Preparation and Electrocatalytic Activity of a Cobalt Mixed Nitrogen 3D Carbon Nanostructure @ Carbon Felt toward an All-Vanadium Redox Flow Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZIF-67@CF and Co-CN@CF
2.2. Material Characterization
2.3. Electrochemical Characterization
2.4. Single-Cell Test
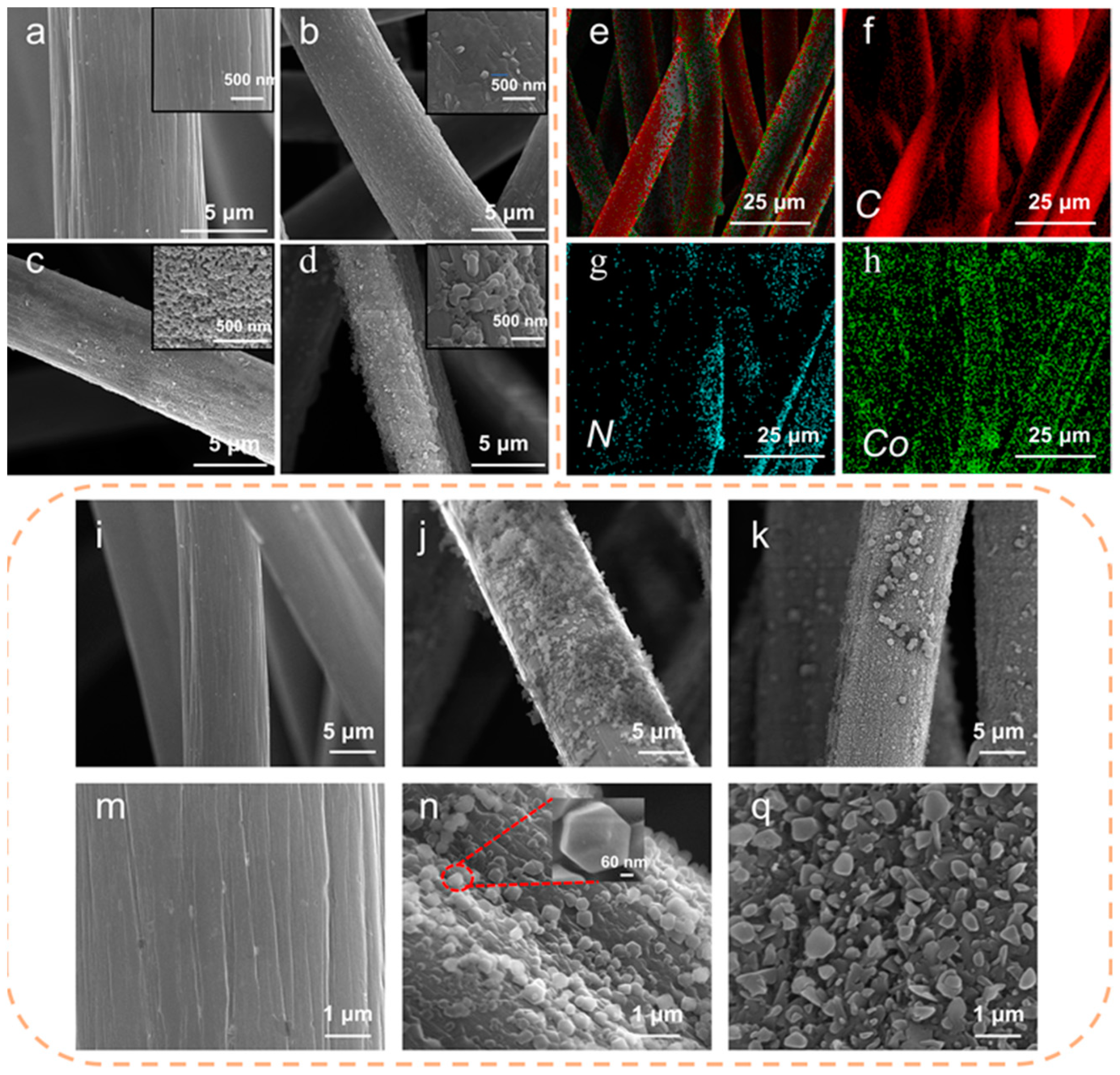
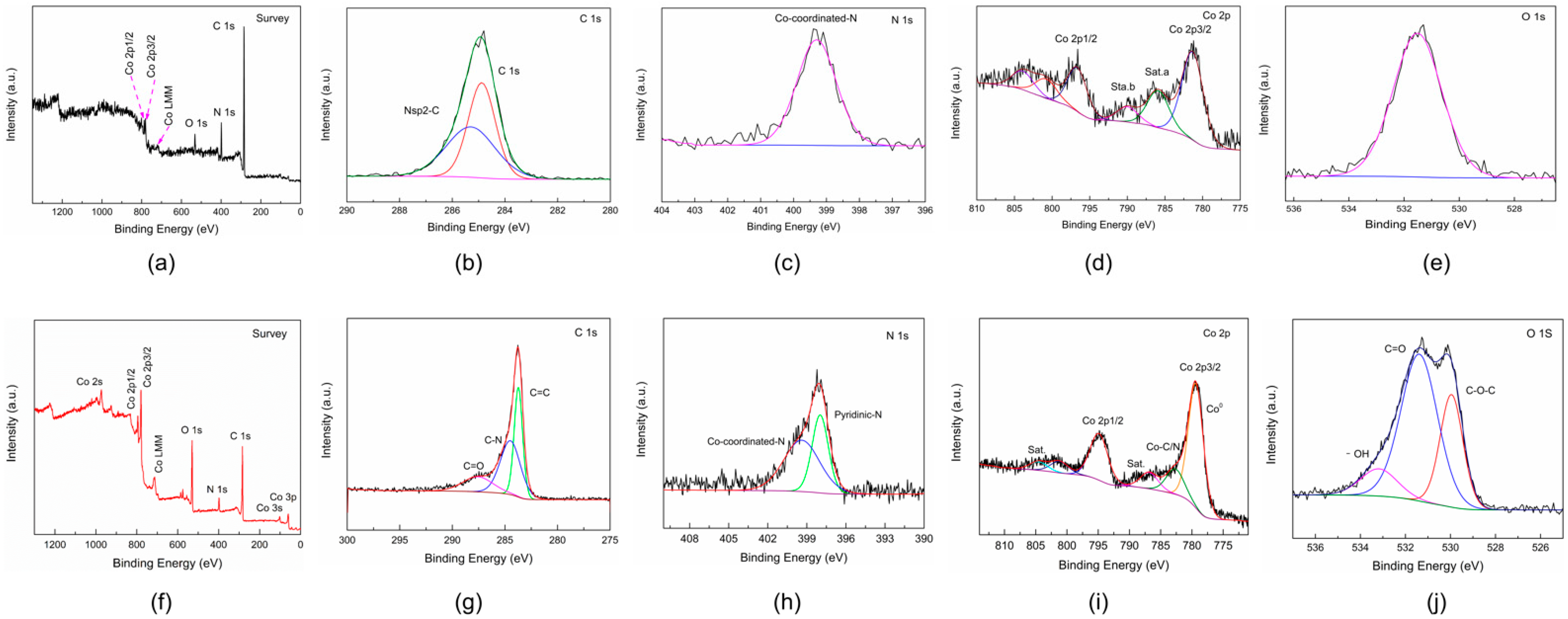
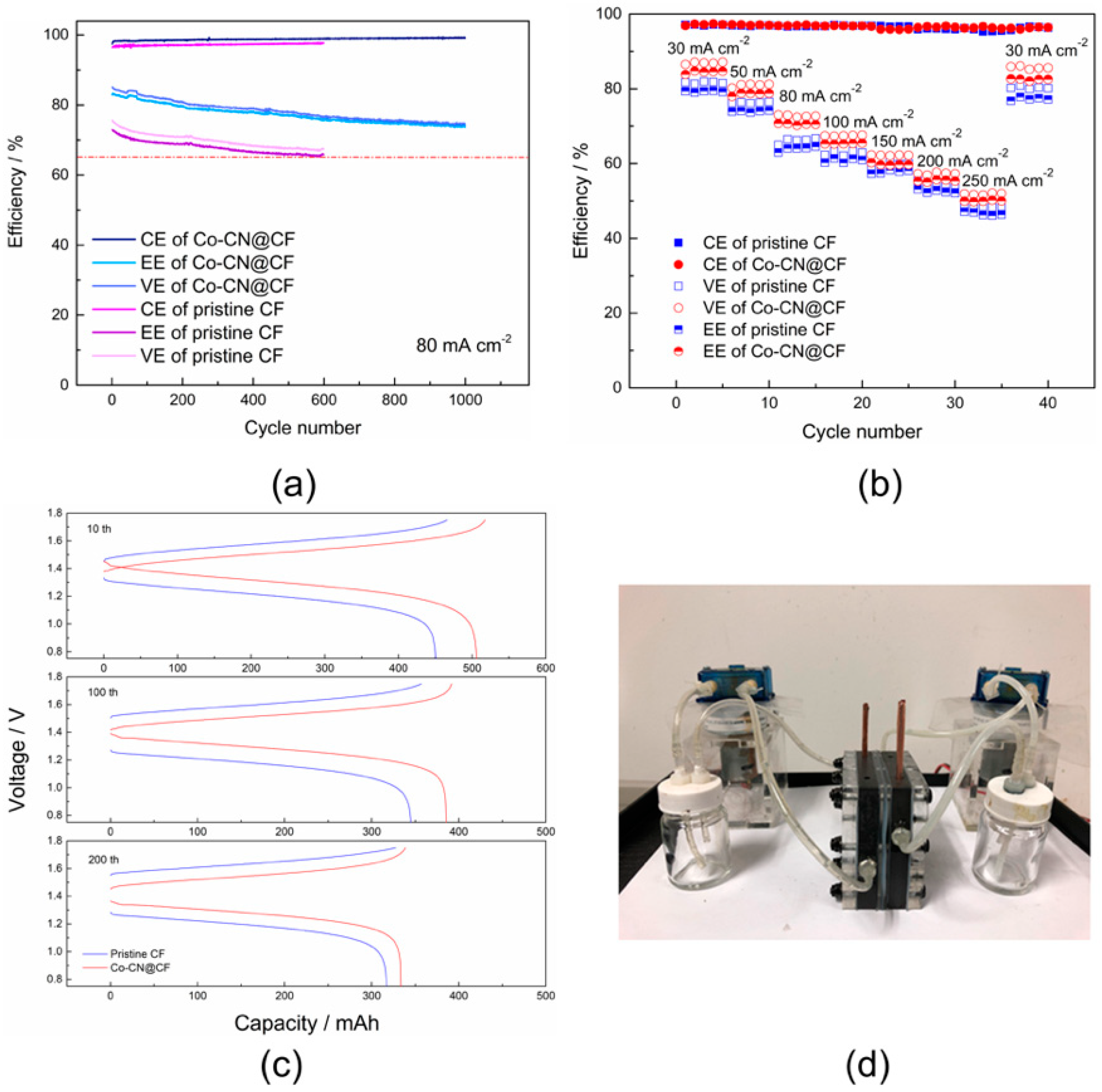
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Noto, V.D. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changkun, Z.; Leyuan, Z.; Yu, D.; Sangshan, P.; Xuelin, G.; Yu, Z.; Gaohong, H.; Guihua, Y. Progress and prospects of next-generation redox flow batteries. Energy Storage Mater. 2018, 15, 324–350. [Google Scholar]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Minke, C.; Turek, T. Materials, system designs and modelling approaches in techno-economic assessment of all-vanadium redox flow batteries ? A review. J. Power Sources 2017, 376, 66–81. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; Ponce de Len, C.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Hu, B.; DeBruler, C.; Rhodes, Z.; Liu, T.L. Long-Cycling Aqueous Organic Redox Flow Battery (AORFB) toward Sustainable and Safe Energy Storage. J. Am. Chem. Soc. 2016, 139, 1207–1214. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal? Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Zhang, B.W.; Sheng, T.; Wang, Y.X.; Chou, S.L.; Davey, K.; Dou, S.X.; Qiao, S.Z. Long-Life Room-Temperature Sodium-Sulfur Batteries by Virtue of Transition-Metal-Nanocluster-Sulfur Interactions. Angew. Chem.-Int. Edit. 2019, 58, 1484–1488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wei, Y.L.; Cao, P.F.; Lin, M.C. Energy storage system: Current studies on batteries and power condition system. Renew. Sust. Energ. Rev. 2018, 82, 3091–3106. [Google Scholar] [CrossRef]
- Li, Z.; Weng, G.; Zou, Q.; Cong, G.; Lu, Y.C. A high-energy and low-cost polysulfide/iodide redox flow battery. Nano Energy 2016, 30, 283–292. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.; Suh, C.; Er, S.; Gerhardt, M.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A metal-free organic-inorganic aqueous flow battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yin, Y.; Xie, C.; Zhang, H.; Yao, Y.; Li, X. Advanced materials for zinc-based flow battery: Development and challenge. Adv. Mater. 2019, 31, 1902025. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, H. Review of the development of first-generation redox flow batteries: Iron-chromium system. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Beh, E.S.; De Porcellinis, D.; Gracia, R.L.; Xia, K.T.; Gordon, R.G.; Aziz, M.J. A Neutral pH Aqueous Organic-Organometallic Redox Flow Battery with Extremely High Capacity Retention. ACS Energy Lett. 2017, 2, 639–644. [Google Scholar] [CrossRef]
- Opar, D.O.; Nankya, R.; Lee, J.; Jung, H. Assessment of three-dimensional nitrogen-doped mesoporous graphene functionalized carbon felt electrodes for high-performance all vanadium redox flow batteries. Appl. Surf. Sci. 2020, 531, 147391. [Google Scholar] [CrossRef]
- Deng, Q.; Huang, P.; Zhou, W.X.; Ma, Q.; Zhou, N.; Xie, H.; Ling, W.; Zhou, C.J.; Yin, Y.X.; Wu, X.W.; et al. A High-Performance Composite Electrode for Vanadium Redox Flow Batteries. Adv. Energy Mater. 2017, 7, 7. [Google Scholar] [CrossRef]
- Wu, Y.P.; Holze, R. Electrocatalysis at Electrodes for Vanadium Redox Flow Batteries. Batteries 2018, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Zhang, Q.; Wu, C.; Liu, Y.; Ding, M.; Ye, J.; Cheng, Y.; Jia, C. Graphene coated carbon felt as a high-performance electrode for all vanadium redox flow batteries. Surf. Coat. Technol. 2019, 358, 153–158. [Google Scholar] [CrossRef]
- Sun, B.T.; Skyllas-Kazacos, M. Chemical modification of graphite electrode materials for vanadium redox flow battery application part II. Acid treatments. Electrochim. Acta 2010, 37, 1253–1260. [Google Scholar] [CrossRef]
- Sun, B.T.; Skyllas-Kazacos, M. Modification of graphite electrode materials for vanadium redox flow battery application? I. Thermal treatment. Electrochim. Acta 1992, 37, 1253–1260. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, J.; Zhou, H.; Qiu, X. KOH etched graphite felt with improved wettability and activity for vanadium flow batteries. Electrochim. Acta 2016, 218, 15–23. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Y.; Luo, X.-D.; Sun, C.-Y.; Chen, N. Polarization Effects of a Rayon and Polyacrylonitrile Based Graphite Felt for Iron-Chromium Redox Flow Batteries. ChemElectroChem 2019, 6, 3175–3188. [Google Scholar] [CrossRef]
- Zhang, W.; Xi, J.; Li, Z.; Zhou, H.; Liu, L.; Wu, Z.; Qiu, X. Electrochemical activation of graphite felt electrode for VO2+/VO2+ redox couple application. Electrochim. Acta 2013, 89, 429–435. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Sanchez, A.; Blanco, C.; Granda, M.; Menendez, R.; Santamaria, R. Enhanced performance of a Bi-modified graphite felt as the positive electrode of a vanadium redox flow battery. Electrochem. Commun. 2011, 13, 1379–1382. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Zhao, X.; Ma, Y.; Su, J.; Xiang, C.; Zhao, K.; Ding, M.; Jia, C.; Sun, L. Hybrid membranes dispersed with superhydrophilic TiO2 nanotubes toward ultra-stable and high-performance vanadium redox flow batteries. Adv.Energy Mater. 2020, 10, 1904041. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zeng, Y.K.; Zhu, X.B.; Wei, L.; Zhao, T.S. A high-performance dual-scale porous electrode for vanadium redox flow batteries. J. Power Sources 2016, 325, 329–336. [Google Scholar] [CrossRef]
- Busacca, C.; Di Blasi, O.; Giacoppo, G.; Briguglio, N.; Antonucci, V.; Di Blasi, A. High performance electrospun nickel manganite on carbon nanofibers electrode for vanadium redox flow battery. Electrochim. Acta 2020, 355, 136755. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, S.W.; Yim, T.; Kim, J.G.; Choi, J.W.; Kim, J.H.; Park, M.S.; Kim, Y.J. A new strategy for integrating abundant oxygen functional groups into carbon felt electrode for vanadium redox flow batteries. Sci. Rep. 2014, 4, 6. [Google Scholar] [CrossRef]
- Kim, J.; Lim, H.; Jyoung, J.-Y.; Lee, E.-S.; Yi, J.S.; Lee, D. High electrocatalytic performance of N and O atomic co-functionalized carbon electrodes for vanadium redox flow battery. Carbon 2017, 111, 592–601. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, C.; Liu, J.; Sun, T.; Yu, S.; Li, H. In situ grown tungsten trioxide nanoparticles on graphene oxide nanosheet to regulate ion selectivity of membrane for high performance vanadium redox flow battery. Adv. Funct. Mater. 2022, 32, 2109427. [Google Scholar] [CrossRef]
- Ye, J.; Yu, S.; Zheng, C.; Sun, T.; Liu, J.; Li, H. Advanced hybrid membrane for vanadium redox flow battery created by polytetrafluoroethylene layer and functionalized silicon carbide nanowires. Chem. Eng. J. 2022, 427, 131413. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.S.; Kim, J.H.; Hwang, U.; Lee, N.J.; Jeong, G.; Kim, Y.J. Novel catalytic effects of Mn3O4 for all vanadium redox flow batteries. Chem. Commun. 2012, 48, 5455–5457. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.M.; Huang, R.H.; Huang, C.Y.; Hsueh, K.L.; Shieu, F.S. A Kinetic Study of the Platinum/Carbon Anode Catalyst for Vanadium Redox Flow Battery. J. Electrochem. Soc. 2013, 160, A690–A696. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.J.; Wang, L.; He, Z.X.; Li, Y.H.; Jiang, Y.Q.; Meng, W.; Dai, L. Electrocatalytic activity of cobalt phosphide-modified graphite felt toward VO2+/VO2+ redox reaction. Appl. Surf. Sci. 2018, 436, 1030–1037. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2010, 64, 8553–8557. [Google Scholar] [CrossRef]
- Qi, S.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam? Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. Int. Ed. 2011, 50, 672–675. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Li, Z.; Hao, L.; Qin, L. Preparation and Electrocatalytic Activity of a Cobalt Mixed Nitrogen 3D Carbon Nanostructure @ Carbon Felt toward an All-Vanadium Redox Flow Battery. Appl. Sci. 2022, 12, 2304. https://doi.org/10.3390/app12052304
Su J, Li Z, Hao L, Qin L. Preparation and Electrocatalytic Activity of a Cobalt Mixed Nitrogen 3D Carbon Nanostructure @ Carbon Felt toward an All-Vanadium Redox Flow Battery. Applied Sciences. 2022; 12(5):2304. https://doi.org/10.3390/app12052304
Chicago/Turabian StyleSu, Jun, Zongyang Li, Longlong Hao, and Lilu Qin. 2022. "Preparation and Electrocatalytic Activity of a Cobalt Mixed Nitrogen 3D Carbon Nanostructure @ Carbon Felt toward an All-Vanadium Redox Flow Battery" Applied Sciences 12, no. 5: 2304. https://doi.org/10.3390/app12052304





