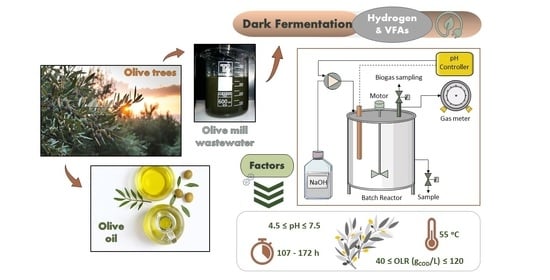
Thermophilic Dark Fermentation of Olive Mill Wastewater in Batch Reactors: Effect of pH and Organic Loading
Abstract
:1. Introduction
2. Materials and Methods
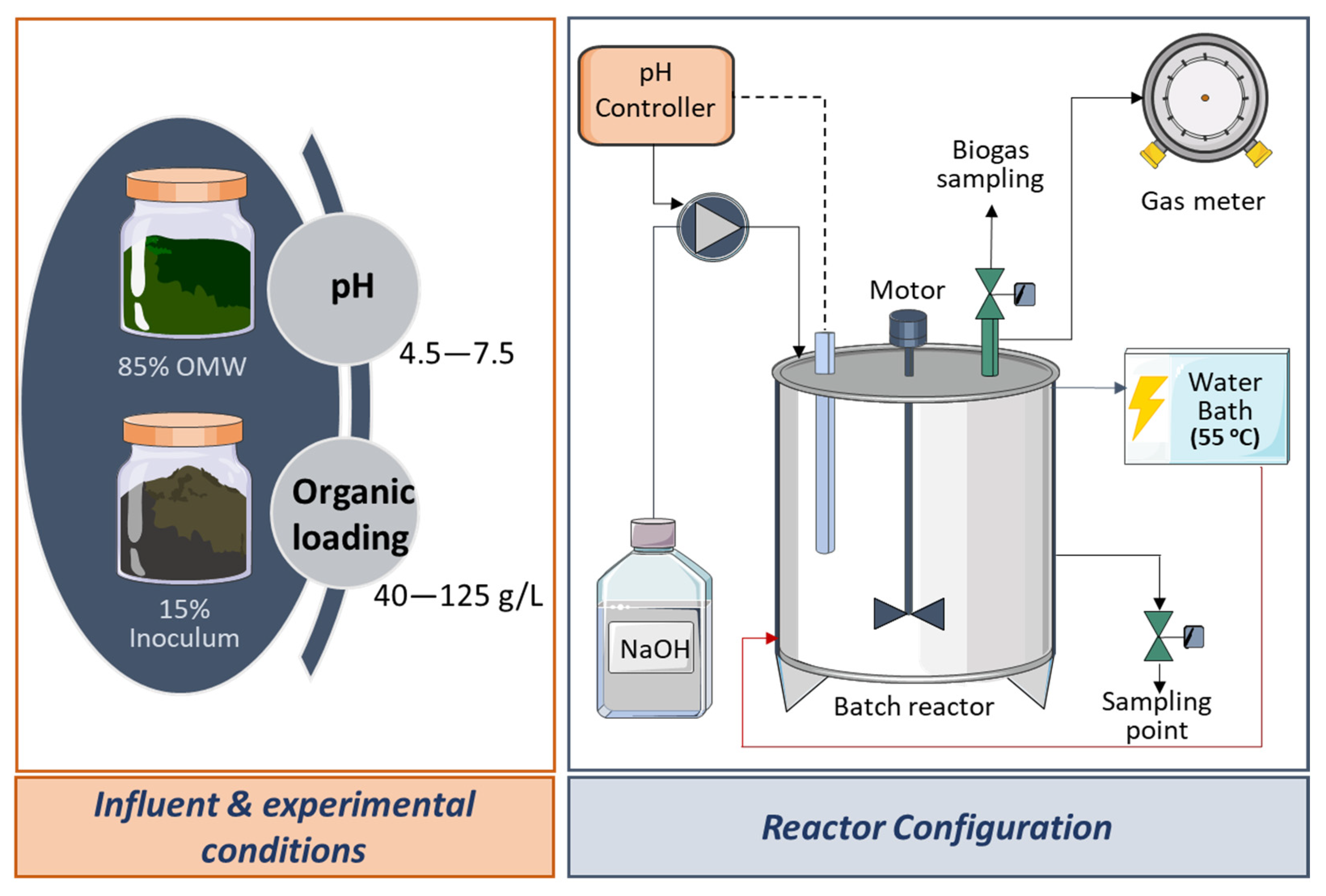
2.1. Experimental Set-Up and Procedure Descrpition
2.2. Inoculum and Substrate
2.3. Analytical Techniques
2.4. Figures Design
3. Results and Discussion
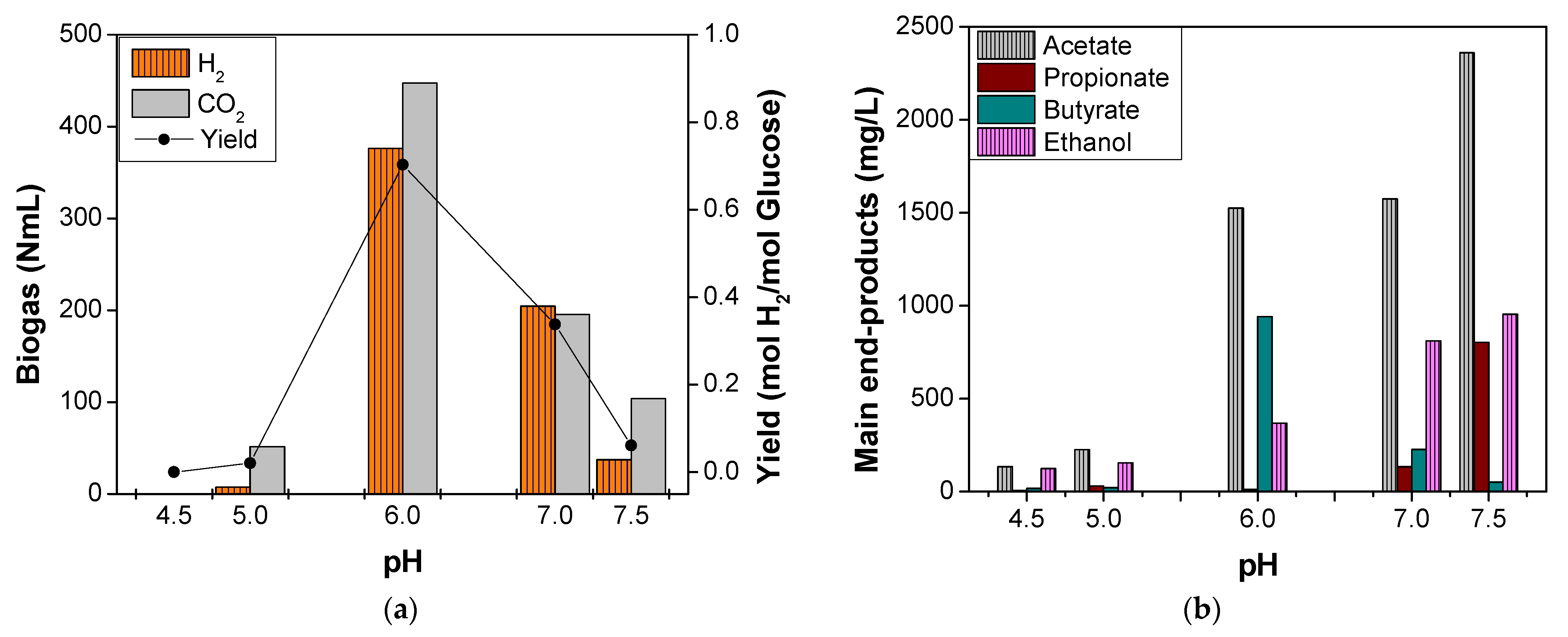
3.1. Effect of pH on OMW Thermophilic DF
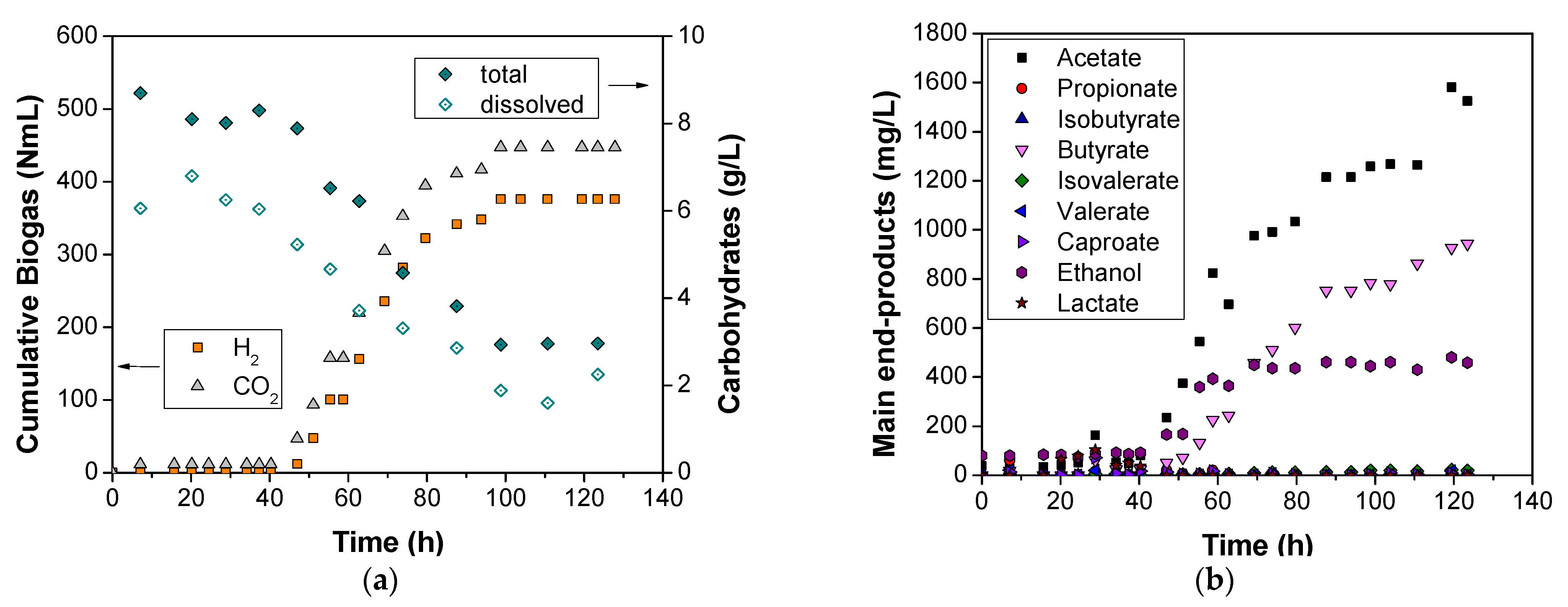
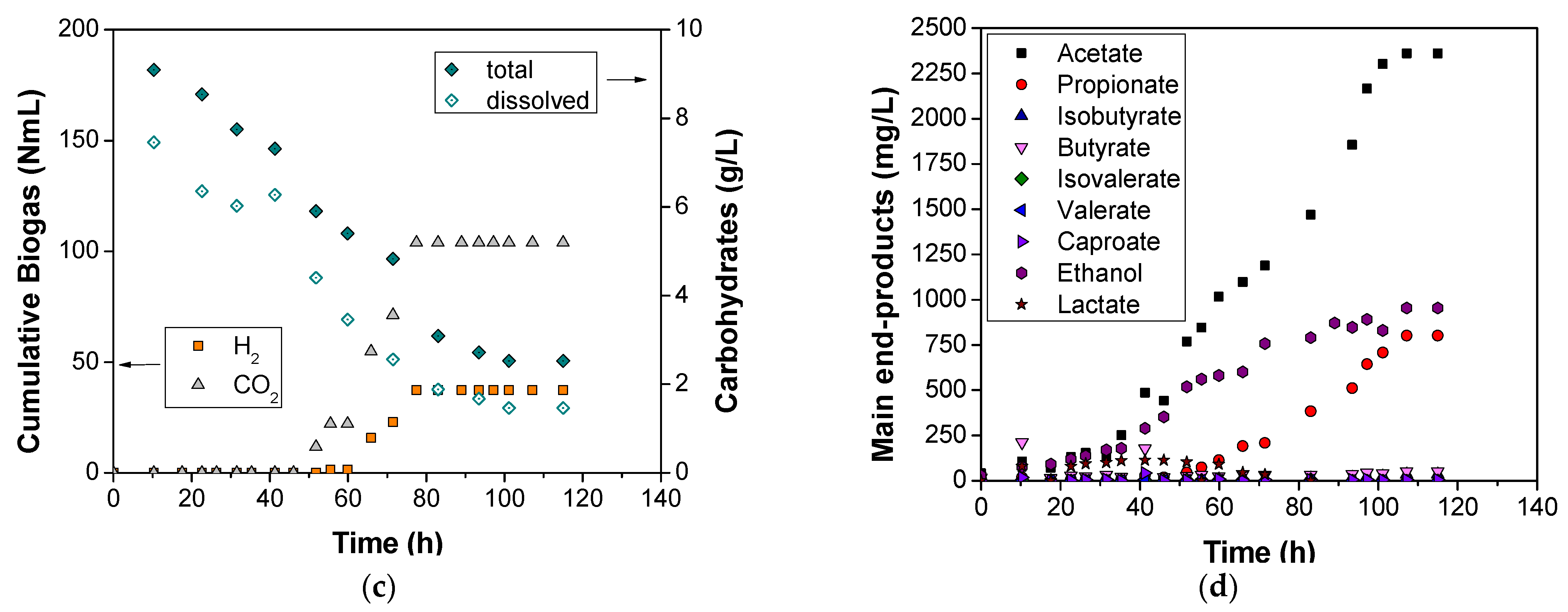
3.2. Hydrogen and Metabolites Evolution at pH 6.0 and 7.5
3.3. Organic Loading Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbandeh, M. Production Volume of Olive Oil Worldwide from 2012/13 to 2021/22. Available online: https://www.statista.com/statistics/613466/olive-oil-production-volume-worldwide/#statisticContainer (accessed on 7 January 2022).
- World’s Olive Oil Production Has Tripled. Available online: https://www.internationaloliveoil.org/worlds-olive-oil-production-has-tripled/ (accessed on 7 January 2022).
- Papanastasis, V.P.; Mantzanas, K.; Dini-Papanastasi, O.; Ispikoudis, I. Traditional agroforestry systems and their evolution in Greece. In Agroforestry in Europe; Springer: Berlin/Heidelberg, Germany, 2009; pp. 89–109. [Google Scholar]
- European, C. Market Situation in the Olive Oil and Table Olives Sectors. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/plants_and_plant_products/documents/market-situation-olive-oil-table-olives_en.pdf (accessed on 8 January 2022).
- Klonaris, S.; Agiangkatzoglou, A. Competitiveness of Greek virgin olive oil in the main destination markets. Br. Food J. 2018, 120, 80–95. [Google Scholar] [CrossRef]
- Aravani, V.P.; Sun, H.; Yang, Z.; Liu, G.; Wang, W.; Anagnostopoulos, G.; Syriopoulos, G.; Charisiou, N.D.; Goula, M.A.; Kornaros, M. Agricultural and livestock sector’s residues in Greece & China: Comparative qualitative and quantitative characterization for assessing their potential for biogas production. Renew. Sustain. Energy Rev. 2022, 154, 111821. [Google Scholar]
- Tsigkou, K.; Kornaros, M. Development of a high-rate anaerobic thermophilic upflow packed bed reactor for efficient bioconversion of diluted three-phase olive mill wastewater into methane. Fuel 2022, 310, 122263. [Google Scholar] [CrossRef]
- Koutsos, T.M.; Chatzistathis, T.; Balampekou, E.I. A new framework proposal, towards a common EU agricultural policy, with the best sustainable practices for the re-use of olive mill wastewater. Sci. Total Environ. 2018, 622–623, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Azzam, M.O.J.; Hazaimeh, S.A. Olive mill wastewater treatment and valorization by extraction/concentration of hydroxytyrosol and other natural phenols. Process Saf. Environ. Prot. 2021, 148, 495–523. [Google Scholar] [CrossRef]
- Tsigkou, K.; Terpou, A.; Treu, L.; Kougias, P.G.; Kornaros, M. Thermophilic anaerobic digestion of olive mill wastewater in an upflow packed bed reactor: Evaluation of 16S rRNA amplicon sequencing for microbial analysis. J. Environ. Manag. 2022, 301, 113853. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Alajlouni, M.A.; Ghariabeh, M.A.; Rusan, M.J. Short-Term Effects of Olive Mill Wastewater Land Spreading on Soil Physical and Hydraulic Properties. Water Air Soil Pollut. 2019, 230, 208. [Google Scholar] [CrossRef]
- Oz, N.A.; Uzun, A.C. Ultrasound pretreatment for enhanced biogas production from olive mill wastewater. Ultrason. Sonochem. 2015, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, E.; Eroğlu, İ.; Gündüz, U.; Yücel, M. Treatment of olive mill wastewater by different physicochemical methods and utilization of their liquid effluents for biological hydrogen production. Biomass Bioenergy 2009, 33, 701–705. [Google Scholar] [CrossRef]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Galliou, F.; Markakis, N.; Fountoulakis, M.S.; Nikolaidis, N.; Manios, T. Production of organic fertilizer from olive mill wastewater by combining solar greenhouse drying and composting. Waste Manag. 2018, 75, 305–311. [Google Scholar] [CrossRef]
- Ntaikou, I.; Antonopoulou, G.; Vayenas, D.; Lyberatos, G. Assessment of electrocoagulation as a pretreatment method of olive mill wastewater towards alternative processes for biofuels production. Renew. Energy 2020, 154, 1252–1262. [Google Scholar] [CrossRef]
- Zakoura, M.; Kopsahelis, A.; Tsigkou, K.; Ntougias, S.; Ali, S.S.; Kornaros, M. Performance evaluation of three mesophilic upflow anaerobic sludge blanket bioreactors treating olive mill wastewater: Flocculent and granular inocula tests, organic loading rate effect and anaerobic consortia structure. Fuel 2022, 313, 122951. [Google Scholar] [CrossRef]
- Tsigkou, K.; Sakarika, M.; Kornaros, M. Inoculum origin and waste solid content influence the biochemical methane potential of olive mill wastewater under mesophilic and thermophilic conditions. Biochem. Eng. J. 2019, 151, 107301. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Extraction of antioxidants from olive mill wastewater and electro-coagulation of exhausted fraction to reduce its toxicity on anaerobic digestion. J. Hazard. Mater. 2008, 151, 531–539. [Google Scholar] [CrossRef]
- Eroğlu, E.; Eroğlu, İ.; Gündüz, U.; Türker, L.; Yücel, M. Biological hydrogen production from olive mill wastewater with two-stage processes. Int. J. Hydrogen Energy 2006, 31, 1527–1535. [Google Scholar] [CrossRef]
- Mugnai, G.; Borruso, L.; Mimmo, T.; Cesco, S.; Luongo, V.; Frunzo, L.; Fabbricino, M.; Pirozzi, F.; Cappitelli, F.; Villa, F. Dynamics of bacterial communities and substrate conversion during olive-mill waste dark fermentation: Prediction of the metabolic routes for hydrogen production. Bioresour. Technol. 2021, 319, 124157. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-hydrogen Production. Waste Biomass Valorization 2020, 11, 539–551. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.D.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.-H.; Pandey, A. Dark fermentation: Production and utilization of volatile fatty acid from different wastes—A review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef] [PubMed]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Zagklis, D.; Papadionysiou, M.; Tsigkou, K.; Tsafrakidou, P.; Zafiri, C.; Kornaros, M. Effect of pH on the Economic Potential of Dark Fermentation Products from Used Disposable Nappies and Expired Food Products. Appl. Sci. 2021, 11, 4099. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Potential of bio-hydrogen production from dark fermentation of crop residues: A review. Int. J. Hydrogen Energy 2019, 44, 17346–17362. [Google Scholar] [CrossRef]
- Cahyari, K.; Hidayat, M.; Syamsiah, S. Optimization of hydrogen production from fruit waste through mesophilic and thermophilic dark fermentation: Effect of substrate-to-inoculum ratio. Malaysian J. Anal. Sci. 2019, 23, 116–123. [Google Scholar]
- Cakır, A.; Ozmihci, S.; Kargi, F. Comparison of bio-hydrogen production from hydrolyzed wheat starch by mesophilic and thermophilic dark fermentation. Int. J. Hydrogen Energy 2010, 35, 13214–13218. [Google Scholar] [CrossRef]
- Al-Bsoul, A.; Al-Shannag, M.; Tawalbeh, M.; Al-Taani, A.A.; Lafi, W.K.; Al-Othman, A.; Alsheyab, M. Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Sci. Total Environ. 2020, 700, 134576. [Google Scholar] [CrossRef]
- Aboutaleb, E.; Kamel, G.; Hellal, M. Investigation of effective treatment techniques for olive mill wastewater. Egypt. J. Chem. 2018, 61, 415–422. [Google Scholar]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Screening of commercial catalysts for steam reforming of olive mill wastewater. Renew. Energy 2021, 169, 765–779. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Matei Ghimbeu, C.; Jellai, S.; El-Bassi, L.; Jeguirim, M. Olive Mill by-Products Thermochemical Conversion via Hydrothermal Carbonization and Slow Pyrolysis: Detailed Comparison between the Generated Hydrochars and Biochars Characteristics. Processes 2022, 10, 231. [Google Scholar] [CrossRef]
- Haddad, K.; Jeguirim, M.; Jellali, S.; Thevenin, N.; Ruidavets, L.; Limousy, L. Biochar production from Cypress sawdust and olive mill wastewater: Agronomic approach. Sci. Total Environ. 2021, 752, 141713. [Google Scholar] [CrossRef]
- Jaouad, Y.; Villain-Gambier, M.; Mandi, L.; Marrot, B.; Ouazzani, N. Comparison of aerobic processes for olive mill wastewater treatment. Water Sci. Technol. 2020, 81, 1914–1926. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific: Oxford, UK; Boston, MA, USA, 1994; 238p. [Google Scholar]
- Joseffson, B. Rapid spectrophotometric determination of total carbohydrates. In Methods Seawater Analysis; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie GmbH: Deerfield Beach, FL, USA; Weinheim, Germany; Basel, Switzerland, 1983; pp. 340–342. [Google Scholar]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Zafiri, C.; Kornaros, M. Dark Fermentation of Sweet Sorghum Stalks, Cheese Whey and Cow Manure Mixture: Effect of pH, Pretreatment and Organic Load. Processes 2021, 9, 1017. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.L.; Esposito, G.; Pirozzi, F. Dark fermentation of complex waste biomass for biohydrogen production by pretreated thermophilic anaerobic digestate. J. Environ. Manag. 2015, 152, 43–48. [Google Scholar] [CrossRef]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Wang, B.; Huang, J.C. Ethanol-type fermentation from carbohydrate in high rate acidogenic reactor. Biotechnol. Bioeng. 1997, 54, 428–433. [Google Scholar] [CrossRef]
- Stavropoulos, K.P.; Kopsahelis, A.; Zafiri, C.; Kornaros, M. Effect of pH on Continuous Biohydrogen Production from End-of-Life Dairy Products (EoL-DPs) via Dark Fermentation. Waste Biomass Valorization 2016, 7, 753–764. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Zhou, J.; Cen, K. Inhibitory effects of furan derivatives and phenolic compounds on dark hydrogen fermentation. Bioresour. Technol. 2015, 196, 250–255. [Google Scholar] [CrossRef]
- Luo, L.; Kaur, G.; Wong, J.W.C. A mini-review on the metabolic pathways of food waste two-phase anaerobic digestion system. Waste Manag. Res. 2019, 37, 333–346. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Isipato, M.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. Control of fermentation duration and pH to orient biochemicals and biofuels production from cheese whey. Bioresour. Technol. 2019, 289, 121722. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Liu, Y.; Wang, Y.; She, Z.; Gao, M.; Zhao, Y. Effect of salinity and pH on dark fermentation with thermophilic bacteria pretreated swine wastewater. J. Environ. Manag. 2020, 271, 111023. [Google Scholar] [CrossRef] [PubMed]
- Magrini, F.E.; de Almeida, G.M.; da Maia Soares, D.; Fuentes, L.; Ecthebehere, C.; Beal, L.L.; da Silveira, M.M.; Paesi, S. Effect of different heat treatments of inoculum on the production of hydrogen and volatile fatty acids by dark fermentation of sugarcane vinasse. Biomass Convers. Biorefinery 2021, 11, 2443–2456. [Google Scholar] [CrossRef]
- Mechery, J.; Thomas, D.M.; Kumar, C.S.; Joseph, L.; Sylas, V.P. Biohydrogen production from acidic and alkaline hydrolysates of paddy straw using locally isolated facultative bacteria through dark fermentation. Biomass Convers. Biorefinery 2021, 11, 1263–1272. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, Y.; Ren, L.; Li, M.; Tang, R.; Jiang, Y.; Hou, J. Early warning indicators and microbial community dynamics during unstable stages of continuous hydrogen production from food wastes by thermophilic dark fermentation. Int. J. Hydrogen Energy 2019, 44, 30000–30013. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Sen, B.; Lin, C.Y. Overcoming propionic acid inhibition of hydrogen fermentation by temperature shift strategy. Int. J. Hydrogen Energy 2014, 39, 19232–19241. [Google Scholar] [CrossRef]
- Rangela, C.; Sastoqueb, J.; Calderonb, J.; Mosquerab, J.; Velasquezd, P.; Cabezabc, I.; Acevedobd, P. Hydrogen Production by Dark Fermentation Process: Effect of Initial Organic Load. Chem. Eng. 2020, 79, 133–138. [Google Scholar]
- Hodaifa, G.; Gallardo, P.A.R.; García, C.A.; Kowalska, M.; Seyedsalehi, M. Chemical oxidation methods for treatment of real industrial olive oil mill wastewater. J. Taiwan Inst. Chem. Eng. 2019, 97, 247–254. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chiang, C.-C.; Nguyen, M.-L.T.; Lay, C.-H. Enhancement of fermentative biohydrogen production from textile desizing wastewater via coagulation-pretreatment. Int. J. Hydrogen Energy 2017, 42, 12153–12158. [Google Scholar] [CrossRef]

| Method | Characteristics | Ref. |
|---|---|---|
| Membrane ultrafiltration | Purification of the substrate; however, some boundaries are presented usually due to fouling problems. | [14] |
| Combination of ultrasound and advanced oxidation processes | Reduction in organic content; however, the residual stream needs further treatment. | [32] |
| Electro-coagulation | High organic content removal; however, the residual stream needs further treatment. | [33] |
| Steam reforming | High total organic carbon conversion and H2 yields; however the performance is strongly depended on the catalyst. | [34] |
| Hydrothermal carbonization | Production of hydrochars for agricultural applications. | [35] |
| Slow pyrolysis | Biochar production, which can be applied as an organic fertilizer with promising results (e.g., K bioavailability). | [36] |
| Anaerobic digestion | Biofuel (methane) production; however the inoculum is usually stressed by the phenolics presence. | [7] |
| Dark fermentation | Production of H2 and conversion of the substrate mainly to VFAs (and/or ethanol). | [21] |
| Aerobic process | Good performance on organic compounds treatment and polyphenols degradation, however the organic load should be relatively low. | [37] |
| Compost production | Production of a nutrient-rich fertilizer; however, reduction in moisture is mandatory. | [15] |
| Run | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| pH | 4.5 | 5.0 | 6.0 | 7.0 | 7.5 | 6.0 | 6.0 |
| Average organic loading (g COD/L) | 40 | 40 | 40 | 40 | 40 | 125 | 80 |
| Parameter | Unit | Average Value ± SD |
|---|---|---|
| pH | - | 8.89 ± 0.36 |
| Total solids | g/L | 39.05 ± 5.07 |
| Volatile solids | g/L | 33.26 ± 3.79 |
| Total suspended solids | g/L | 30.95 ± 4.70 |
| Volatile suspended solids | g/L | 27.13 ± 3.15 |
| Parameter | Unit | Average Value ± SD |
|---|---|---|
| pH | - | 5.41 ± 0.12 |
| Total COD | gO2/L | 125.63 ± 15.70 |
| Dissolved COD | gO2/L | 40.21 ± 0.04 |
| Total solids | g/L | 110.45 ± 1.20 |
| Volatile solids | g/L | 95.28 ± 1.22 |
| Total suspended solids | g/L | 22.67 ± 0.20 |
| Volatile suspended solids | g/L | 22.43 ± 0.24 |
| Total carbohydrates | gglucose/L | 15.36 ± 0.93 |
| Dissolved carbohydrates | gglucose/L | 11.40 ± 0.12 |
| Phenolic compounds | gsyringic acid/L | 1.28 ± 0.05 |
| TKN | g/L | 0.03 ± 0.003 |
| Ammonium nitrogen | g/L | 0.59 ± 0.05 |
| Fats and Oils | g/L | 2.23 ± 0.45 |
| Total phosphorus | g/L | 0.48 ± 0.01 |
| Dissolved phosphorus | g/L | 0.38 ± 0.01 |
| Alkalinity | gCaCO3/L | 0.98 ± 0.19 |
| Substrate | Reactor Type | Temperature | H2 Yield | Other Metabolites | Reference |
|---|---|---|---|---|---|
| Cheese whey | batch | 39 ± 1 °C | 162 L/kg TOC | Max lactate yield (23 mmol/g TOC) | [48] |
| Swine wastewater pretreated with thermophilic bacteria | batch | 36 °C | 7.0 mL H2/g VSS | Acetate > Propionate > Butyrate > Valerate | [49] |
| Sugarcane vinasse (Pretreated 90 °C for 10 min) | batch | 37 °C | 4.75 mmol H2/g CODinfluent | Butyrate > Propionate > Acetate | [50] |
| Expired food products and used disposable nappies hydrolysate | batch | 37 ± 0.2 °C | 1.33 mol H2/mol glucose | Mainly Acetate and butyrate | [28] |
| Paddy straw after acid pretreatment | batch | mesophilic | 1.03 mol H2/mol glucose | Final VFAs of 3.45 g/L | [51] |
| OMW–Cheese whey–liquid cow manure (55:40:5 v/v/v) | batch | 37 °C | 0.642 mol H2/mol glucose | Butyrate >> Propionate > Acetate | [52] |
| Hydrothermal pretreated food wastes | CSTR | 55 ± 1 °C | 10.38 mL g−1 vs. h−1 Gas production rate (47% H2) | Butyrate > Acetate | [53] |
| Fruit/vegetable wastes and disposable nappies hydrolysate | CSTR | 37 ± 0.5 °C | 1.93 ± 0.17 L H2/Lfeed | 11.7 ± 3.4 g VFAs/L | [24] |
| OMW | batch | 55 ± 0.5 °C | 0.7 mol H2/mol glucose | Acetate > Butyrate > Ethanol | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsigkou, K.; Sivolapenko, N.; Kornaros, M. Thermophilic Dark Fermentation of Olive Mill Wastewater in Batch Reactors: Effect of pH and Organic Loading. Appl. Sci. 2022, 12, 2881. https://doi.org/10.3390/app12062881
Tsigkou K, Sivolapenko N, Kornaros M. Thermophilic Dark Fermentation of Olive Mill Wastewater in Batch Reactors: Effect of pH and Organic Loading. Applied Sciences. 2022; 12(6):2881. https://doi.org/10.3390/app12062881
Chicago/Turabian StyleTsigkou, Konstantina, Natalia Sivolapenko, and Michael Kornaros. 2022. "Thermophilic Dark Fermentation of Olive Mill Wastewater in Batch Reactors: Effect of pH and Organic Loading" Applied Sciences 12, no. 6: 2881. https://doi.org/10.3390/app12062881