Computer Simulations of Dual-Antenna Microwave Ablation and Comparison to Experimental Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
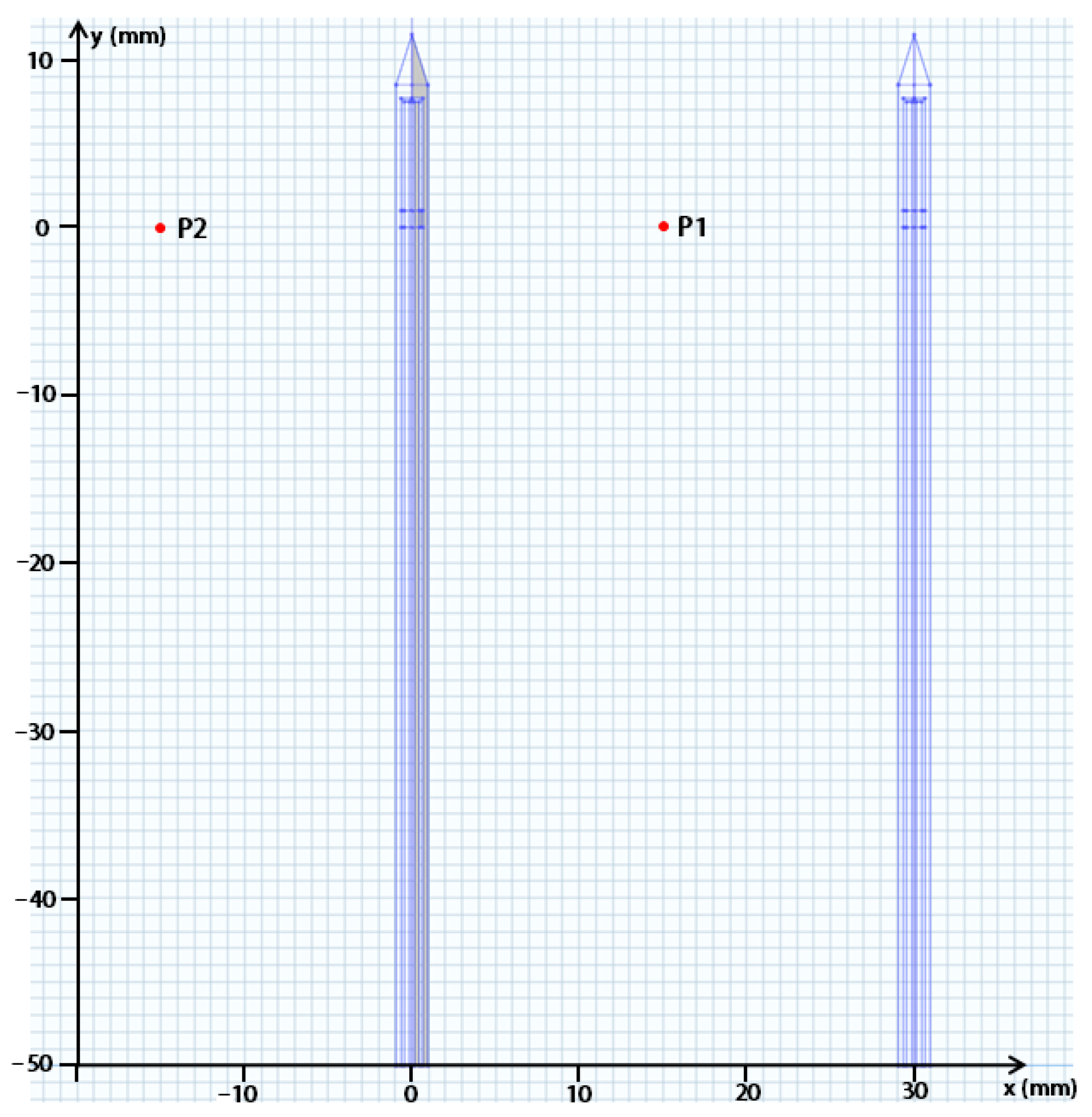
2.2. Finite Element Modeling
2.3. Model Validation Based on Ex Vivo Experiments
2.4. Influence of Heating Parameters and Blood Perfusion on Coagulation Zone
3. Results
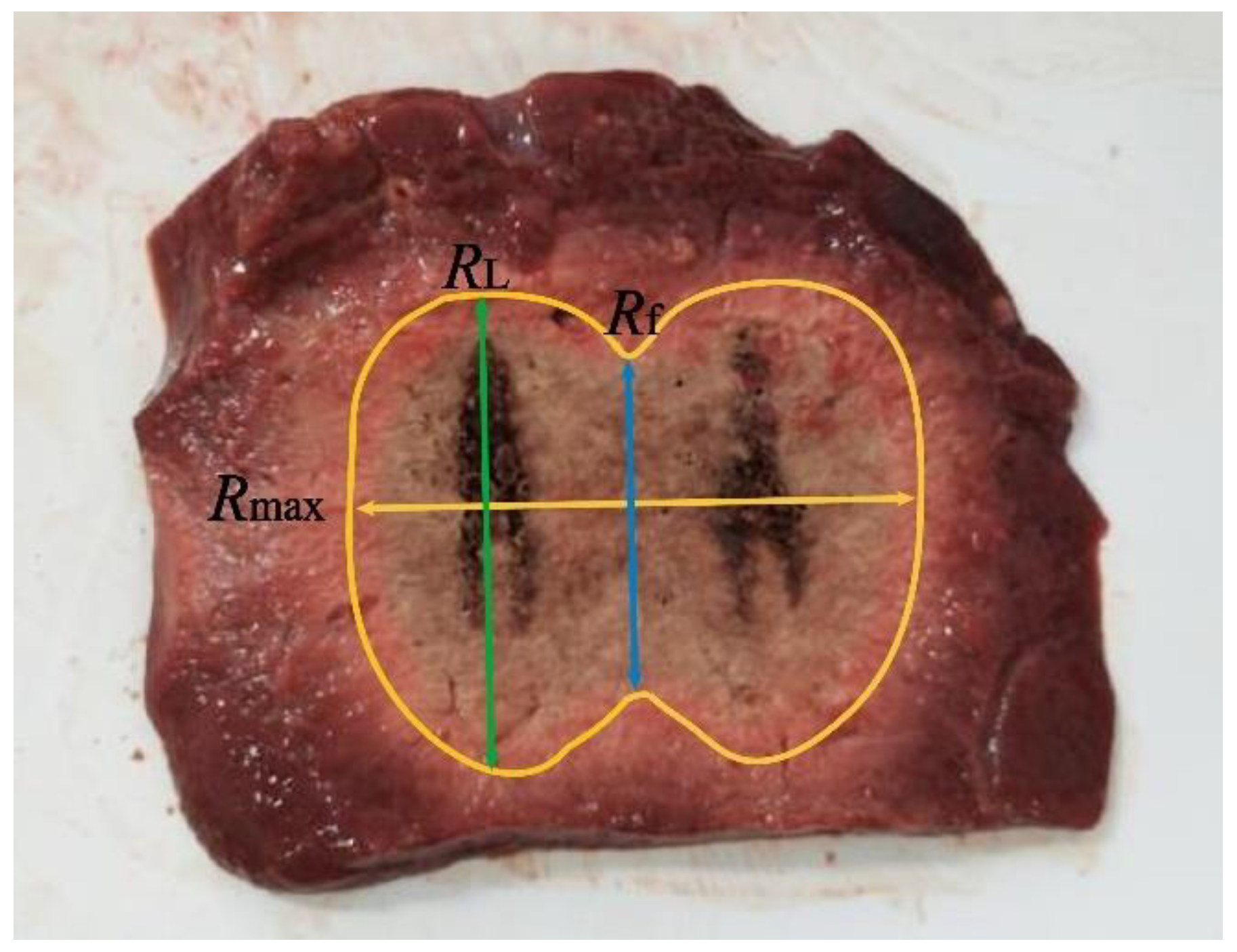
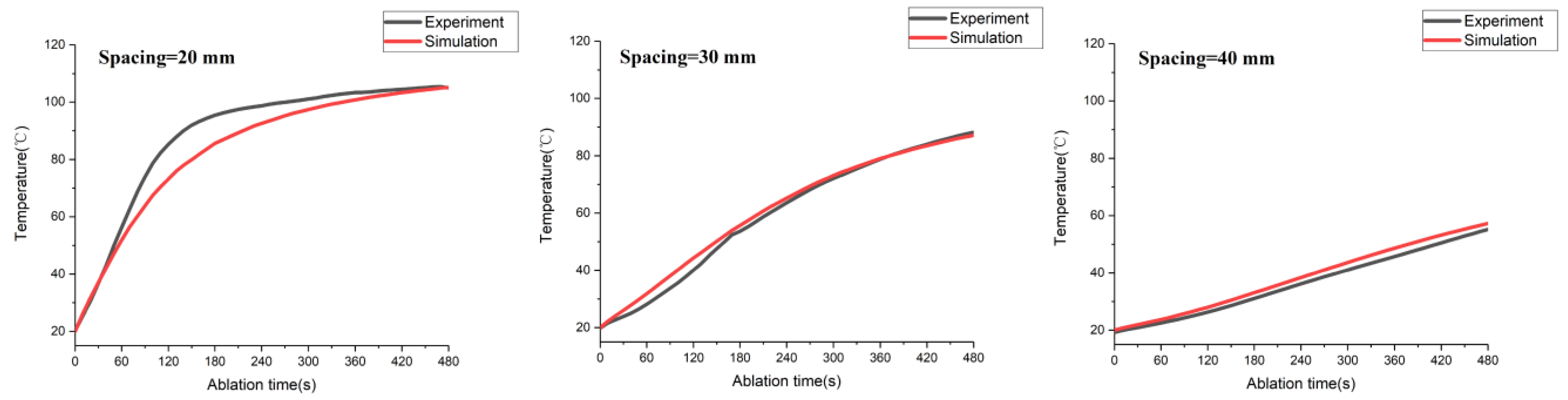
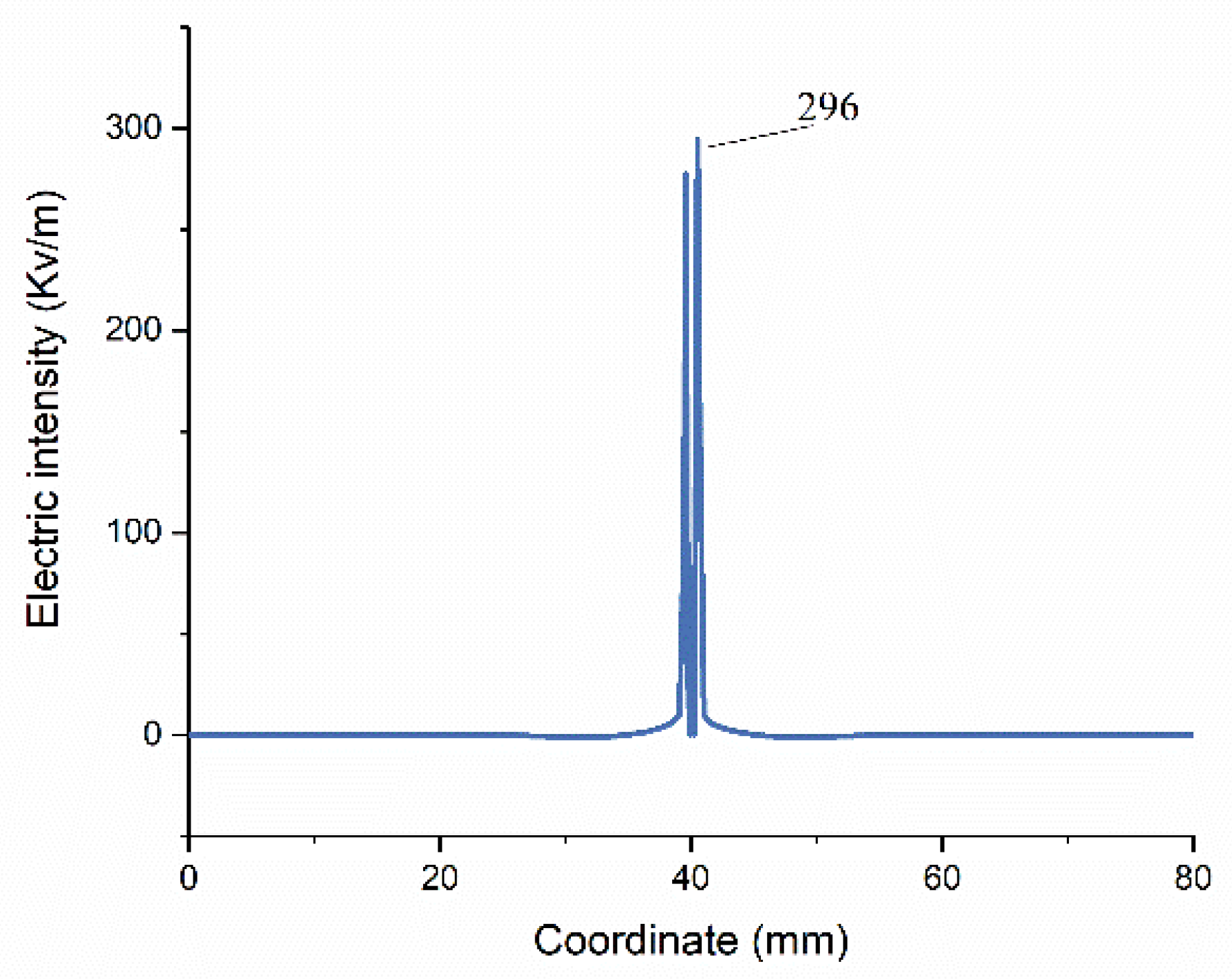
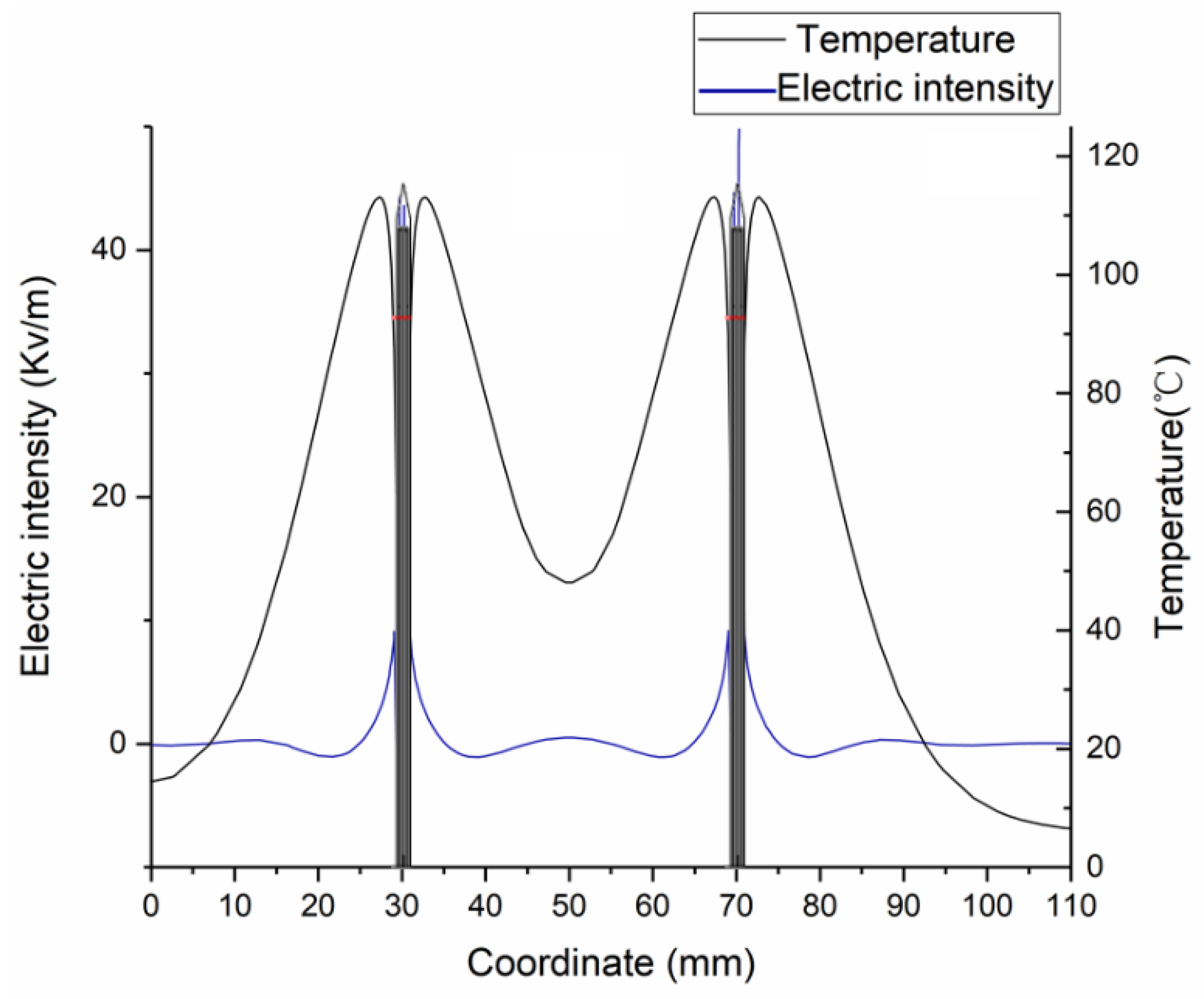
3.1. FEM Model Verification by Ex Vivo Experiments
3.1.1. Experimental Validation Based on Temperature Distribution
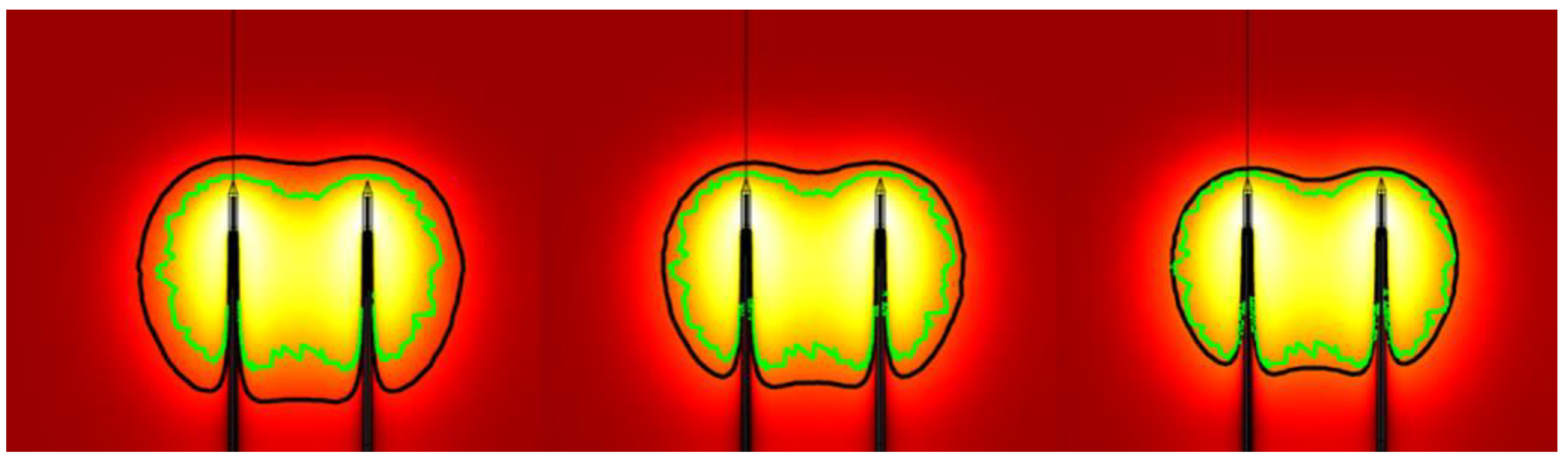
3.1.2. Experimental Validation Based on Coagulation Zone Models
3.2. Influences of Heating Parameters on MWA Coagulation Zone
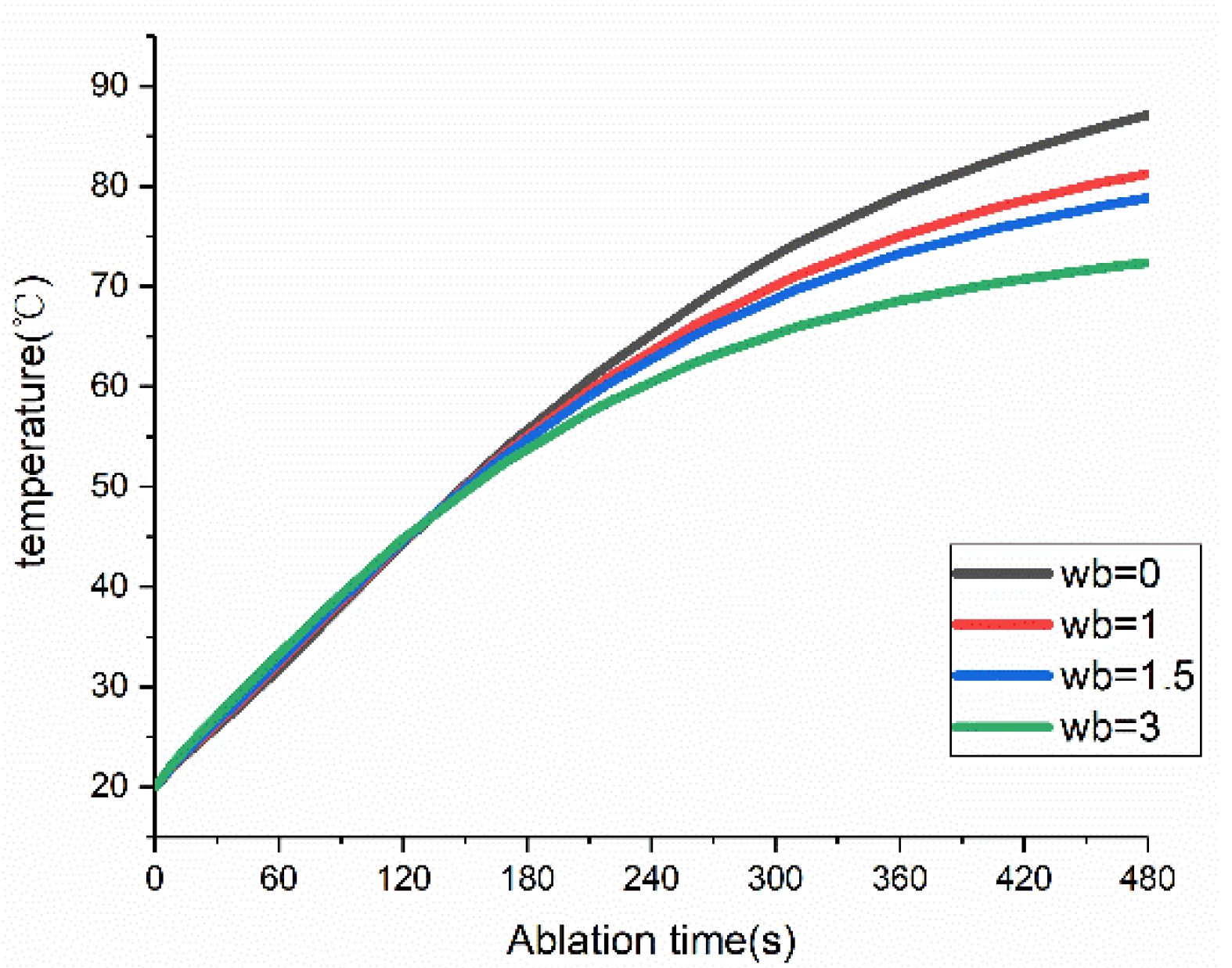
3.3. Influences of Blood Perfusion on MWA Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961–987. [Google Scholar] [CrossRef]
- Tanis, E.; Spliethoff, J.W.; Evers, D.J.; Langhout, G.C.; Snaebornsson, P.; Prevoo, W.; Hendriks, B.H.W.; Ruers, T.J.M. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur. J. Surg. Oncol. 2016, 42, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; An, V.; Ha, H.; Phan, S.; Lam, V.; Pleass, H. Hepatic resection for malignant liver tumors in the elderly: A systematic review and meta-analysis. ANZ J. Surg. 2015, 85, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, 990–1005. [Google Scholar] [CrossRef] [Green Version]
- Brace, C.L. Microwave ablation Technology: What every User should know. Curr. Probl. Diagn. Radiol. 2009, 38, 61–67. [Google Scholar] [CrossRef] [Green Version]
- de Baère, T.; Roux, C.; Deschamps, F.; Tselikas, L.; Guiu, B. Evaluation of a New CT-Guided Robotic System for Percutaneous Antenna Insertion for Thermal Ablation of Liver Tumors: A Prospective Pilot Study. Cardiovasc. Interv. Radiol. 2022, 45, 1701–1709. [Google Scholar] [CrossRef]
- Giampiero, F.; Franca, M.M.; Laura, R.; Francesco, G.; Eugenio, C.; Fulvia, T.; Ilario, D.S. Role of Contrast-Enhanced Ultrasound in the Detection of Complications After Ultrasound-Guided Liver Interventional Procedures. J. Ultrasound Med. 2020, 40, 1665–1673. [Google Scholar] [CrossRef]
- Wright, A.S.; Lee, F.T.; Mahvi, D.M. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann. Surg. Oncol. 2003, 10, 275–283. [Google Scholar] [CrossRef]
- Toshikuni, N.; Takuma, Y.; Goto, T.; Yamamoto, H. Prognostic Factors in Hepatitis C Patients with a Single Small Hepatocellular Carcinoma after Radiofrequency Ablation. Hepatogastroenterology 2012, 59, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Phasukkit, P.; Tungjitkusolmun, S.; Sangworasil, M. Finite-Element Analysis and In Vitro Experiments of Placement Configurations Using Triple Antennas in Microwave Hepatic Ablation. IEEE Trans. Biomed. Eng. 2019, 56, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Clibbon, K.L.; McCowen, A. Thermal modeling of non-ideal interstitial microwave antenna array hyperthermia for the treatment of cancer. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest, Atlanta, GA, USA, 14–18 June 1993; Volume 2, pp. 1147–1150. [Google Scholar] [CrossRef]
- Yu, N.C.; Lu, D.S.K.; Raman, S.S.; Dupuy, D.E.; Simon, C.J.; Lassman, C.; Aswad, B.I.; Ianniti, D.; Busuttil, R.W. Hepatocellular carcinoma: MWA with multiple straight and loop antenna clusters-Pilot comparison with pathologic findings. Radiology 2006, 239, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.P.; Turner, P.F.; Hamilton, B. Interstitial microwave transition from hyperthermia to ablation: Historical perspectives and current trends in thermal therapy. Int. J. Hyperth. 2010, 26, 415–433. [Google Scholar] [CrossRef]
- Trembly, B.S. The Effects of Driving Frequency and Antenna Length on Power Deposition Within a Microwave Antenna Array Used for Hyperthermia. IEEE Trans. Biomed. Eng. 1985, 32, 152–157. [Google Scholar] [CrossRef]
- Mukherjee, S.; Curto, S.; Albin, N.; Natarajan, B.; Prakash, P. Multiple-antenna MWA: Analysis of non-parallel antenna implants. In Proceedings of the PIE Conference on Energy-Based Treatment of Tissue and Assessment VIII, San Francisco, CA, USA, 8–9 February 2015; p. 93260U. [Google Scholar] [CrossRef]
- Aparna, V.C.; Gandhi, A.S.; Harsh, R. Minimally Invasive MWA Antenna Designs at 915 MHz and 2.45 GHz. In Proceedings of the 28th National Conference on Communications (NCC), Mumbai, India, 24–27 May 2022; pp. 280–284. [Google Scholar] [CrossRef]
- Gas, P. Study on interstitial microwave hyperthermia with multi-slot coaxial antenna. Rev. Roum. Sci. Tech.-Ser. Electrotech. Energetique 2014, 59, 215–224. [Google Scholar] [CrossRef]
- Poch, F.G.M.; Rieder, C.; Ballhausen, H.; Knappe, V.; Ritz, J.; Gemeinhardt, O.; Kreis, M.; Lehmann, K.S. The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int. J. Hyperth. 2016, 32, 749–756. [Google Scholar] [CrossRef]
- Shi, W.; Liang, P.; Zhu, Q.; Yu, X.L.; Shao, Q.J.; Lu, T.; Wang, Y.; Dong, B.W. Microwave ablation: Results with double 915 MHz antennae in ex vivo bovine livers. Eur. J. Radiol. 2011, 79, 214–217. [Google Scholar] [CrossRef]
- Kok, H.P.; van der Zee, J.; Guirado, F.N.; Bakker, A.; Datta, N.R.; Abdel-Rahman, S.; Schmidt, M.; Wust, P.; Crezee, J. Treatment planning facilitates clinical decision making for hyperthermia treatments. Int. J. Hyperth. 2021, 38, 532–551. [Google Scholar] [CrossRef]
- Claudio, A.; Laura, F.; Vanni, L. Tissue shrinkage in microwave ablation of liver: An ex vivo predictive model. Int. J. Hyperth. 2017, 33, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Wu, S.C.; Wu, Z.Y.; Gao, H.J.; Huang, S.Y. Influences of blood flow parameters on temperature distribution during liver tumor microwave ablation. Front. Biosci. Landmark 2021, 26, 504–516. [Google Scholar] [CrossRef]
- Gas, P.; Kurgan, E. Comparative Analysis Between the 2D and 3D Models of Interstitial Microwave Hyperthermia. In Proceedings of the 2016 17th International Conference Computational Problems of Electrical Engineering (CPEE), Sandomierz, Poland, 14–17 September 2016; IEEE Xplore. pp. 1–4. [Google Scholar] [CrossRef]
- Chiang, J.; Wang, P.; Brace, C.L. Computational modeling of microwave tumor ablations. Int. J. Hyperth. 2013, 29, 308–317. [Google Scholar] [CrossRef]
- Arkin, H.; Xu, L.X.; Holmes, K.R. Recent developments in modeling heat transfer in blood-perfused tissues. IEEE Trans. Biomed. Eng. 1994, 41, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, X.; Wu, S.; Zhou, Z.; Bai, Y. 2450-MHz microwave ablation temperature simulation using temperature-dependence feedback of characteristic parameters. Int. J. RF Microw. Comput.-Aided Eng. 2019, 29, e21488. [Google Scholar] [CrossRef] [Green Version]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical models to evaluate the temperature increase induced by ex vivo microwave thermal ablation. Phys. Med. Biol. 2015, 60, 3287–3311. [Google Scholar] [CrossRef]
- Portosi, V.; Loconsole, A.; Valori, M.; Marrocco, V.; Fassi, I.; Bonelli, F.; Pascazio, G.; Lampignano, V.; Fasano, A.; Prudenzano, F. Low-Cost Mini-Invasive Microwave Needle Applicator for Cancer Thermal Ablation: Feasibility Investigation. IEEE Sens. J. 2021, 21, 14027–14034. [Google Scholar] [CrossRef]
- Qadri, A.M.; Chia, N.J.Y.; Ooi, E.H. Effects of saline volume on lesion formation during saline-infused radiofrequency ablation. Appl. Math. Model. 2017, 43, 360–371. [Google Scholar] [CrossRef]
- He, X.; Bhowmick, S.; Bischof, J.C. Thermal therapy in urologic systems: A comparison of Arrhenius and thermal isoeffective dose models in predicting hyperthermic injury. J. Biomech. Eng. 2009, 131, 074507. [Google Scholar] [CrossRef]
- Gas, P.; Kurgan, E. Evaluation of thermal damage of hepatic tissue during thermotherapy based on the arrhenius model. In Proceedings of the 2018 Progress in Applied Electrical Engineering (PAEE), Koscielisko, Poland, 18–22 June 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Rossmanna, C.; Haemmerich, D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Bhowmik, A.; Repaka, R. Thermal analysis of induced damage to the healthy cell during RFA of breast tumor. J. Therm. Biol. 2016, 58, 80–90. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Z.; Dong, L.; Zhang, G.; Wang, Y.; Liang, P. Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: Results in ex vivo porcine livers. Eur. J. Radiol. 2012, 81, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Thorne, A.M.; Ubbink, R.; Brüggenwirth, I.M.A.; Nijsten, M.; Porte, R.; de Meijer, V.E. Hyperthermia-induced changes in liver physiology and metabolism: A rationale for hyperthermic machine perfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, 43–50. [Google Scholar] [CrossRef]
- Kress, R.; Roemer, R. A comparative analysis of thermal blood perfusion measurement techniques. J. Biomech. Eng. 1987, 109, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Dong, B.W.; Yu, X.L.; Yi, D.J.; Cheng, Z.G.; Su, L.; Peng, J.S.; Nan, Q.; Wang, H.J. Computer-aided dynamic simulation of microwave-induced thermal distribution in coagulation of liver cancer. IEEE Trans. Biomed. Eng. 2001, 48, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Melnik, R. Thermal ablation of biological tissues in disease treatment: A review of computational models and future directions. Electromagn. Biol. Med. 2020, 39, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Miller, W.H.; Roemer, R.B.; CETAS, T.C. Errors between two-dimensional and three-dimensional thermal model predictions of hyperthermia treatments. Int. J. Hyperth. 1990, 6, 175–191. [Google Scholar] [CrossRef]
- Schumann, C.; Rieder, C.; Preusser, T. Interactive multi-criteria planning for radiofrequency ablation. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 879–889. [Google Scholar] [CrossRef]
- Karampatzakis, A.; Kühn, S.; Tsanidis, G.; Neufeld, E.; Samaras, T.; Kuster, N. Heating characteristics of antenna arrays used in microwave ablation: A theoretical parametric study. Comput. Biol. Med. 2013, 43, 1321–1327. [Google Scholar] [CrossRef]
- Chiang, J.; Hynes, K.A.; Bedoya, M.; Brace, C.L. A Dual-Slot Microwave Antenna for More Spherical Ablation Zones: Ex Vivo and in vivo Validation. Radiology 2013, 268, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, N.; Baragona, M.; Lavezzo, V.; Maessen, R.; Veroy, K. Simulation study of the cooling effect of blood vessels and blood coagulation in hepatic radio-frequency ablation. Int. J. Hyperth. 2021, 38, 95–104. [Google Scholar] [CrossRef]
- Barnat, N.; Grisey, A.; Gerold, B.; Yon, S.; Anquez, J.; Aubry, J. Vein wall shrinkage induced by thermal coagulation with high-intensity-focused ultrasound: Numerical modeling and in vivo experiments in sheep. Int. J. Hyperth. 2020, 37, 1238–1247. [Google Scholar] [CrossRef] [PubMed]

| Property | Value |
|---|---|
| Outer diameter of the antenna | 1.9 mm |
| Inner diameter of the antenna | 1.3 mm |
| Distance from emission point to tip of antenna | 11.5 mm |
| Total length | 191.5 mm |
| Tissue | (kg/m3) | Cp (J/(kg·K)) | (W/(m·K)) | (S/m) | |
|---|---|---|---|---|---|
| Porcine liver | 1050 | 3628 | 0.565 | 1.8 | 44.3 |
| Spacing | Characteristic Length | Experiment Data | IS-54 Model | Arrhenius Model | Error-1 * | Error-2 ** |
|---|---|---|---|---|---|---|
| 40 mm | Rmax (mm) | 69.9 | 74.00 | 66.00 | 5.87% | −5.58% |
| Rf (mm) | 20.10 | 21.00 | N/A | 4.48% | N/A | |
| RL (mm) | 42.5 | 44.00 | 35.1 | 3.53% | −17.41% | |
| 30 mm | Rmax (mm) | 57.88 | 62.00 | 55.00 | 7.12% | −4.98% |
| Rf (mm) | 37.75 | 40.00 | 24.00 | 5.96% | −36.42% | |
| RL (mm) | 42.63 | 45.00 | 37.00 | 5.56% | −13.21% | |
| 20 mm | Rmax (mm) | 53.13 | 55.00 | 44.00 | 3.52% | −17.18% |
| Rf (mm) | 46.25 | 48.00 | 37.00 | 3.78% | −20.00% | |
| RL (mm) | 47.13 | 48.00 | 37.00 | 1.85% | −21.49% |
| Spacing | 40 W | 50 W | 60 W |
|---|---|---|---|
| 20 mm | 70 | 34 | 28 |
| 30 mm | 110 | 90 | 80 |
| 40 mm | 240 | 210 | 190 |
| (kg/(m3·s) | 1.0 | 1.5 | 3.0 | |
|---|---|---|---|---|
| Antenna Matrix Spacing, Total Input Power | ||||
| 2 cm, 40 W | 18.1% | 25.2% | 41.9% | |
| 2 cm, 50 W | 17.3% | 24.4% | 40.6% | |
| 2 cm, 60 W | 16.9% | 23.8% | 39.8% | |
| 3 cm, 40 W | 18.4% | 25.9% | 43.0% | |
| 3 cm, 50 W | 17.6% | 24.6% | 41.5% | |
| 3 cm, 60 W | 17.0% | 23.8% | 40.1% | |
| 4 cm, 40 W | 20.2% | 28.7% | 48.0% | |
| 4 cm, 50 W | 19.1% | 26.8% | 45.2% | |
| 4 cm, 60 W | 18.1% | 25.6% | 43.4% | |
| Mean | 18.4% | 25.4% | 42.5% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, S.; Gao, H.; Liu, J.; Zhang, Y.; Wu, S. Computer Simulations of Dual-Antenna Microwave Ablation and Comparison to Experimental Measurements. Appl. Sci. 2023, 13, 26. https://doi.org/10.3390/app13010026
Wang J, Huang S, Gao H, Liu J, Zhang Y, Wu S. Computer Simulations of Dual-Antenna Microwave Ablation and Comparison to Experimental Measurements. Applied Sciences. 2023; 13(1):26. https://doi.org/10.3390/app13010026
Chicago/Turabian StyleWang, Jinying, Shengyang Huang, Hongjian Gao, Ju Liu, Yubo Zhang, and Shuicai Wu. 2023. "Computer Simulations of Dual-Antenna Microwave Ablation and Comparison to Experimental Measurements" Applied Sciences 13, no. 1: 26. https://doi.org/10.3390/app13010026





