Evaluation of Adsorbent’s Efficiency by Using Biomarker Approaches in Farm Animals: A Systematic Review
Abstract
:1. Introduction
2. Methods
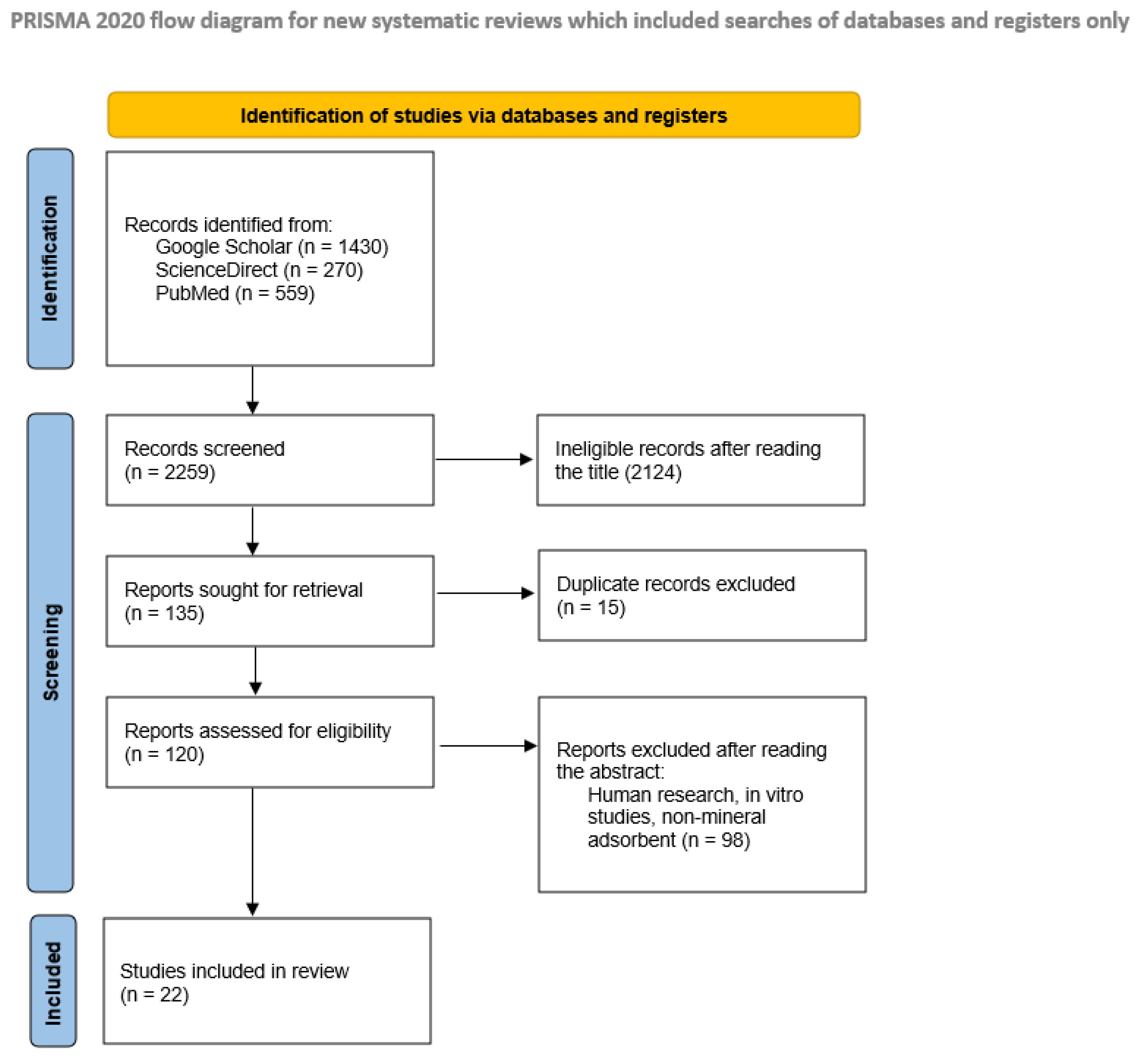
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria and Data Collection
2.3. Calculation of Percent Reduction in Biomarker Concentration Caused by Treatments (RBTC)
3. Study Characteristics
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in Food and Feed. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 89, pp. 297–345. [Google Scholar]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; de Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked Mycotoxins: A Review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.M.Y.; Rivas-Caceres, R.R.; Salem, A.Z.M. Mycotoxin Toxicity and Residue in Animal Products: Prevalence, Consumer Exposure and Reduction Strategies—A Review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-Occurrence of Mycotoxins in Maize Food and Maize-Based Feed from Small-Scale Farms in Brazil: A Pilot Study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarian, M.; Tavakolipour, H.; Bagheri, F.; Fernandes Oliveira, C.A.; Corassin, C.H.; Khaneghah, A.M. Aflatoxin B1 in the Iranian Pistachio Nut and Decontamination Methods: A Systematic Review. Qual. Assur. Saf. Crop. Foods 2020, 12, 15–25. [Google Scholar] [CrossRef]
- Delage, N.; d’Harlingue, A.; Colonna Ceccaldi, B.; Bompeix, G. Occurrence of Mycotoxins in Fruit Juices and Wine. Food Control 2003, 14, 225–227. [Google Scholar] [CrossRef]
- Corassin, C.H.; Borowsky, A.; Ali, S.; Rosim, R.E.; de Oliveira, C.A.F. Occurrence of Aflatoxin M1 in Milk and Dairy Products Traded in São Paulo, Brazil: An Update. Dairy 2022, 3, 842–848. [Google Scholar] [CrossRef]
- Nan, M.; Xue, H.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Batatinha, M.J.; Botura, M.B.; Górniak, S.L. Micotoxinas e Micotoxicoses. In Toxicologia Aplicada à Medicina Veterinária; Manole Santana de Parnaíba: São Paulo, Brazil, 2020; Volume 2, pp. 304–330. ISBN 9788520458976. [Google Scholar]
- Oliveira, C.; Augusto, F.; Bovo, F.; Corassin, C.H.; Jager, A.V.; Reddy, K.R. Recent Trends in Microbiological Decontamination of Aflatoxins in Foodstuffs. In Aflatoxins—Recent Advances and Future Prospects; IntechOpen: Rijeka, Croatia, 2013; pp. 59–92. ISBN 978-953-51-0904-4. [Google Scholar]
- International Agency for Research on Cancer IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; International Agency for Research on Cancer: Lyon, France, 2002; Volume 82, ISBN 9283212827. [Google Scholar]
- Grenier, B.; Loureiro-Bracarense, A.-P.; Lucioli, J.; Pacheco, G.D.; Cossalter, A.-M.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Individual and Combined Effects of Subclinical Doses of Deoxynivalenol and Fumonisins in Piglets. Mol. Nutr. Food Res. 2011, 55, 761–771. [Google Scholar] [CrossRef]
- Miazzo, R.; Peralta, M.F.; Magnoli, C.; Salvano, M.; Ferrero, S.; Chiacchiera, S.M.; Carvalho, E.C.Q.; Rosa, C.A.R.; Dalcero, A. Efficacy of Sodium Bentonite as a Detoxifier of Broiler Feed Contaminated with Aflatoxin and Fumonisin. Poult. Sci. 2005, 84, 1–8. [Google Scholar] [CrossRef]
- Vasiljević, M.; Marinković, D.; Milićević, D.; Pleadin, J.; Stefanović, S.; Trialović, S.; Raj, J.; Petrujkić, B.; Trialović, J.N. Efficacy of a Modified Clinoptilolite Based Adsorbent in Reducing Detrimental Effects of Ochratoxin A in Laying Hens. Toxins 2021, 13, 469. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, C.P.; Frias, N.C.; Carvalho, C.B.; Sá, L.R.M.; Belli, C.B.; Baccarin, R.Y.A. Leukoencephalomalacia Outbreak in Horses Due to Consumption of Contaminated Hay. J. Vet. Intern. Med. 2016, 30, 1879–1881. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Diário Oficial da União No 37; Sesão 1; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2011.
- Ministério da Agricultura. Diário Oficial da União; Seção 1; Ministério da Agricultura: Brasília, Brazil, 1988; p. 21.968.
- European Union. Comission Regulation; Official Journal of the European Union; European Union: Brussels, Belgium, 2006. [Google Scholar]
- Gonçalves, B.L.; Gonçalves, J.L.; Rosim, R.E.; Cappato, L.P.; Cruz, A.G.; Oliveira, C.A.F.; Corassin, C.H. Effects of Different Sources of Saccharomyces Cerevisiae Biomass on Milk Production, Composition, and Aflatoxin M1 Excretion in Milk from Dairy Cows Fed Aflatoxin B1. J. Dairy Sci. 2017, 100, 5701–5708. [Google Scholar] [CrossRef] [Green Version]
- di Gregorio, M.C.; de Neeff, D.V.; Jager, A.V.; Corassin, C.H.; Carão, Á.C.D.P.; de Albuquerque, R.; de Azevedo, A.C.; Oliveira, C.A.F. Mineral Adsorbents for Prevention of Mycotoxins in Animal Feeds. Toxin. Rev. 2014, 33, 125–135. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating Techniques on Controlling Mycotoxins—A Review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Daković, A.; Matijašević, S.; Rottinghaus, G.E.; Ledoux, D.R.; Butkeraitis, P.; Sekulić, Ž. Aflatoxin B1 Adsorption by Natural and Copper Modified Montmorillonite. Colloids Surf. B Biointerfaces 2008, 66, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, E.; Beraldin, R.; Penha, F.G.; Pergher, S.B.C. Caracterização de Argilas Bentonitas e Diatomitas e Sua Aplicação Como Adsorventes. Química Nova 2009, 32, 2064–2067. [Google Scholar] [CrossRef]
- Jager, A.V.; Tonin, F.G.; Baptista, G.Z.; Souto, P.C.M.C.; Oliveira, C.A.F. Assessment of Aflatoxin Exposure Using Serum and Urinary Biomarkers in São Paulo, Brazil: A Pilot Study. Int. J. Hyg. Environ. Health 2016, 219, 294–300. [Google Scholar] [CrossRef]
- di Gregorio, M.C.; Jager, A.V.; Souto, P.C.M.C.; Costa, A.A.; Rottinghaus, G.E.; Passarelli, D.; Budiño, F.E.L.; Corassin, C.H.; Oliveira, C.A.F. Determination of Serum Aflatoxin B1-Lysine to Evaluate the Efficacy of an Aflatoxin-Adsorbing Feed Additive in Pigs Fed an Aflatoxin B1-Contaminated Diet. Mycotoxin Res. 2017, 33, 93–102. [Google Scholar] [CrossRef]
- Raj, J.; Vasiljević, M.; Tassis, P.; Farkaš, H.; Männer, K. Efficacy of a Multicomponent Mycotoxin Detoxifying Agent on Concurrent Exposure to Zearalenone and T-2 Mycotoxin in Weaned Pigs. Livest. Sci. 2020, 242, 104295. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Rodrigues, R.O.; Ledoux, D.R.; McFadden, T.B.; Rottinghaus, G.E.; Borutova, R.; Averkieva, O. Feed Additives Containing Sequestrant Clay Minerals and Inactivated Yeast Reduce Aflatoxin Excretion in Milk of Dairy Cows. J. Dairy Sci. 2019, 102, 6614–6623. [Google Scholar] [CrossRef]
- European Union; European Parliament and of the Council; Official Journal of the European Union. Regulation (EC) No 1831/2003 of the European Parliament and of the Council; European Union: Brussels, Belgium, 2003; Volume 46, p. 29. [Google Scholar]
- European Union; European Parliament and of the Council; Official Journal of the European Union. Regulation (EC) No 1333/2008 Of The European Parliament And Of The Council; European Union: Brussels, Belgium, 2008; Volume 51. [Google Scholar]
- European Union; European Parliament and of the Council; Official Journal of the European Union. Commission Regulation (EC) No 386/2009 of May 2009 amending Regulation (EC) No 1831/2003 of the European Parliament and of the Council; European Union: Brussels, Belgium, 2009; Volume 37, p. 66. [Google Scholar]
- EFSA Panel on Additives or Substances used in Animal Feed (FEEDAP). Statement on the Establishment of Guidelines for the Assessment of Additives from the Functional Group ‘Substances for Reduction of the Contamination of Feed by Mycotoxins’. EFSA J. 2010, 8, 1693. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Z.; Deng, Y.; Zhou, Z.; Hou, J. Toxicity Induced by F. Poae-Contaminated Feed and the Protective Effect of Montmorillonite Supplementation in Broilers. Food Chem. Toxicol. 2014, 74, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.L.; Wang, Y.M.; Zhou, H.L.; Liu, J.X. Effects of Dietary Adsorbent on Milk Aflatoxin M1 Content and the Health of Lactating Dairy Cows Exposed to Long-Term Aflatoxin B1 Challenge. J. Dairy Sci. 2018, 101, 8944–8953. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.L.; Wang, Y.M.; Nennich, T.D.; Li, Y.; Liu, J.X. Transfer of Dietary Aflatoxin B1 to Milk Aflatoxin M1 and Effect of Inclusion of Adsorbent in the Diet of Dairy Cows. J. Dairy Sci. 2015, 98, 2545–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, M.; Wang, E.; Hao, Y.; Ji, S.; Huang, S.; Zhao, L.; Wang, W.; Shao, W.; Wang, Y.; Li, S. Adsorbents Reduce Aflatoxin M1 Residue in Milk of Healthy Dairy Cow Exposed to Moderate Level Aflatoxin B1 in Diet and Its Exposure Risk for Humans. Toxins 2021, 13, 665. [Google Scholar] [CrossRef]
- Pate, R.T.; Paulus Compart, D.M.; Cardoso, F.C. Aluminosilicate Clay Improves Production Responses and Reduces Inflammation during an Aflatoxin Challenge in Lactating Holstein Cows. J. Dairy Sci. 2018, 101, 11421–11434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeff, D.V.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Dakovic, A.; Murarolli, R.A.; Oliveira, C.A.F. In Vitro and In Vivo Efficacy of a Hydrated Sodium Calcium Aluminosilicate to Bind and Reduce Aflatoxin Residues in Tissues of Broiler Chicks Fed Aflatoxin B1. Poult. Sci. 2013, 92, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, O.C.M.; Han, J.H.; Staples, C.R.; Adesogan, A.T. Effect of Adding a Mycotoxin-Sequestering Agent on Milk Aflatoxin M1 Concentration and the Performance and Immune Response of Dairy Cattle Fed an Aflatoxin B1-Contaminated Diet. J. Dairy Sci. 2012, 95, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, A.P.; Monge, M.P.; Miazzo, R.D.; Cavaglieri, L.R.; Magnoli, C.E.; Merkis, C.I.; Cristofolini, A.L.; Dalcero, A.M.; Chiacchiera, S.M. Effect of Low Levels of Aflatoxin B1 on Performance, Biochemical Parameters, and Aflatoxin B1 in Broiler Liver Tissues in the Presence of Monensin and Sodium Bentonite. Poult. Sci. 2011, 90, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kutz, R.E.; Sampson, J.D.; Pompeu, L.B.; Ledoux, D.R.; Spain, J.N.; Vázquez-Añón, M.; Rottinghaus, G.E. Efficacy of Solis, NovasilPlus, and MTB-100 to Reduce Aflatoxin M1 Levels in Milk of Early to Mid Lactation Dairy Cows Fed Aflatoxin B1. J. Dairy Sci. 2009, 92, 3959–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzi, L.; Simioli, M.; Roncada, P.; Zaghini, A. Aflatoxin B1 and Clinoptilolite in Feed for Laying Hens: Effects on Egg Quality, Mycotoxin Residues in Livers, and Hepatic Mixed-Function Oxygenase Activities. J. Food Prot. 2003, 66, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Horky, P.; Gruberova, H.A.; Aulichova, T.; Malyugina, S.; Slama, P.; Pavlik, A.; Skladanka, J.; Skoric, M.; Skalickova, S. Protective Effect of a New Generation of Activated and Purified Bentonite in Combination with Yeast and Phytogenic Substances on Mycotoxin Challenge in Pigs. PLoS ONE 2021, 16, e0259132. [Google Scholar] [CrossRef] [PubMed]
- Wakade, B.A.; Ingole, S.D.; Bharucha, S.V.; Nagvekar, A.S. Effect of Toxin Binders on Immunity and Aflatoxin M1 Residues in Milk in Buffaloes. Indian J. Anim. Sci. 2019, 89, 944–950. [Google Scholar]
- Mugerwa, S.; Kabirizi, J.; Zziwa, E. Effect of Supplementing Lactating Goats Fed on Aflatoxin Contaminated Feed with Calcium Bentonite and Activated Charcoal on Aflatoxin M1 Concentration, Excretion and Carryover in Milk. Uganda J. Agric. Sci. 2016, 16, 83. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Deng, Q.; Gu, K.; Wang, J. Detoxification of Aflatoxin B1 by Lactic Acid Bacteria and Hydrated Sodium Calcium Aluminosilicate in Broiler Chickens. Livest. Sci. 2018, 208, 28–32. [Google Scholar] [CrossRef]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for Exposure as a Tool for Efficacy Testing of a Mycotoxin Detoxifier in Broiler Chickens and Pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef] [Green Version]
- Soufiani, G.R.N.; Razmara, M.; Kermanshahi, H.; Barrientos Velázquez, A.L.; Daneshmand, A. Assessment of Aflatoxin B1 Adsorption Efficacy of Natural and Processed Bentonites: In Vitro and in Vivo Assays. Appl. Clay Sci. 2016, 123, 129–133. [Google Scholar] [CrossRef]
- Rao, S.B.N.; Chopra, R.C. Influence of Sodium Bentonite and Activated Charcoal on Aflatoxin M1 Excretion in Milk of Goats. Small Rumin. Res. 2001, 41, 203–213. [Google Scholar] [CrossRef]
- Fowler, J.; Li, W.; Bailey, C. Effects of a Calcium Bentonite Clay in Diets Containing Aflatoxin When Measuring Liver Residues of Aflatoxin B1 in Starter Broiler Chicks. Toxins 2015, 7, 3455–3464. [Google Scholar] [CrossRef] [Green Version]
- Travassos, G.F.; Coelho, A.B. Padrão de Substituição Entre Carnes No Consumo Domiciliar do Brasil. Rev. Econ. Sociol. Rural 2017, 55, 285–304. [Google Scholar] [CrossRef]
- Takagi, M.; Uno, S.; Kokushi, E.; Sato, F.; Wijayagunawardane, M.M.P.; Fink-Gremmels, J. Measurement of Urinary Concentrations of the Mycotoxins Zearalenone and Sterigmatocystin as Biomarkers of Exposure in Mares. Reprod. Domest. Anim. 2018, 53, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: An Update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Franco, L.T.; Ismail, A.; Amjad, A.; Oliveira, C.A.F. de Occurrence of Toxigenic Fungi and Mycotoxins in Workplaces and Human Biomonitoring of Mycotoxins in Exposed Workers: A Systematic Review. Toxin. Rev. 2021, 40, 576–591. [Google Scholar] [CrossRef]
- Zheng, M.Z.; Richard, J.L.; Binder, J. A Review of Rapid Methods for the Analysis of Mycotoxins. Mycopathologia 2006, 161, 261–273. [Google Scholar] [CrossRef]
- Phillips, T.D.; Afriyie-Gyawu, E.; Williams, J.; Huebner, H.; Ankrah, N.A.; Ofori-Adjei, D.; Jolly, P.; Johnson, N.; Taylor, J.; Marroquin-Cardona, A.; et al. Reducing Human Exposure to Aflatoxin through the Use of Clay: A Review. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 2008, 25, 134–145. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Alvito, P.; Assunção, R.; Oliveira, C.A.F. Assessment of Mycotoxin Exposure and Risk Characterization Using Occurrence Data in Foods and Urinary Biomarkers in Brazil. Food Chem. Toxicol. 2019, 128, 21–34. [Google Scholar] [CrossRef]
| Species (N) | Mycotoxin Offered (µg/kg) | Adsorbent (Concentration) | Biomarkers | Collected Samples | Mean RBTC in Treatments (%) | Analytical Method | Reference |
|---|---|---|---|---|---|---|---|
| Pigs (24) | AFB1 (1100) | HSCAS a (0.5%) | AFB1-lisine | Serum | 63.64 | UPLC-MS/MS | [25] |
| Piglets (112) | T-2 (500) | T1—MMDA b (1 g/kg) T2—MMDA (3 g/kg) | T-2 HT-2 | Liver, kidney, spleen, and genital tract | T1—17.11 T2—20.05 | Fluorescence-Polarization-Immunoassay | [26] |
| ZEN (350) | T1—MMDA (1 g/kg) T2—MMDA (3 g/kg) | ZEN | Liver, kidney, spleen, and genital tract | T1—28.19 T2—49.11 | |||
| Dairy cows (32) | AFB1 (2800) | Toxy-Nil c (100 g/day) Unique Plus d (100 g/day) | AFM1 e AF | Milk, urine, and feces | TN—52 UP—42.03 | HPLC-FLD | [27] |
| Broilers (160) | T-2 (4000) HT-2 (667) | Montmorillonite e (5 g/kg) | T-2 HT-2 | Heart, liver, lung, kidney, muscular stomach, small intestine, muscle, and brain | T-2 liver—60.25 T-2 kidney—18.34 Other samples could not be calculated | LC-MS/MS | [33] |
| Dairy cows (40) | AFB1 (20) | Solis Molis f (0.25% DM) | AFM1 | Milk | 31.58 | LC-MS/MS | [34] |
| Dairy cows (24) | T1—AFB1 (20) T2—AFB1 (40) | Solis Molis f (0.25% DM) | AFM1 | Milk | T1—16 T2—1.92 | LC-MS/MS | [35] |
| Dairy cows (40) | AFB1 (8) | Adsorbent 1 g (0.07% DM) Adsorbent 2 h (0.07% DM) | AFM1 | Milk | Adsorbent 1—50.53 Adsorbent 2—45.16 | LC-MS/MS | [36] |
| Dairy cows (60) | AFB1 (100) | T1—Aluminosilicate clay i (113 g) T2—Aluminosilicate clay i (227 g) | AFM1 AFB1 and AFM1 AFB1 and AFM1 | Milk, urine, and feces | T1—12.17 T2—21.82 | LC-FLD | [37] |
| Broiler chicks (100) | AFB1 (2500) | HSCAS (0.5%) | AFB1, AFB2, AFM1, AFG1, AFG2, AFL | Liver and kidney | AF liver—82.78 AF kidney—47.62 | LC-FLD | [38] |
| Dairy cows (8) | AFB1 (75) | T1—Calibrin A j (0.2%) T2—Calibrin A j (1%) | AFM1 | Milk | T1—+ 12.28 * T2—19.28 | Radioimmunoassay | [39] |
| Broilers (160) | AFB1 (50) | Sodium bentonite k (0.3%) | AFB1 | Liver | 62.5 | LC-MS/MS | [40] |
| Dairy cows (12) | AFB1 (112) | Solis® l NovasilPlus® m MTB-100® n | AFM1 | Milk | SO—44.8 NOV—47.91 MTB—4.17 | LC-FLD | [41] |
| Laying hens (48) | T1—OTA (1000) T2—OTA (250) | Minazel Plus® o (0.2%) | OTA | Egg | T1—67 T2—ND * | LC-MS/MS | [14] |
| Laying hens (96) | AFB1 (2500) | Clinoptilolite p (2%) | AFB1 | Liver | < LoQ * | Enzyme immunoassay | [42] |
| Pigs (96) | DON (5000) | T1—activated bentonite q (1.5 kg/ton) T2 r—(2 kg/ton) T3 s—(3.5 kg/ton) | DON total/free DON-GlcA | Urine | T1—DON total and free—14.64 T2—DON total and free—26.12 T3—DON total and free—9.01 | ELISA | [43] |
| Buffaloes (48) | AFB1 (1771) | T1 t—(50 g/d) T2 u—(50 g/d) T3 u—(25 g/d) | AFM1 | Milk | T1—5.6 T2—2.12 T3—11.57 | ELISA | [44] |
| Goats (9) | AFB1 (100) | T2—calcium bentonite (1%) T3—activated charcoal (1%) | AFM1 | Milk | T2—6.7 T3—13.46 | HPLC | [45] |
| Broilers (480) | AFB1 (40) | HSCAS v (3 g/kg) | AFB1 | Serum Liver Kidney Spleen Bursa of Fabricius Thymus Excreta | Serum—56,31 Liver—64.12 Kidney—44.25 Spleen—47.95 Bursa of Fabricius—65.94 Thymus—45.84 Excreta—37.32 | ELISA | [46] |
| Broilers (160) | AFB1 (2000) DON (500) OTA (250) | Escent® S w (0.237 g/kg) | DON-S AFB1 OTA | Excreta Plasma | DON-S plasma—49 AFB1 plasma—40 OTA plasma—+13 Excreta—n.a. * | LC-MS/MS | [47] |
| Pigs (8) | DON (36) ZEN (3) | Escent® S w (0.1 g/kg) | DON ZEN DON-GlcA ZEN GlcA | Urine Feces Plasma Plasma and urine | DON urine—26 ZEN feces—+21 DON-GlcA plasma—13 ZEN-GlcA plasma—12 ZEN GlcA urine—4 | LC-MS/MS | |
| Dairy cows (12) | AFB1 (1,95) | T1—unprocessed bentonite (0.6%) T2—G.BindTM—processed bentonite (0.6%) T3—commercial bentonite (0.6%) | AFM1 | Milk | NC * | ELISA | [48] |
| Goats (9) | AFB1 (100) | T2—sodium bentonite (1%) T3—activated charcoal (1%) | AFM1 | Milk | T2—+12.17 T3—3.02 | HPLC | [49] |
| Broilers (366) | AFB1 (600) AFB1 (1200) AFB1 (1800) | Calcium bentonite TX4 (0.2%) | AFB1 | Liver | 35.03 | LC-MS | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, L.G.D.; Ali, S.; de Oliveira, C.A.F. Evaluation of Adsorbent’s Efficiency by Using Biomarker Approaches in Farm Animals: A Systematic Review. Appl. Sci. 2022, 12, 13000. https://doi.org/10.3390/app122413000
Freire LGD, Ali S, de Oliveira CAF. Evaluation of Adsorbent’s Efficiency by Using Biomarker Approaches in Farm Animals: A Systematic Review. Applied Sciences. 2022; 12(24):13000. https://doi.org/10.3390/app122413000
Chicago/Turabian StyleFreire, Lucas Gabriel Dionisio, Sher Ali, and Carlos Augusto Fernandes de Oliveira. 2022. "Evaluation of Adsorbent’s Efficiency by Using Biomarker Approaches in Farm Animals: A Systematic Review" Applied Sciences 12, no. 24: 13000. https://doi.org/10.3390/app122413000






