saraswathi, M.S.A.; Rana, D.; Nagendran, A. Fabrication of cellulose acetate nanocomposite membranes using 2D layered nanomaterials for macromolecular separation. Int. J. Biol. Macromol. 2018, 107, 1607–1612. [Google Scholar] [CrossRef]
- Ye, X.; Xiao, H.; Wang, Y.; Ke, L.; Luo, W.; Huang, X.; Shi, B. Efficient separation of viscous emulsion through amphiprotic collagen nanofibers-based membrane. J. Membr. Sci. 2019, 588, 117209. [Google Scholar] [CrossRef]
- Pandey, R.P.; Kallem, P.; Rasheed, P.A.; Mahmoud, K.A.; Banat, F.; Lau, W.J.; Hasan, S.W. Enhanced water flux and bacterial resistance in cellulose acetate membranes with quaternary ammoniumpropylated polysilsesquioxane. Chemosphere 2022, 289, 133144. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Barikani, M.; Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. Part B Eng. 2019, 161, 320–327. [Google Scholar] [CrossRef]
- El-Din, L.N.; El-Gendi, A.; Ismail, N.; Abed, K.; Ahmed, A.I. Evaluation of cellulose acetate membrane with carbon nanotubes additives. J. Ind. Eng. Chem. 2015, 26, 259–264. [Google Scholar] [CrossRef]
- Janeca, A.; Rodrigues, F.S.C.; Gonçalves, M.C.; Faria, M. Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions. Membranes 2021, 11, 825. [Google Scholar] [CrossRef]
- Rajesh, S.; Jayalakshmi, A.; Senthilkumar, S.; Sankar, H.S.H.; Mohan, D.R. Performance Evaluation of Poly(amide-imide) Incorporated Cellulose Acetate Ultrafiltration Membranes in the Separation of Proteins and Its Fouling Propensity by AFM Imaging. Ind. Eng. Chem. Res. 2011, 50, 14016–14029. [Google Scholar] [CrossRef]
- Yin, J.; Fan, H.; Zhou, J. Cellulose acetate/poly(vinyl alcohol) and cellulose acetate/crosslinked poly(vinyl alcohol) blend membranes: Preparation, characterization, and antifouling properties. Desalination Water Treat. 2016, 57, 10572–10584. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Macni, C.R.M.; Caparanga, A.R.; Huang, S.-H.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Mitigating the fouling of mixed-matrix cellulose acetate membranes for oil–water separation through modification with polydopamine particles. Chem. Eng. Res. Des. 2020, 159, 195–204. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Yang, F. Exploration of permeability and antifouling performance on modified cellulose acetate ultrafiltration membrane with cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 190–199. [Google Scholar] [CrossRef]
- Su, J.; Yang, Q.; Teo, J.F.; Chung, T.-S. Cellulose acetate nanofiltration hollow fiber membranes for forward osmosis processes. J. Membr. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Shang, M.; Shi, B. Study on preparation and performances of cellulose acetate forward osmosis membrane. Chem. Pap. 2018, 72, 3159–3167. [Google Scholar] [CrossRef]
- Li, F.; Fei, P.; Cheng, B.; Meng, J.; Liao, L. Synthesis, characterization and excellent antibacterial property of cellulose acetate reverse osmosis membrane via a two-step reaction. Carbohydr. Polym. 2019, 216, 312–321. [Google Scholar] [CrossRef]
- Tahazadeh, S.; Mohammadi, T.; Tofighy, M.A.; Khanlari, S.; Karimi, H.; Emrooz, H.B.M. Development of cellulose acetate/metal-organic framework derived porous carbon adsorptive membrane for dye removal applications. J. Membr. Sci. 2021, 638, 119692. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Mohamed, I.M.; Huang, W.; Liu, C. Membrane fouling mitigation for enhanced water flux and high separation of humic acid and copper ion using hydrophilic polyurethane modified cellulose acetate ultrafiltration membranes. React. Funct. Polym. 2020, 150, 104538. [Google Scholar] [CrossRef]
- Ye, S.H.; Watanabe, J.; Iwasaki, Y.; Ishihara, K. Antifouling blood purification membrane composed of cellulose acetate and phospholipid polymer. Biomaterials 2003, 24, 4143–4152. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, Y.; Zheng, W.; Yu, H.; Liu, Y.; Xu, L. Asymmetric Sc-PLA Membrane with Multi-scale Microstructures: Wettability, Antifouling, and Oil–Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 55520–55526. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Hegab, H.M.; Nassar, L.; Wadi, V.S.; Naddeo, V.; Yousef, A.F.; Banat, F.; Hasan, S.W. Asymmetrical ultrafiltration membranes based on polylactic acid for the removal of organic substances from wastewater. J. Water Process Eng. 2022, 45, 102510. [Google Scholar] [CrossRef]
- Alam, A.K.M.M.; Ewaldz, E.; Xiang, C.; Qu, W.; Bai, X. Tunable Wettability of Biodegradable Multilayer Sandwich-Structured Electrospun Nanofibrous Membranes. Polymers 2020, 12, 2092. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Nasef, M.M.; Fouad, H.; Karim, Z. Poly(lactic acid)/poly(butylene succinate) dual-layer membranes with cellulose nanowhisker for heavy metal ion separation. Int. J. Biol. Macromol. 2021, 192, 654–664. [Google Scholar] [CrossRef]
- Dasari, A.; Quirós, J.; Herrero, B.; Boltes, K.; García-Calvo, E.; Rosal, R. Antifouling membranes prepared by electrospinning polylactic acid containing biocidal nanoparticles. J. Membr. Sci. 2012, 405, 134–140. [Google Scholar] [CrossRef]
- Xix-Rodriguez, C.; Varguez-Catzim, P.; Alonzo-García, A.; Rodriguez-Fuentes, N.; Vázquez-Torres, H.; González-Diaz, A.; Aguilar-Vega, M.; González-Díaz, M.O. Amphiphilic poly(lactic acid) membranes with low fouling and enhanced hemodiafiltration. Sep. Purif. Technol. 2021, 259, 118124–118135. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, H.; Liu, F.; Yu, X.; Wang, Y.; Wang, Y. A new strategy to simultaneously improve the permeability, heat-deformation resistance and antifouling properties of polylactide membrane via bio-based β-cyclodextrin and surface crosslinking. J. Membr. Sci. 2016, 513, 166–176. [Google Scholar] [CrossRef]
- Ao, C.; Zhao, J.; Li, Q.; Zhang, J.; Huang, B.; Wang, Q.; Gai, J.; Chen, Z.; Zhang, W.; Lu, C. Biodegradable all-cellulose composite membranes for simultaneous oil/water separation and dye removal from water. Carbohydr. Polym. 2020, 250, 116872. [Google Scholar] [CrossRef]
- Abolhassani, M.; Griggs, C.S.; Gurtowski, L.A.; Mattei-Sosa, J.A.; Nevins, M.; Medina, V.F.; Morgan, T.A.; Greenlee, L.F. Scalable Chitosan-Graphene Oxide Membranes: The Effect of GO Size on Properties and Cross-Flow Filtration Performance. ACS Omega 2017, 2, 8751–8759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xiao, P.; Chen, P.; Zhang, L.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Chen, T. Functionalization of Biodegradable PLA Nonwoven Fabric as Superoleophilic and Superhydrophobic Material for Efficient Oil Absorption and Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 5968–5973. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Lin, H.; Zhong, Y.; Qin, Y.; Li, T.; Liu, F. Robust superhydrophilic polylactide (PLA) membranes with a TiO2 nano-particle inlaid surface for oil/water separation. J. Mater. Chem. A 2017, 5, 6538–6545. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Yuan, H.; Su, M.; Shao, C.; Liu, C.; Guo, Z.; Shen, C.; Liu, X. Simple fabrication of superhydrophobic PLA with honeycomb-like structures for high-efficiency oil-water separation. Chin. Chem. Lett. 2020, 31, 365–368. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Zhang, Y.; Gao, K.; Dai, L.; Shen, M.; Wang, W.; Wang, J.; Ni, Y. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr(VI) reduction. Carbohydr. Polym. 2021, 258, 117676. [Google Scholar] [CrossRef]
- Kollarigowda, R.H.; Abraham, S.; Montemagno, C.D. Antifouling Cellulose Hybrid Biomembrane for Effective Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 29812–29819. [Google Scholar] [CrossRef]
- Kian, L.; Jawaid, M.; Alamery, S.; Vaseashta, A. Fabrication and Characterization of Novel Poly(D-lactic acid) Nanocomposite Membrane for Water Filtration Purpose. Nanomaterials 2021, 11, 255. [Google Scholar] [CrossRef]
- Kumar, M.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef]
- Abu-Saied, M.; Wycisk, R.; Abbassy, M.M.; El-Naim, G.A.; El-Demerdash, F.; Youssef, M.; Bassuony, H.; Pintauro, P.N. Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions—Fabrication and sorption studies. Carbohydr. Polym. 2017, 165, 149–158. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mousavi, S.M.; Saljoughi, E.; Kiani, S. Biodegradable membrane based on polycaprolactone/polybutylene succinate: Characterization and performance evaluation in wastewater treatment. J. Appl. Polym. Sci. 2021, 138, e50332–e50345. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, R.; Yang, X.; Chen, F.; Ning, X. Bicomponent PLA Nanofiber Nonwovens as Highly Efficient Filtration Media for Particulate Pollutants and Pathogens. Membranes 2021, 11, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Bahremand, A.H.; Mousavi, S.M.; Ahmadpour, A.; Taherian, M. Biodegradable blend membranes of poly (butylene succinate)/cellulose acetate/dextran: Preparation, characterization and performance. Carbohydr. Polym. 2017, 173, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, H.; Maghami, S.; Isfahani, A.P.; Sadeghi, M. Influence of Blend Composition and Silica Nanoparticles on the Morphology and Gas Separation Performance of PU/PVA Blend Membranes. Membranes 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Xuan, H.; Ge, L. Double network self-healing chitosan/dialdehyde starch-polyvinyl alcohol film for gas separation. Appl. Surf. Sci. 2019, 469, 213–219. [Google Scholar] [CrossRef]
- Tirouni, I.; Sadeghi, M.; Pakizeh, M. Separation of C3H8 and C2H6 from CH4 in polyurethane–zeolite 4Å and ZSM-5 mixed matrix membranes. Sep. Purif. Technol. 2015, 141, 394–402. [Google Scholar] [CrossRef]
- Isfahani, A.P.; Ghalei, B.; Wakimoto, K.; Bagheri, R.; Sivaniah, E.; Sadeghi, M. Plasticization resistant crosslinked polyurethane gas separation membranes. J. Mater. Chem. A 2016, 4, 17431–17439. [Google Scholar] [CrossRef]
- AlruwailI, B.M.; Saeed, U.; Ahmad, I.; Al-Turaif, H.; Aboalkhair, H.; AlsaiarI, A.O. Development of Multiwalled Carbon Nanotube-Reinforced Biodegradable Polylactic Acid/Polybutylene Succinate Blend Membrane. Membranes 2021, 11, 760. [Google Scholar] [CrossRef]
- Koh, H.C.; Park, J.S.; Jeong, M.A.; Hwang, H.Y.; Hong, Y.T.; Ha, S.Y.; Nam, S.Y. Preparation and gas permeation properties of biodegradable polymer/layered silicate nanocomposite membranes. Desalination 2008, 233, 201–209. [Google Scholar] [CrossRef]
- Jiamjirangkul, P.; Inprasit, T.; Intasanta, V.; Pangon, A. Metal organic framework-integrated chitosan/poly(vinyl alcohol) (PVA) nanofibrous membrane hybrids from green process for selective CO2 capture and filtration. Chem. Eng. Sci. 2020, 221, 115650. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Sudesh, K.; Tan, S.H. Poly(3-hydroxybutyrate)-functionalised multi-walled carbon nanotubes/chitosan green nanocomposite membranes and their application in pervaporation. Sep. Purif. Technol. 2011, 76, 419–427. [Google Scholar] [CrossRef]
- Mokhtarzadeh, S.; Hakimpour, F.; Sarvari, R.; Agbolaghi, S.; Mansourpanah, Y. Nanocomposite membranes based on sodium alginate/poly(ε-caprolactone)/graphene oxide for methanol, ethanol and isopropanol dehydration via pervaporation. Polym. Bull. 2020, 77, 3367–3387. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Anjanapura, R.V.; Han, J.M.; Aminabhavi, T.M. Functionalized Graphene Sheets Embedded in Chitosan Nanocomposite Membranes for Ethanol and Isopropanol Dehydration via Pervaporation. Ind. Eng. Chem. Res. 2014, 53, 14474–14484. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Potential of chitosan-based membranes for the separation of essential oil components by target-organophilic pervaporation. Carbohydr. Polym. 2020, 247, 116676. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.; Adhikari, B. Porous polyurethane urea membranes for pervaporation separation of phenol and chlorophenols from water. Chem. Eng. J. 2008, 138, 215–223. [Google Scholar] [CrossRef]
- Additive, O.; Methyl, M. Application of Polymer Membranes for a Purification. Polymer 2020, 12, 2218. [Google Scholar]
- Moulik, S.; Bukke, V.; Sajja, S.C.; Sridhar, S. Chitosan-polytetrafluoroethylene composite membranes for separation of methanol and toluene by pervaporation. Carbohydr. Polym. 2018, 193, 28–38. [Google Scholar] [CrossRef]
- Tomietto, P.; Russo, F.; Galiano, F.; Loulergue, P.; Salerno, S.; Paugam, L.; Audic, J.-L.; De Bartolo, L.; Figoli, A. Sustainable fabrication and pervaporation application of bio-based membranes: Combining a polyhydroxyalkanoate (PHA) as biopolymer and Cyrene™ as green solvent. J. Membr. Sci. 2022, 643, 120061. [Google Scholar] [CrossRef]
- Chen, X.; Li, J. Bioinspired by cell membranes: Functional polymeric materials for biomedical applications. Mater. Chem. Front. 2020, 4, 750–774. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers—A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Krajewska, B. Membrane-based processes performed with use of chitin/chitosan materials. Sep. Purif. Technol. 2005, 41, 305–312. [Google Scholar] [CrossRef]
- Muzzarelli, C.; Muzzarelli, R.A. Natural and artificial chitosan–inorganic composites. J. Inorg. Biochem. 2002, 92, 89–94. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and biodegradable chitosan/agarose film revealing slightly acidic pH for potential applications in regenerative medicine as artificial skin graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef]
- Bernards, D.A.; Desai, T.A. Nanotemplating of Biodegradable Polymer Membranes for Constant-Rate Drug Delivery. Adv. Mater. 2010, 22, 2358–2362. [Google Scholar] [CrossRef]
- Gao, A.; Liu, F.; Xue, L. Preparation and evaluation of heparin-immobilized poly (lactic acid) (PLA) membrane for hemodialysis. J. Membr. Sci. 2014, 452, 390–399. [Google Scholar] [CrossRef]
- Ma, L.; Huang, L.; Zhang, Y.; Zhao, L.; Xin, Q.; Ye, H.; Li, H. Hemocompatible poly(lactic acid) membranes prepared by immobilizing carboxylated graphene oxide via mussel-inspired method for hemodialysis. RSC Adv. 2018, 8, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Song, X.; Ji, H.; Zhao, W.; Sun, S.; Zhao, C. Hemocompatibility enhancement of polyethersulfone membranes: Strategies and challenges. Adv. Membr. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2021, 9, 1438. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2020, 113, 106530. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Eldin, R.E.S.; Serea, E.S.A.; Gomaa, N.M.; Aboelmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Ishak, M.R. Water barrier properties of biodegradable films reinforced with nanocellulose for food packaging application: A review. In Proceedings of the 6th Postgraduate Seminar on Natural Fiber Reinforced Polymer Composites, Selangor, Malaysia, 4 December 2018; pp. 55–59. [Google Scholar]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849–104860. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.; Dutta, P. Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Râpă, M.; Miteluţ, A.C.; Tănase, E.E.; Grosu, E.; Popescu, P.; Popa, M.E.; Rosnes, J.T.; Sivertsvik, M.; Darie-Niţă, R.N.; Vasile, C. Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos. Part B Eng. 2016, 102, 112–121. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2020, 60, 171–202. [Google Scholar] [CrossRef]
- Modi, J.; Choumal, A.; Vyas, D.; Shah, D.; Joshi, K.; Patel, K.; Iyer, K. Materials Today: Proceedings Sustainable technology for modern era effluent treatment: Microbial fuel cell. Mater. Today: Proc. 2022, 57, 1781–1788. [Google Scholar]
- Liu, Q.; Lan, F.; Chen, J.; Zeng, C.; Wang, J. A review of proton exchange membrane fuel cell water management: Membrane electrode assembly. J. Power Sources 2022, 517, 230723. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- González-Pabón, M.J.; Figueredo, F.; Martínez-Casillas, D.C.; Cortón, E. High-performance biodegradable membrane for point of need paper-based micro-scale microbial fuel cell analytical devices. bioRxiv 2018, 351890. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Smitha, B.; Sridhar, S.; Khan, A.A. Synthesis and characterization of poly(vinyl alcohol)-based membranes for direct methanol fuel cell. J. Appl. Polym. Sci. 2005, 95, 1154–1163. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A. Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Umar, M.; Nawaz, H.; Gong, H.; Li, J. Cross-linked chitosan coated biodegradable porous electrospun membranes for the removal of synthetic dyes. React. Funct. Polym. 2021, 166, 104995. [Google Scholar] [CrossRef]
- Rahaman, H.; Islam, A.; Islam, M.; Rahman, A.; Alam, S.N. Biodegradable composite adsorbent of modified cellulose and chitosan to remove heavy metal ions from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Torstensen, J.; Helberg, R.M.; Deng, L.; Gregersen, W.; Syverud, K. PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int. J. Greenh. Gas Control 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Serafin, J.; Antosik, A.; Wilpiszewska, K.; Czech, Z. Preparation of Activated Carbon from the Biodegradable film for CO2 Capture Applications. Pol. J. Chem. Technol. 2018, 20, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Langari, S.; Saljoughi, E.; Mousavi, S.M. Chitosan/polyvinyl alcohol/amino functionalized multiwalled carbon nanotube pervaporation membranes: Synthesis, characterization, and performance. Polym. Adv. Technol. 2018, 29, 84–94. [Google Scholar] [CrossRef]
- Galiano, F.; Ghanim, A.H.; Rashid, K.T.; Marino, T.; Simone, S.; Alsalhy, Q.F.; Figoli, A. Preparation and characterization of green polylactic acid (PLA) membranes for organic/organic separation by pervaporation. Clean Technol. Environ. Policy 2019, 21, 109–120. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Gu, J.; Sun, Y.; Ji, X. Cross-linking of poly(vinyl alcohol) with N,N′-methylene bisacrylamide via a radical reaction to prepare pervaporation membranes. RSC Adv. R. Soc. Chem. 2015, 5, 19859–19864. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Wang, J.; Li, S.-Y.; Suen, S.-Y. Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications. Membranes 2021, 11, 970. [Google Scholar] [CrossRef]
- Msahel, A.; Galiano, F.; Pilloni, M.; Russo, F.; Hafiane, A.; Castro-Muñoz, R.; Kumar, V.; Gedanken, A.; Ennas, G.; Porat, Z.; et al. Exploring the Effect of Iron Metal-Organic Framework Particles in Polylactic Acid Membranes for the Azeotropic Separation of Organic/Organic Mixtures by Pervaporation. Membranes 2021, 11, 65. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Masaki, K.; Ishikawa, M. Pervaporation separation of aqueous organic mixtures through agarose membranes. J. Membr. Sci. 2002, 205, 293–300. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S.I.; Cho, C.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar] [CrossRef]
- Chen, S.; Hao, Y.; Cui, W.; Chang, J.; Zhou, Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J. Mater. Sci. 2013, 48, 6567–6577. [Google Scholar] [CrossRef] [Green Version]
- Piscioneri, A.; Campana, C.; Salerno, S.; Morelli, S.; Bader, A.; Giordano, F.; Drioli, E.; De Bartolo, L. Biodegradable and synthetic membranes for the expansion and functional differentiation of rat embryonic liver cells. Acta Biomater. 2011, 7, 171–179. [Google Scholar] [CrossRef]
- Li, F.; Su, Y.; Pi, G.; Ma, P.X.; Lei, B. Biodegradable, Biomimetic Elastomeric, Photoluminescent, and Broad-Spectrum Antibacterial Polycitrate-Polypeptide-based Membrane toward Multifunctional Biomedical Implants. ACS Biomater. Sci. Eng. 2018, 4, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, Y.; Yang, X.; Mei, F.; Ma, Q.; Chen, G.; Ryu, S.; Deng, X. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J. Biomed. Mater. Res. Part A 2009, 90A, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Guo, B.; Shao, Y.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Wang, S.A. Preparation of graphene-based poly(vinyl alcohol)/chitosan nanocomposites membrane for alkaline solid electrolytes membrane. J. Membr. Sci. 2015, 477, 49–57. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y.; Buchheit, R.G. A high performance direct borohydride fuel cell employing cross-linked chitosan membrane. J. Power Sources 2011, 196, 8257–8264. [Google Scholar] [CrossRef]
- Kalaiselvimary, J.; Prabhu, M.R. Fabrications and investigation of physicochemical and electrochemical properties of heteropoly acid-doped sulfonated chitosan-based polymer electrolyte membranes for fuel cell applications. Polym. Bull. 2019, 76, 1401–1422. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N.; Masdar, M.S. New composite membrane poly(vinyl alcohol)/graphene oxide for direct ethanol–proton exchange membrane fuel cell. J. Appl. Polym. Sci. 2019, 136, 46928. [Google Scholar] [CrossRef]
- Harewood, A.J.T.; Popuri, S.R.; Cadogan, E.I.; Lee, C.-H.; Wang, C.-C. Bioelectricity generation from brewery wastewater in a microbial fuel cell using chitosan/biodegradable copolymer membrane. Int. J. Environ. Sci. Technol. 2017, 14, 1535–1550. [Google Scholar] [CrossRef]
- Azevedo, H.; Reis, R. Understanding the Enzymatic Degradation of Biodegradable Polymers and Strategies to Control Their Degradation Rate. In Biodegradable Systems in Tissue Engineering and Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2004; pp. 177–202. [Google Scholar]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Amulya, K.; Katakojwala, R.; Ramakrishna, S.; Mohan, S.V. Low carbon biodegradable polymer matrices for sustainable future. Compos. Part C Open Access 2021, 4, 100111. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Nikhil, G.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Patel, F.M.-W.M.; Schleich, J.; Hüsing, B.; Angerer, G.; Wolf, O.; Crank, M. Techno-economic Feasibility of Large-scale Production of Bio-based Polymers in Europe, Institute for Prospective Technological Studies. Teh. Rep. EUR 2005, 22103. Available online: https://bioplastique-gmat.weebly.com/uploads/1/4/2/0/14200175/feasibility_of_production_of_bio-based_polymers_in_europe.pdf (accessed on 23 July 2021).

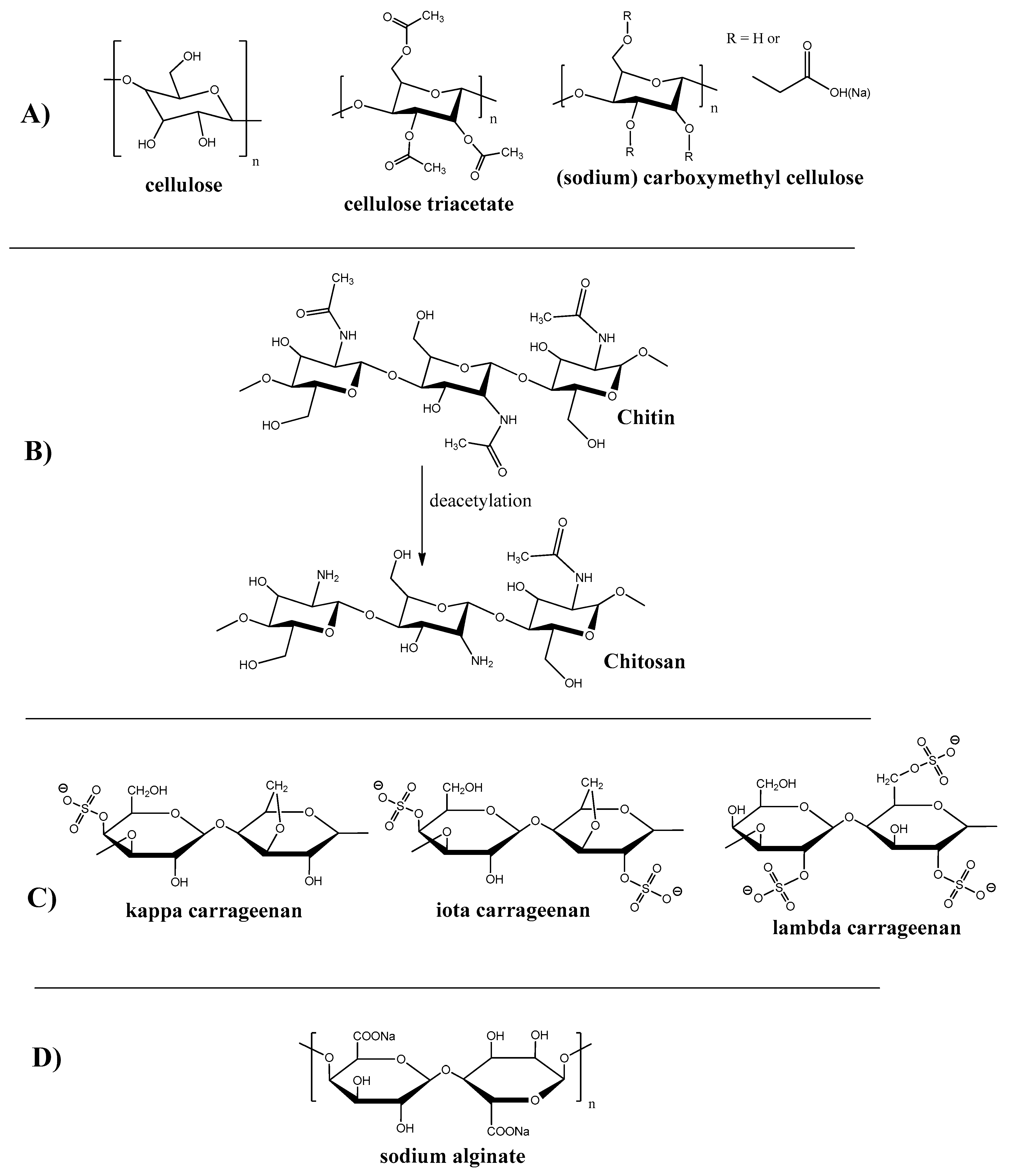
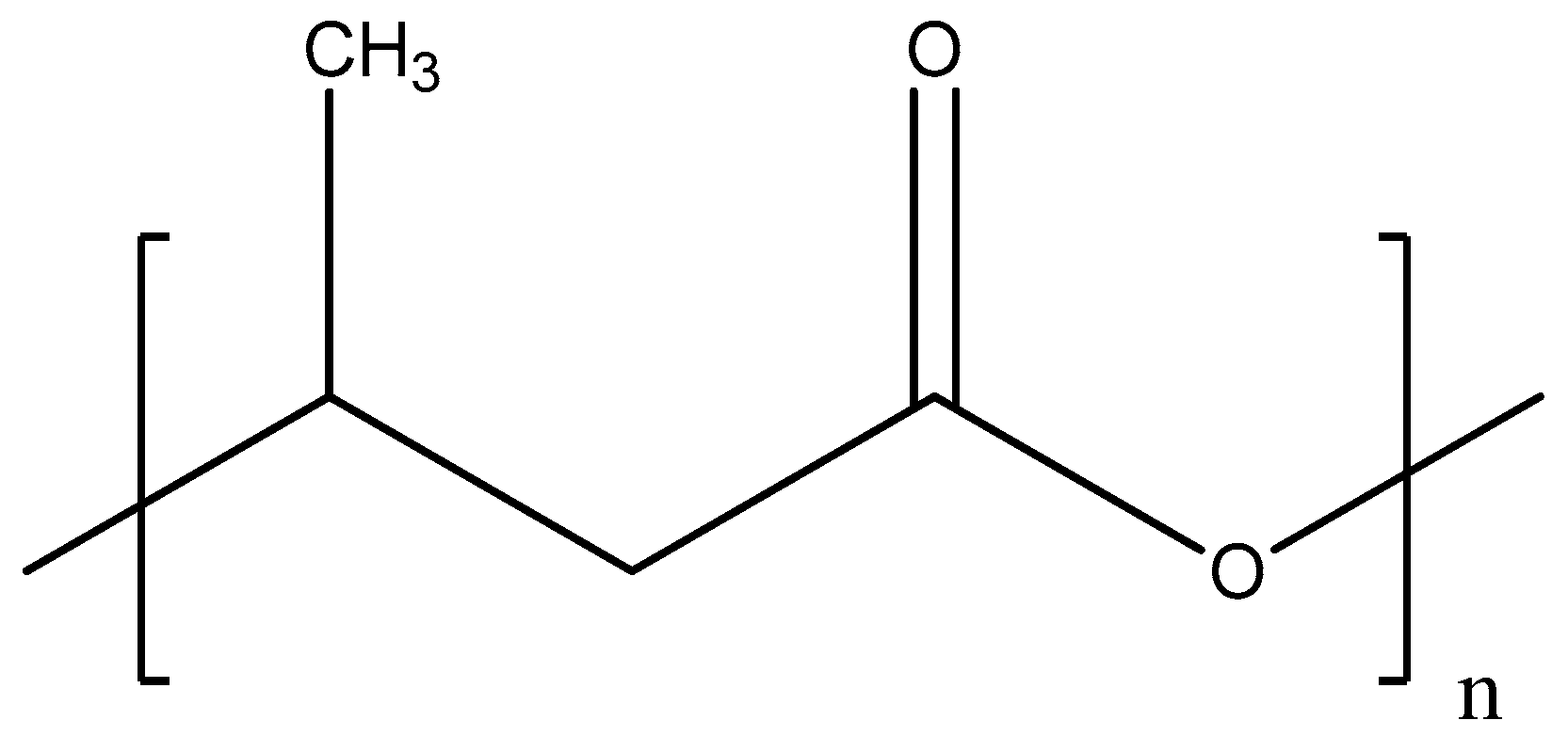
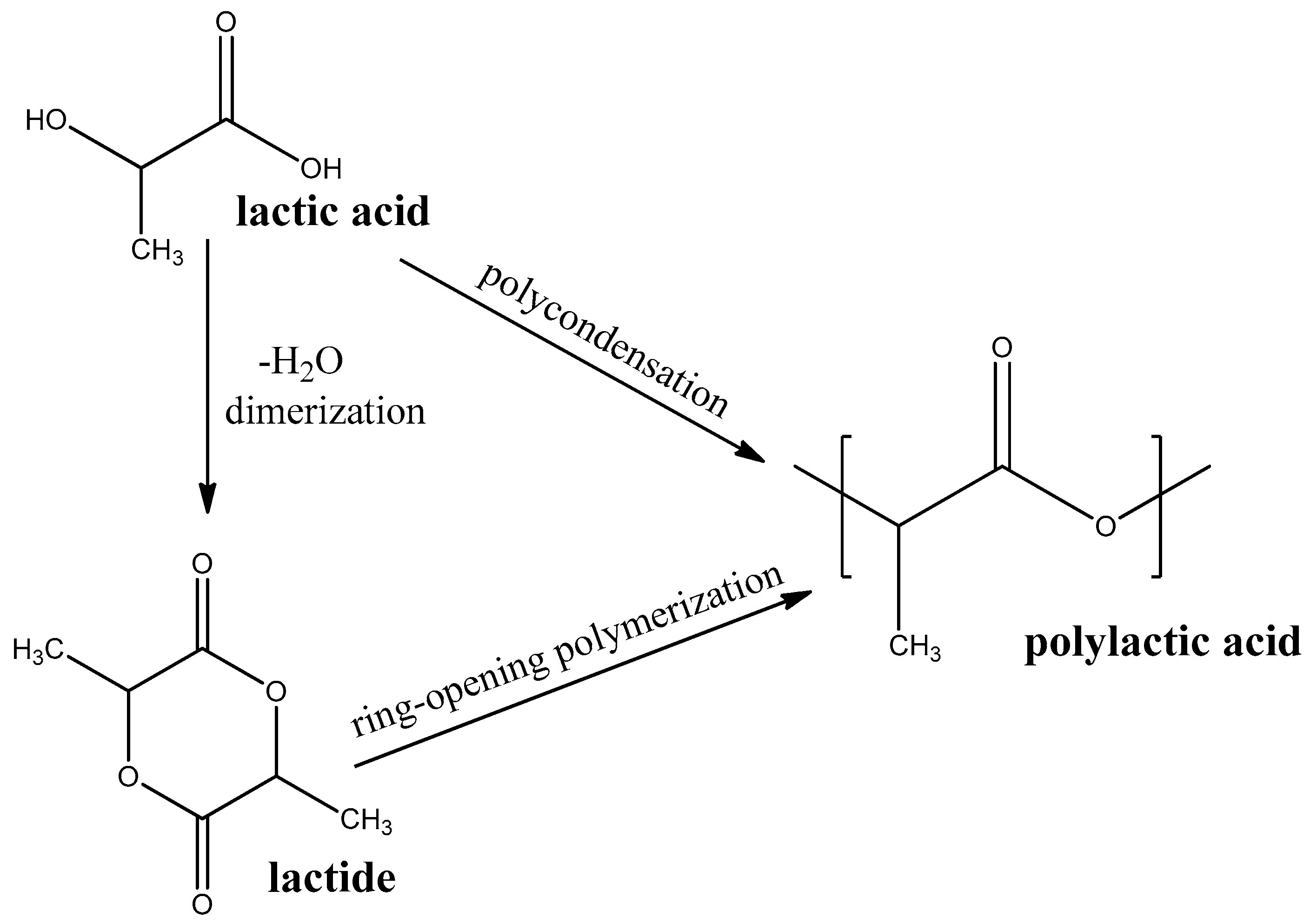
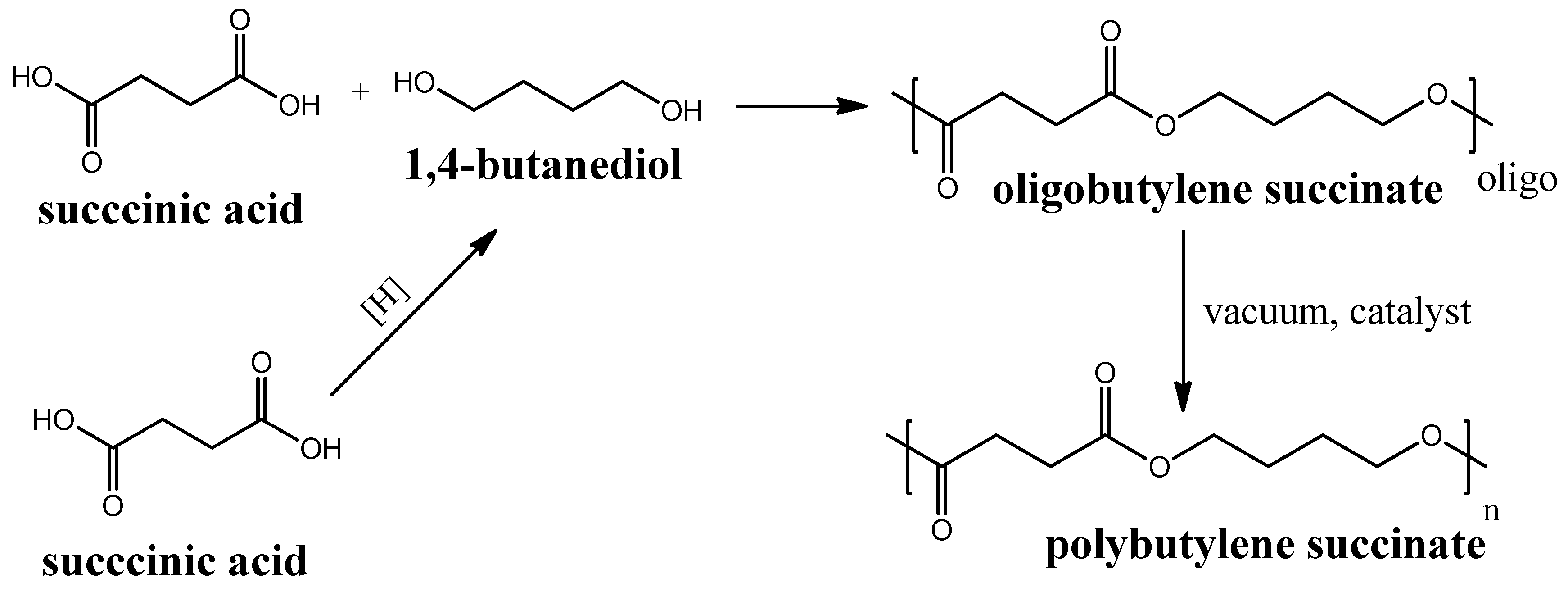
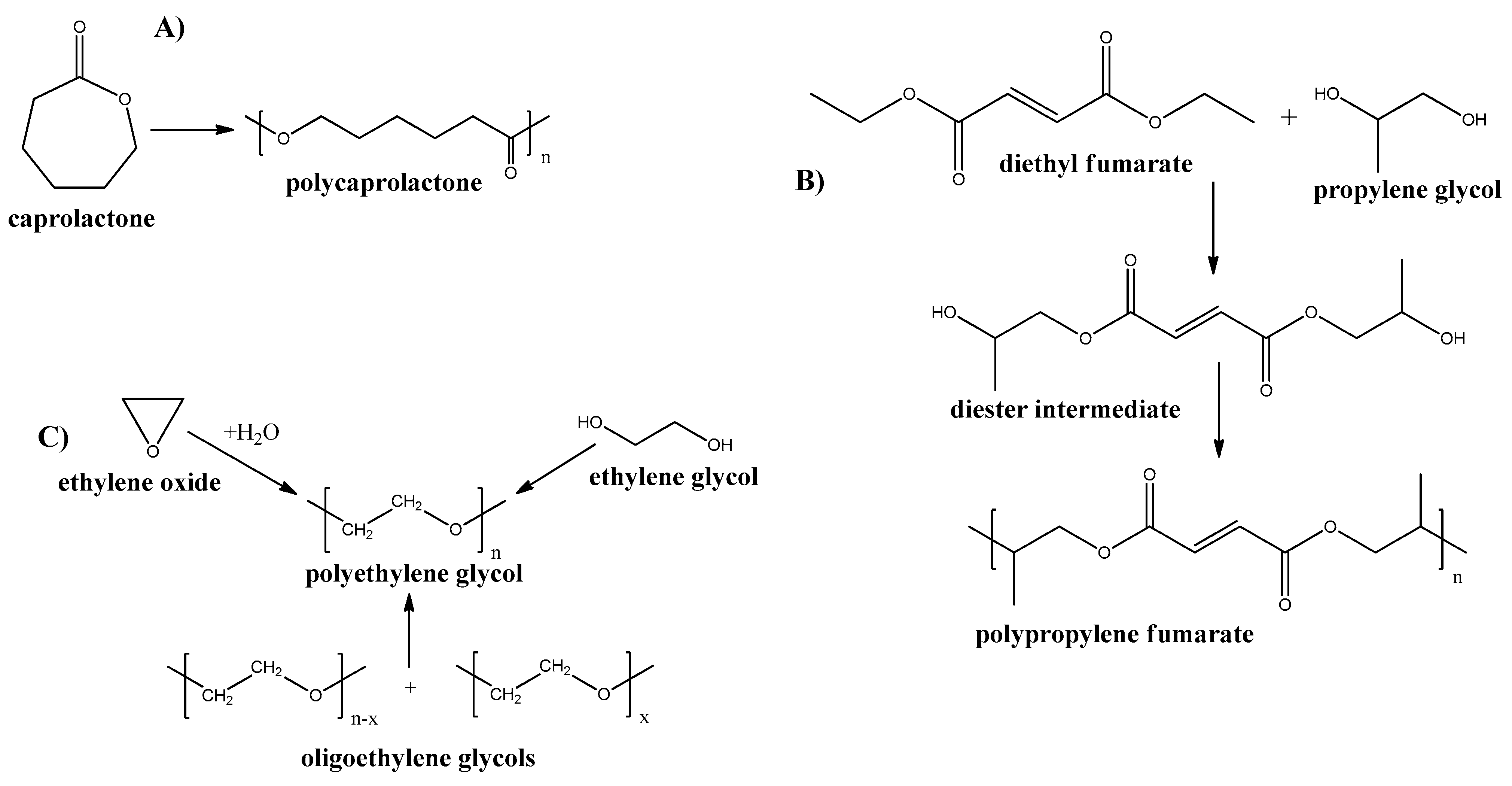
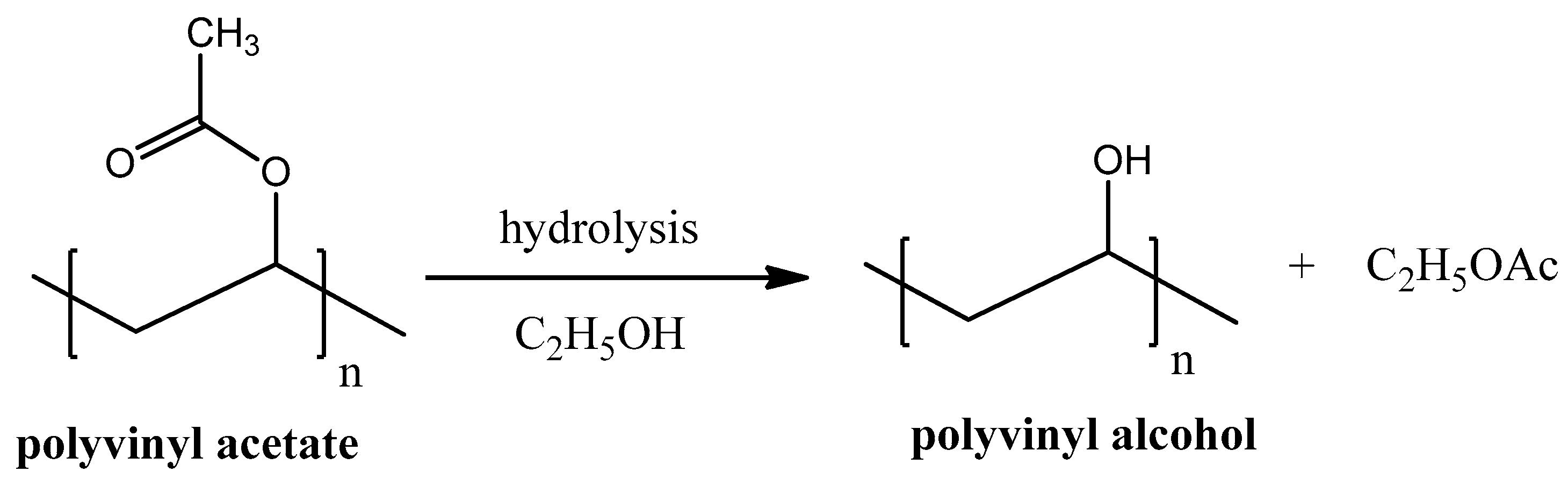

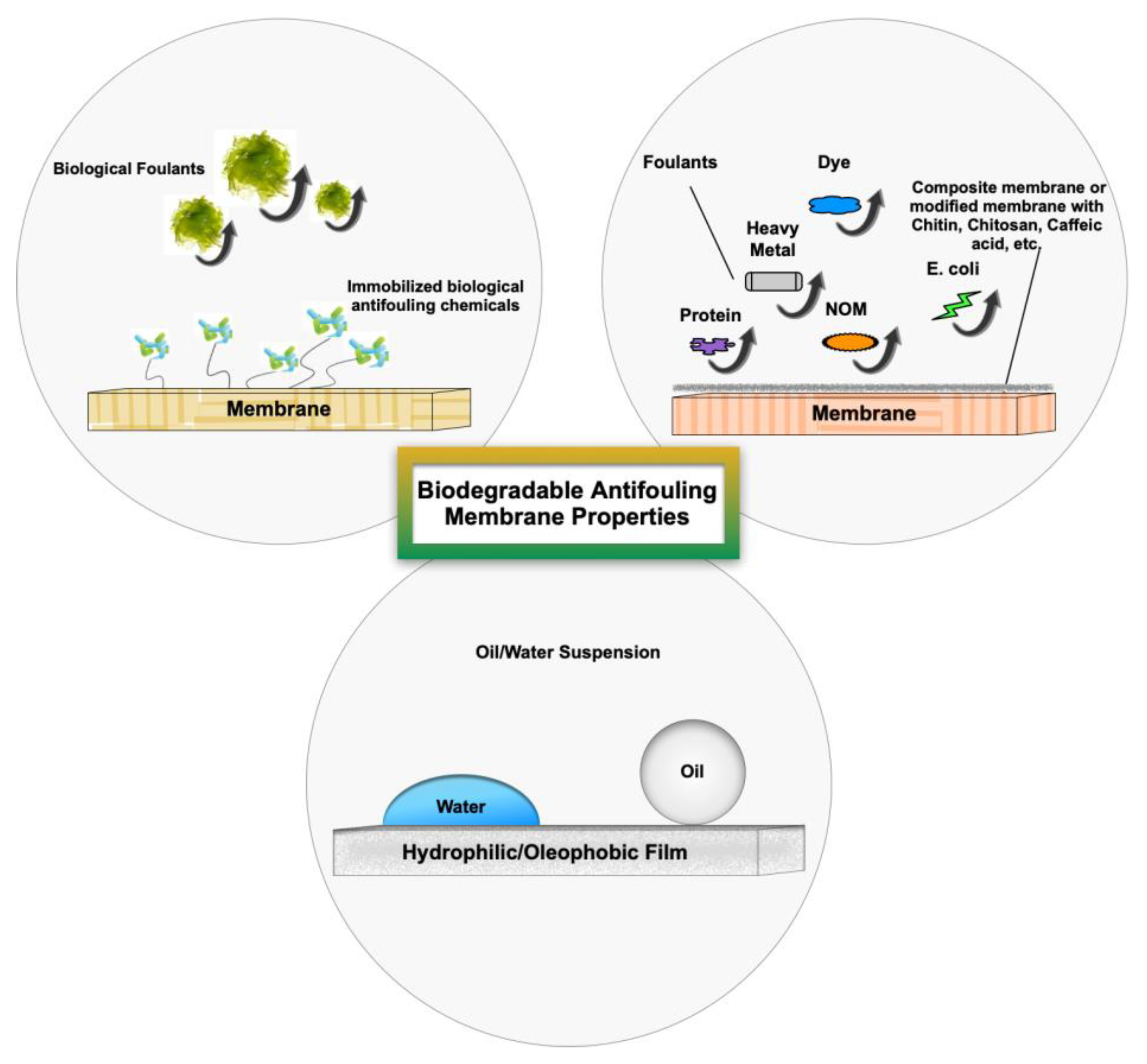
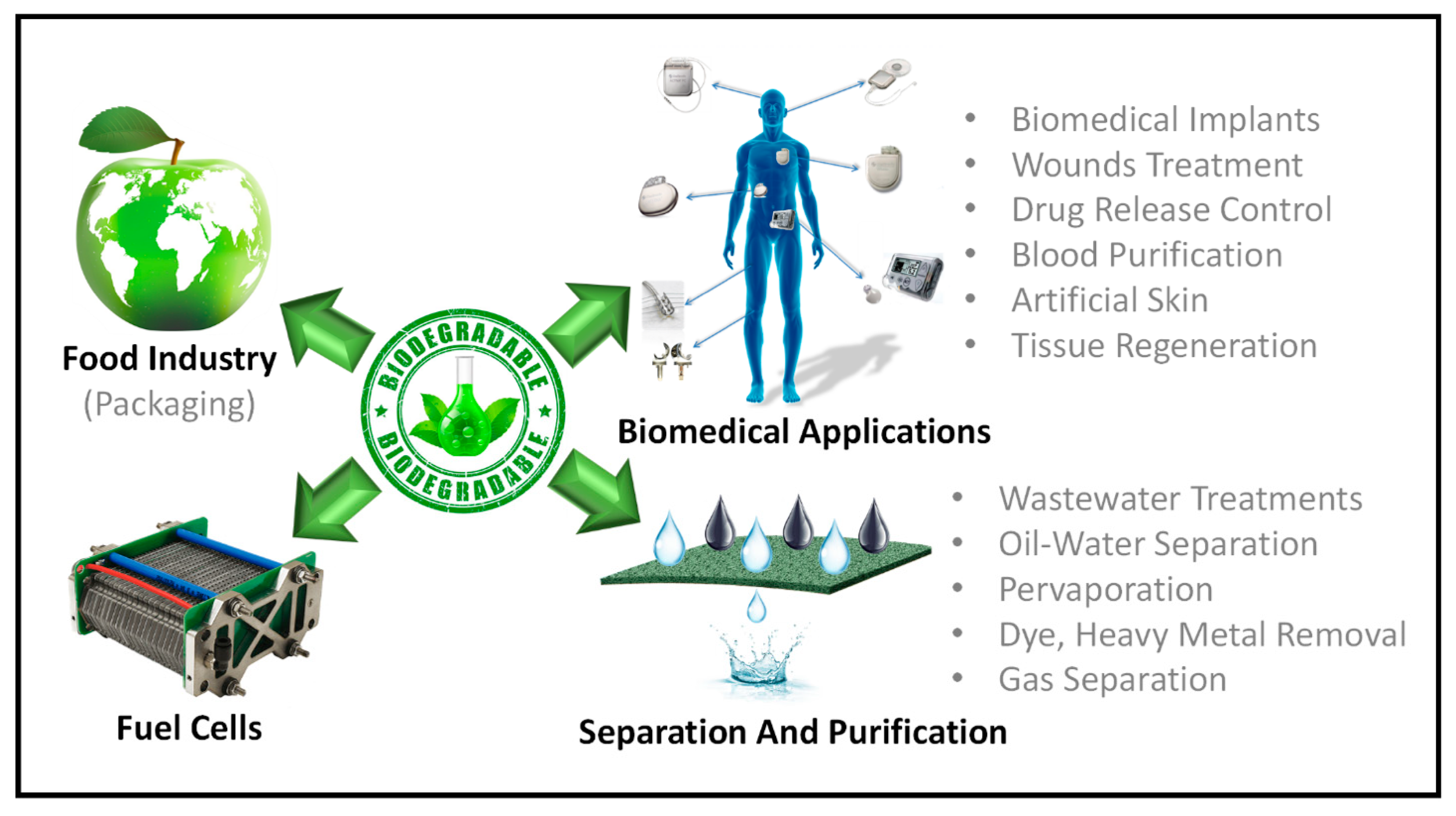
| No. | Membrane Material | Synthesis Method | Main Applications |
|---|---|---|---|
| Bio-sourced | |||
| 1 | Cellulose derivatives (acetates, carboxymethyl etc.) | Processing of cellulose containing biomaterials (plants, trees) | Water treatment and separation, gas separation, biomedical applications |
| 2 | Chitin and chitosan | Processing of chitin containing biomaterials (insects, shrimps, crabs etc.). Deacetylation results in chitosan | Ion exchange, water treatment and separation, dye removal |
| 3 | Carrageenan | Processing of biomaterials (algae) | Dyes and heavy metals removal |
| 4 | Starch | Processing of starch containing biomaterials (potato, corn, etc.) | Used as additive and source of other materials |
| 5 | Cyclodextrin | Enzymatic conversion of starch | Water treatment |
| 6 | Alginate | Processing of biomaterials (algae) | Dyes and heavy metals removal |
| 7 | Silk fibroin | Processing of biomaterials (insects cocoons) | Heavy metals removal |
| 8 | Collagen | Processing of biomaterials (animal connecting tissues) | Water separation, pervaporation |
| 9 | Polyhydroxybutyrate | Glucose and starch conversion by microorganisms | Biomedical application, dye removal, microfiltration |
| Synthesized from bio-sourced chemicals | |||
| 1 | Polylactic acid | Polycondensation or ring-opening polymerization of lactic acid, produced by aerobic fermentation of glucose or starch. | Water separation, tissue regeneration |
| 2 | Polybutylene succinate | Polycondensation of succinic acid and 1,4-butanediol. Succinic acid is produced by biomass fermentation. 1,4-butanediol is produced by succinic acid hydrogenation | Pervaporation |
| Petrochemical | |||
| 1 | Poly-ε-caprolactone | Ring-opening polymerization of ε-caprolactone | Biomedical applications |
| 2 | Polypropylene fumarate | Polycondensation of fumarate with propylene glycol | Biomedical applications |
| 3 | Poly-ethylene glycol | Polycondensation of ethylene glycol or ring-opening polymerization of ethylene oxide | Drug release systems |
| 4 | Poly-vinyl alcohol | Hydrolysis of polyvinyl acetate | Wound dressing |
| 5 | Polyurethane and polyurethane urea | Polycondensation of diisocyanates with diols and diamines | Biomedical application, agriculture |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehsani, M.; Kalugin, D.; Doan, H.; Lohi, A.; Abdelrasoul, A. Bio-Sourced and Biodegradable Membranes. Appl. Sci. 2022, 12, 12837. https://doi.org/10.3390/app122412837
Ehsani M, Kalugin D, Doan H, Lohi A, Abdelrasoul A. Bio-Sourced and Biodegradable Membranes. Applied Sciences. 2022; 12(24):12837. https://doi.org/10.3390/app122412837
Chicago/Turabian StyleEhsani, Masoume, Denis Kalugin, Huu Doan, Ali Lohi, and Amira Abdelrasoul. 2022. "Bio-Sourced and Biodegradable Membranes" Applied Sciences 12, no. 24: 12837. https://doi.org/10.3390/app122412837





