Selective Chlorination and Extraction of Valuable Metals from Iron Precipitation Residues
Abstract
:Featured Application
Abstract
1. Introduction
2. Theoretical Considerations and Thermodynamic Calculations
3. Materials and Methods
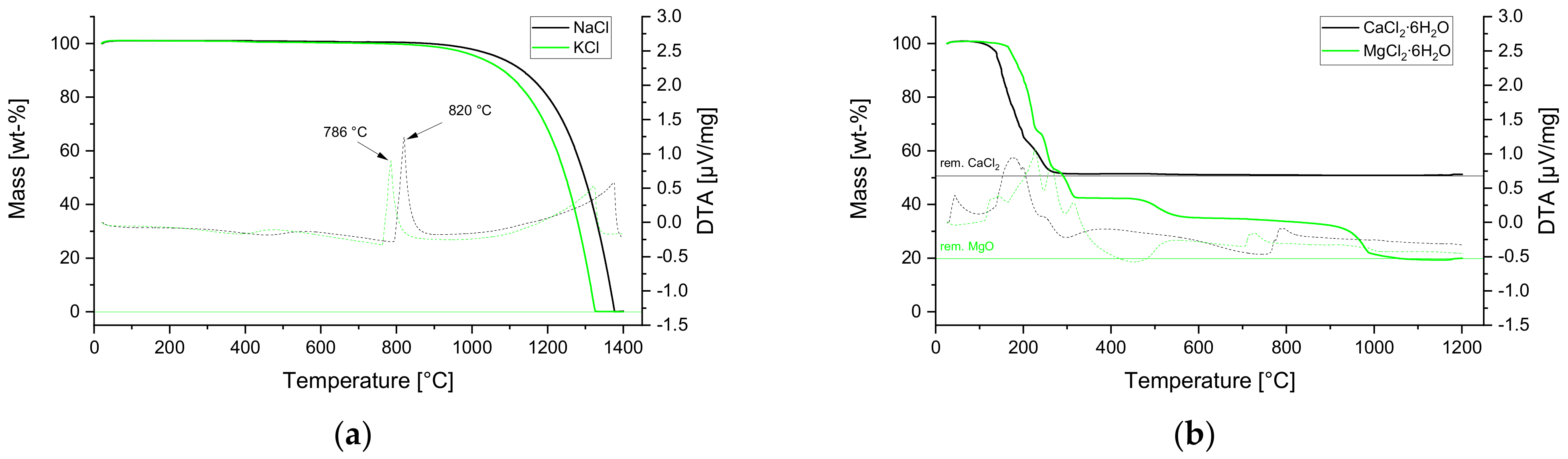
3.1. STA Campaigns with Pure Substances
3.2. Campaigns with Industrial Iron Precipitation Residues
4. Results
4.1. STA Trials
4.2. Campaigns with Industrial Residues in a Muffle Furnace
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayliss, P.; Kolitsch, U.; Nickel, E.H.; Pring, A. Alunite supergroup: Recommended nomenclature. Mineral. Mag. 2010, 74, 919–927. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and Their Application in Hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Sinclair, R.J. The Extractive Metallurgy of Zinc; Australasian Institute of Mining and Metallurgy: Carlton South, VIC, Australia, 2005; ISBN 9781613442166. [Google Scholar]
- Calla-Choque, D.; Nava-Alonso, F.; Fuentes-Aceituno, J.C. Acid decomposition and thiourea leaching of silver from hazardous jarosite residues: Effect of some cations on the stability of the thiourea system. J. Hazard. Mater. 2016, 317, 440–448. [Google Scholar] [CrossRef] [PubMed]
- González-Ibarra, A.A.; Nava-Alonso, F.; Uribe-Salas, A.; Castillo-Ventureño, E.N. Decomposition kinetics of industrial jarosite in alkaline media for the recovery of precious metals by cyanidation. Can. Metall. Q. 2016, 55, 448–454. [Google Scholar] [CrossRef]
- Ju, S.; Zhang, Y.; Zhang, Y.; Xue, P.; Wang, Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J. Hazard. Mater. 2011, 192, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Orko, I.; Kangas, P.; Lundström, M.; Koukkari, P. Hydrometallurgical Processing of Jarosite Waste to Value-Added Products. In 3rd Symposium on Urban Mining and Circular Economy, SUM2016; VTT Technical Research Centre of Finland Ltd.: Espoo, Finland, 2016. [Google Scholar]
- Linsong, W.; Peng, Z.; Yu, F.; Sujun, L.; Yue, Y.; Li, W.; Wei, S. Recovery of metals from jarosite of hydrometallurgical nickel production by thermal treatment and leaching. Hydrometallurgy 2020, 198, 105493. [Google Scholar] [CrossRef]
- Hoeber, L.; Steinlechner, S. A comprehensive review of processing strategies for iron precipitation residues from zinc hydrometallurgy. Clean. Eng. Technol. 2021, 4, 100214. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Hazardous jarosite use in developing non-hazardous product for engineering application. J. Hazard. Mater. 2006, 137, 1589–1599. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Recycling hazardous jarosite waste using coal combustion residues. Mater. Charact. 2010, 61, 1342–1355. [Google Scholar] [CrossRef]
- Pappu, A.; Thakur, V.K.; Patidar, R.; Asolekar, S.R.; Saxena, M. Recycling marble wastes and Jarosite wastes into sustainable hybrid composite materials and validation through Response Surface Methodology. J. Clean. Prod. 2019, 240, 118249. [Google Scholar] [CrossRef]
- Mymrin, V.; Vazquez Vaamonde, A. New construction materials from Spanish jarosite processing wastes. Miner. Eng. 1999, 12, 1399–1402. [Google Scholar] [CrossRef]
- Mymrin, V.A.; Ponte, H.A.; Impinnisi, P.R. Potential application of acid jarosite wastes as the main component of construction materials. Constr. Build. Mater. 2005, 19, 141–146. [Google Scholar] [CrossRef]
- Mehra, P.; Gupta, R.C.; Thomas, B.S. Properties of concrete containing jarosite as a partial substitute for fine aggregate. J. Clean. Prod. 2016, 120, 241–248. [Google Scholar] [CrossRef]
- Katsioti, M.; Boura, P.; Agatzini, S.; Tsakiridis, P.E.; Oustadakis, P. Use of jarosite/alunite precipitate as a substitute for gypsum in Portland cement. Cem. Concr. Compos. 2005, 27, 3–9. [Google Scholar] [CrossRef]
- Winters, J.; Vos, L.; Canoo, C. Goethite: From Residue to Secondary Building Material-Union Minière’s Graveliet ® Process. In Proceedings of the Lead-Zinc 2000 Symposium which was part of the TMS Fall Extraction & Process Metallurgy Meeting, Pittsburgh, PA, USA, 22–25 October 2000; Dutrizac, J.E., Ed.; TMS: Warrendale, PA, USA, 2000; pp. 903–916, ISBN 9781118805558. [Google Scholar]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Reductive leaching of jarosites by Aeromonas hydrophila. Miner. Eng. 2016, 95, 21–28. [Google Scholar] [CrossRef]
- Castro, L.; García-Balboa, C.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Effectiveness of anaerobic iron bio-reduction of jarosite and the influence of humic substances. Hydrometallurgy 2013, 131–132, 29–33. [Google Scholar] [CrossRef]
- Mäkinen, J.; Salo, M.; Hassinen, H.; Kinnunen, P. Comparison of Reductive and Oxidative Bioleaching of Jarosite for Valuable Metals Recovery. Solid State Phenom. 2017, 262, 24–27. [Google Scholar] [CrossRef]
- Boháček, J.; Šubrt, J.; Hanslík, T.; Tláskal, J. Preparing particulate magnetites with pigment properties from suspensions of basic iron(III) sulphates with the structure of jarosite. J. Mater. Sci. 1993, 28, 2827–2832. [Google Scholar] [CrossRef]
- Dutrizac, J.E. Converting jarosite residues into compact hematite products. JOM 1990, 42, 36–39. [Google Scholar] [CrossRef]
- Röpenack, A.; Böhmer, W.; Rieger, H. Verfahren zur Aufarbeitung von Jarosit-Haltigen Rückständen. European Patent Application 90202618.6, 2 October 1990. [Google Scholar]
- Cruells, M.; Roca, A.; Patiño, F.; Salinas, E.; Rivera, I. Cyanidation kinetics of argentian jarosite in alkaline media. Hydrometallurgy 2000, 55, 153–163. [Google Scholar] [CrossRef]
- Patiño, F.; Cruells, M.; Roca, A.; Salinas, E.; Pérez, M. Kinetics of alkaline decomposition and cyanidation of argentian ammonium jarosite in lime medium. Hydrometallurgy 2003, 70, 153–161. [Google Scholar] [CrossRef]
- Patiño, F.; Salinas, E.; Cruells, M.; Roca, A. Alkaline decomposition–cyanidation kinetics of argentian natrojarosite. Hydrometallurgy 1998, 49, 323–336. [Google Scholar] [CrossRef]
- Patiño, F.; Viñals, J.; Roca, A.; Núñez, C. Alkaline decomposition-cyanidation kinetics of argentian plumbojarosite. Hydrometallurgy 1994, 34, 279–291. [Google Scholar] [CrossRef]
- Hage, J.L.T.; Schuiling, R.D.; Vriend, S.P. Production of Magnetite from Sodiumjarosite under Reducing Hydrothermal Conditions. The Reduction of Fe III to Fe II with Cellulose. Can. Metall. Q. 1999, 38, 267–276. [Google Scholar] [CrossRef]
- Han, H.; Sun, W.; Hu, Y.; Jia, B.; Tang, H. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy. J. Hazard. Mater. 2014, 278, 49–54. [Google Scholar] [CrossRef]
- Riley, A.L.; Pepper, S.E.; Canner, A.J.; Brown, S.F.; Ogden, M.D. Metal recovery from jarosite waste—A resin screening study. Sep. Sci. Technol. 2018, 53, 22–35. [Google Scholar] [CrossRef]
- Rodriguez, N.; Machiels, L.; Onghena, B.; Spooren, J.; Binnemans, K. Selective recovery of zinc from goethite residue in the zinc industry using deep-eutectic solvents. RSC Adv. 2020, 10, 7328–7335. [Google Scholar] [CrossRef] [Green Version]
- Creedy, S.; Glinin, A.; Matusewicz, R.; Hughes, S.; Reuter, M. Ausmelt Technology for Treating Zinc Residues. World Metall. Erzmetall 2013, 66, 230. [Google Scholar]
- Salminen, J.; Nyberg, J.; Imris, M.; Heegaard, B.M. Smelting Jarosite and Sulphur Residue in a Plasma Furnace. In PBZN 2020: The 9th International Symposium on Lead and Zinc Processing; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 391–403. ISBN 978-3-030-37069-5. [Google Scholar]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Spada, E. Jarosite wastes reduction through blast furnace sludges for cast iron production. J. Environ. Chem. Eng. 2019, 7, 102966. [Google Scholar] [CrossRef]
- Piga, L.; Stoppa, L.; Massidda, R. Recycling of industrial goethite wastes by thermal treatment. Resour. Conserv. Recycl. 1995, 14, 11–20. [Google Scholar] [CrossRef]
- Pelino, M.; Cantalini, C.; Abbruzzese, C.; Plescia, P. Treatment and recycling of goethite waste arising from the hydrometallurgy of zinc. Hydrometallurgy 1996, 40, 25–35. [Google Scholar] [CrossRef]
- Pelino, M.; Cantalini, C.; Boattini, P.P.; Abbruzzese, C.; Rincon, J.; Garcia Hernandez, J.E. Glass-ceramic materials obtained by recycling goethite industrial wastes. Resour. Conserv. Recycl. 1994, 10, 171–176. [Google Scholar] [CrossRef]
- Steinlechner, S.; Antrekowitsch, J. Thermodynamic Considerations for a Pyrometallurgical Extraction of Indium and Silver from a Jarosite Residue. Metals 2018, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Wegscheider, S.; Steinlechner, S.; Leuchtenmüller, M. Innovative Concept for the Recovery of Silver and Indium by a Combined Treatment of Jarosite and Electric Arc Furnace Dust. JOM 2017, 69, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, H.; Zhang, G.; Kang, J.; Wang, C. Comprehensive recovery and recycle of jarosite residues from zinc hydrometallurgy. Chem. Eng. J. Adv. 2020, 3, 100023. [Google Scholar] [CrossRef]
- Guan, J.; Wang, S.; Ren, H.; Guo, Y.; Yuan, H.; Yan, X.; Guo, J.; Gu, W.; Su, R.; Liang, B.; et al. Indium recovery from waste liquid crystal displays by polyvinyl chloride waste. RSC Adv. 2015, 5, 102836–102843. [Google Scholar] [CrossRef]
- Park, K.-S.; Sato, W.; Grause, G.; Kameda, T.; Yoshioka, T. Recovery of indium from In2O3 and liquid crystal display powder via a chloride volatilization process using polyvinyl chloride. Thermochim. Acta 2009, 493, 105–108. [Google Scholar] [CrossRef]
- Takahashi, K.; Sasaki, A.; Dodbiba, G.; Sadaki, J.; Sato, N.; Fujita, T. Recovering Indium from the Liquid Crystal Display of Discarded Cellular Phones by Means of Chloride-Induced Vaporization at Relatively Low Temperature. Metall. Mater. Trans. A 2009, 40, 891–900. [Google Scholar] [CrossRef]
- Gustafsson, A.M.; Steenari, B.-M.; Ekberg, C. Recycling of CIGS solar cell waste materials—separation of copper, indium and gallium by high-temperature chlorination reaction with ammonium chloride. Sep. Sci. Technol. 2015, 38, 2415–2425. [Google Scholar] [CrossRef]
- Li, H.; Ma, A.; Srinivasakannan, C.; Zhang, L.; Li, S.; Yin, S. Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process. J. Alloys Compd. 2018, 763, 241–249. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Tian, Q.; Zhang, L. Pyrite enhanced chlorination roasting and its efficacy in gold and silver recovery from gold tailing. Sep. Purif. Technol. 2020, 250, 117168. [Google Scholar] [CrossRef]
- Jaafar, I.; Griffiths, A.J.; Hopkins, A.C.; Steer, J.M.; Griffiths, M.H.; Sapsford, D.J. An evaluation of chlorination for the removal of zinc from steelmaking dusts. Miner. Eng. 2011, 24, 1028–1030. [Google Scholar] [CrossRef]
- Kurashima, K.; Matsuda, K.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. A combined kinetic and thermodynamic approach for interpreting the complex interactions during chloride volatilization of heavy metals in municipal solid waste fly ash. Waste Manag. 2019, 87, 204–217. [Google Scholar] [CrossRef]
- Wang, S.-J.; He, P.-J.; Lu, W.-T.; Shao, L.-M.; Zhang, H. Comparison of Pb, Cd, Zn, and Cu chlorination during pyrolysis and incineration. Fuel 2017, 194, 257–265. [Google Scholar] [CrossRef]
- Nowak, B.; Frías Rocha, S.; Aschenbrenner, P.; Rechberger, H.; Winter, F. Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: Influence of the chloride type. Chem. Eng. J. 2012, 179, 178–185. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Xiang, J.; Hu, S.; Yao, H. Mechanism on heavy metals vaporization from municipal solid waste fly ash by MgCl2⋅6H2O. Waste Manag. 2016, 49, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Silver Price. Available online: https://www.goldpreis.de/silberpreis/ (accessed on 29 December 2021).
- Gold Price. Available online: https://www.goldpreis.de/ (accessed on 29 December 2021).
- Bismuth Price. Available online: https://price.metal.com/Bismuth-Selenium-Tellurium (accessed on 29 December 2021).
- Copper Price. Available online: https://www.finanzen.at/rohstoffe/kupferpreis (accessed on 29 December 2021).
- Indium Price. Available online: http://www.indium-preis.de/ (accessed on 29 December 2021).
- Lead Price. Available online: https://www.finanzen.at/rohstoffe/bleipreis (accessed on 29 December 2021).
- Tin Price. Available online: https://www.finanzen.at/rohstoffe/zinnpreis (accessed on 29 December 2021).
- Zinc Price. Available online: https://www.finanzen.at/rohstoffe/zinkpreis (accessed on 29 December 2021).
- Zboril, R.; Mashlan, M.; Papaefthymiou, V.; Hadjipanayis, G. Thermal decomposition of Fe2(SO4)3: Demonstration of Fe2O3 polymorphism. J. Radioanal. Nucl. Chem. 2003, 255, 413–417. [Google Scholar] [CrossRef]
- Pelovski, Y.; Pietkova, W.; Gruncharov, I.; Pacewska, B.; Pysiak, J. The thermal decomposition of aluminum sulfate in different gas phase environments. Thermochim. Acta 1992, 205, 219–224. [Google Scholar] [CrossRef]
- Scheidema, M.N.; Taskinen, P. Decomposition Thermodynamics of Magnesium Sulfate. Ind. Eng. Chem. Res. 2011, 50, 9550–9556. [Google Scholar] [CrossRef]








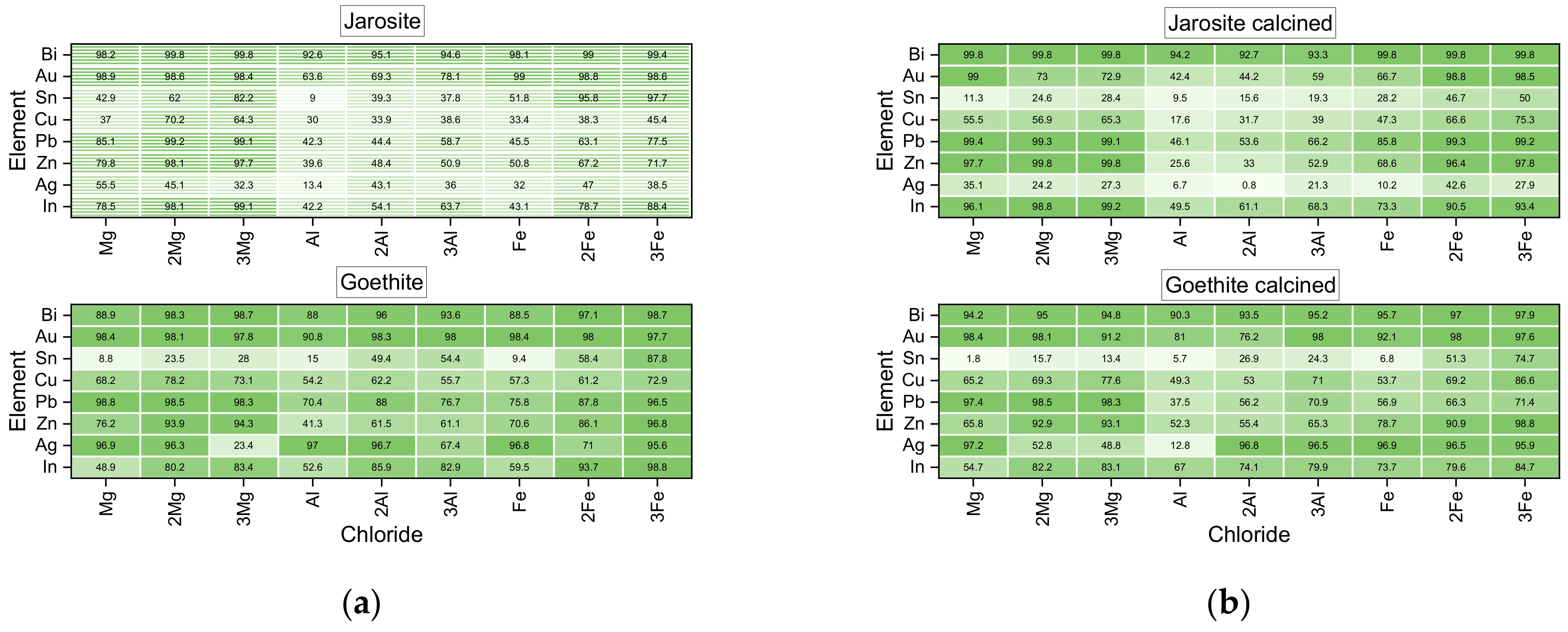

| Concentration [wt %] | Jarosite | Goethite | Ni-Jarosite | Jarosite Calcined | Goethite Calcined | Ni-Jarosite Calcined |
|---|---|---|---|---|---|---|
| Structural Elements | ||||||
| Fe | 25.6 ± 1.8 | 29.1 ± 1.9 | 36.6 ± 0.3 | 39.9 ± 1.7 | 36.6 ± 1.3 | 61.9 ± 2.3 |
| S | 13.83 ± 0.24 | 7.16 ± 0.33 | 6.38 ± 0.08 | 7.86 ± 0.04 | 6.61 ± 0.02 | 0.19 ± 0 |
| K | 2.77 ± 0.05 | 0.2 ± 0 | 0.08 ± 0.01 | 4.03 ± 0.05 | 0.23 ± 0.05 | 0.12 ± 0 |
| Na | 1.46 ± 0.06 | <0.05 | 0.01 ± 0 | 2.24 ± 0.15 | 0.06 ± 0.01 | 0.15 ± 0.01 |
| Ca | 0.53 ± 0.02 | 4.78 ± 0.24 | 0.01 ± 0 | 0.78 ± 0.01 | 6.08 ± 0.03 | 0.01 ± 0 |
| As | 0.42 ± 0.01 | 0.19 ± 0.01 | 0.26 ± 0 | 0.63 ± 0.01 | 0.23 ± 0 | 0.45 ± 0.01 |
| Mg | 0.19 ± 0 | 0.15 ± 0 | <0.01 | 0.30 ± 0 | 0.18 ± 0 | <0.01 |
| Al | 0.37 ± 0 | 0.89 ± 0.03 | 0.19 ± 0.02 | 0.56 ± 0 | 1.10 ± 0 | 0.26 ± 0 |
| Valuable Elements | ||||||
| Zn | 3.57 ± 0.07 | 9.60 ± 0.21 | 0 | 5.37 ± 0.05 | 11.90 ± 0.08 | 0 |
| Pb | 1.26 ± 0 | 0.68 ± 0 | 0.06 ± 0 | 1.9 ± 0 | 0.84 ± 0 | 0.08 ± 0 |
| Ni | 3.08 ± 0.04 | 5.43 ± 0.26 | ||||
| [ppm] | ||||||
| In | 591 ± 3 | 410 ± 9 | 0 | 882 ± 11 | 495 ± 9 | 0 |
| Ag | 282 ± 40 | 136 ± 32 | 0 | 403 ± 79 | 194 ± 26 | 0 |
| Cu | 2713 ± 25 | 9113 ± 67 | 47 ± 2 | 4053 ± 5 | 11,000 | 68 ± 2 |
| Sn | 1703 ± 26 | 308 ± 6 | <20 | 2617 ± 52 | 376 ± 5 | <20 |
| Bi | 941 ± 8 | 181 ± 2 | 17 ± 1 | 1440 ± 20 | 224 ± 3 | 28 ± 0 |
| [ppb] | ||||||
| Au | 367 ± 9 | 264 ± 13 | 73 ± 10 | 547 ± 18 | 333 ± 103 | 114 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höber, L.; Witt, K.; Steinlechner, S. Selective Chlorination and Extraction of Valuable Metals from Iron Precipitation Residues. Appl. Sci. 2022, 12, 3590. https://doi.org/10.3390/app12073590
Höber L, Witt K, Steinlechner S. Selective Chlorination and Extraction of Valuable Metals from Iron Precipitation Residues. Applied Sciences. 2022; 12(7):3590. https://doi.org/10.3390/app12073590
Chicago/Turabian StyleHöber, Lukas, Kerrin Witt, and Stefan Steinlechner. 2022. "Selective Chlorination and Extraction of Valuable Metals from Iron Precipitation Residues" Applied Sciences 12, no. 7: 3590. https://doi.org/10.3390/app12073590







