Cold Physical Plasma Toxicity in Breast and Oral Squamous Carcinoma In Vitro and in Patient-Derived Cancer Tissue Ex Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Plasma Treatment
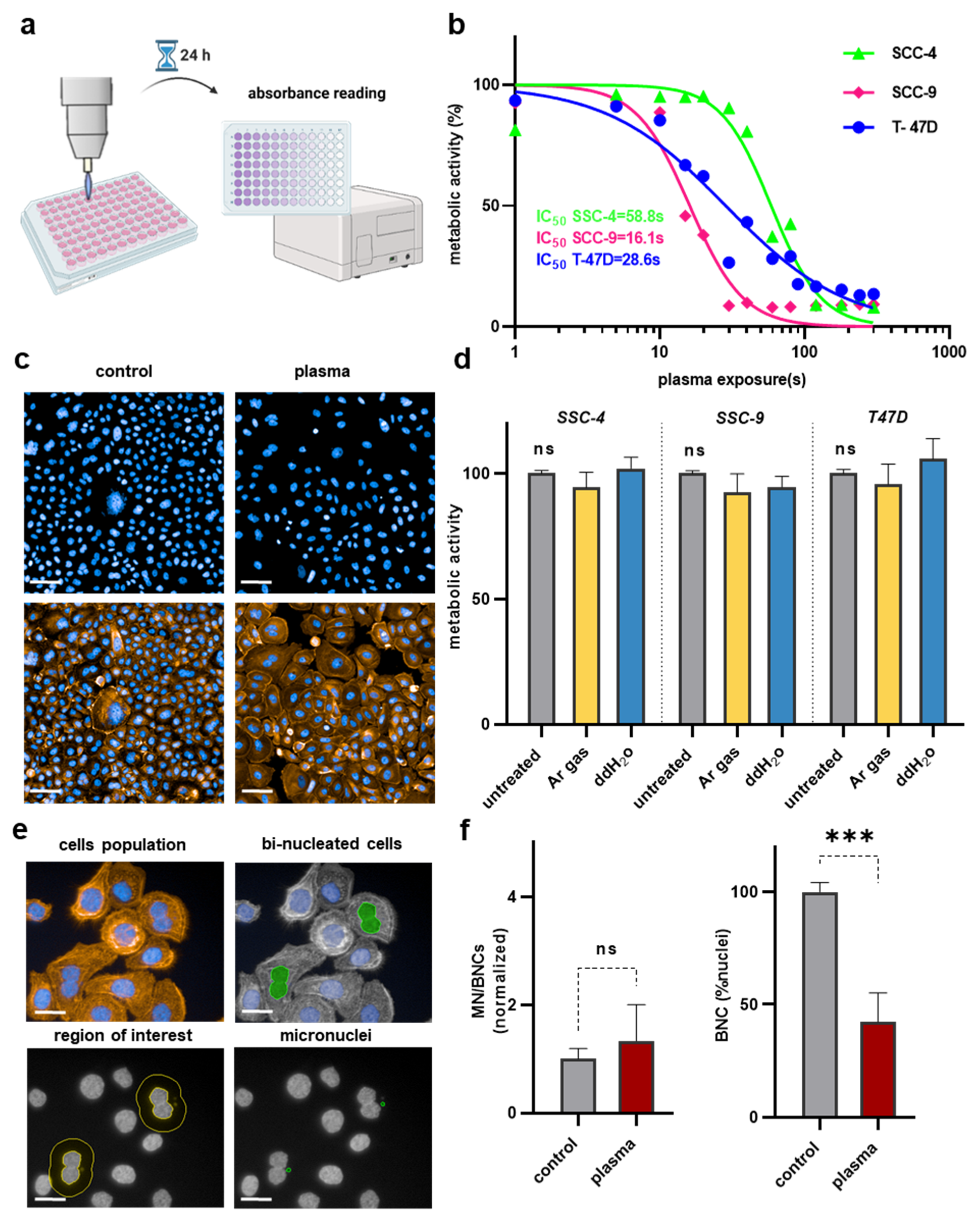
2.3. Metabolic Activity Assay
2.4. OECD-Based Micronucleus Assay
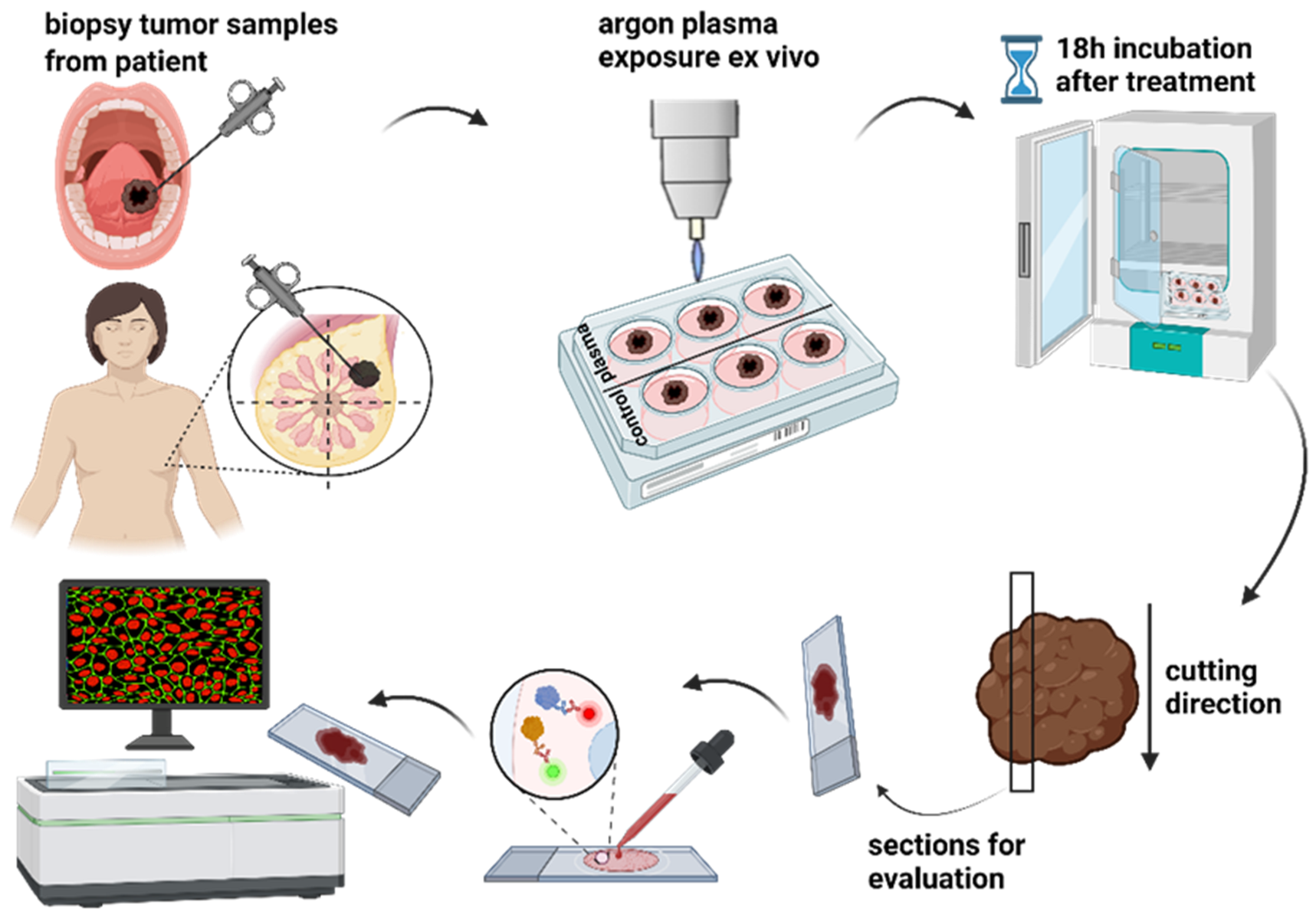
2.5. Ex Vivo Biopsies and Exposure to Cold Physical Plasma
2.6. Tissue Section Preparation, Immunofluorescence Staining, Imaging, and Data Evaluation
2.7. Statistical Data Analysis
3. Results
3.1. Argon Plasma Showed Treatment Time-Dependent Toxicity in T-47D, SCC-4, and SCC-9 Cells but Did Not Lead to Genotoxic Effects in Human Keratinocytes
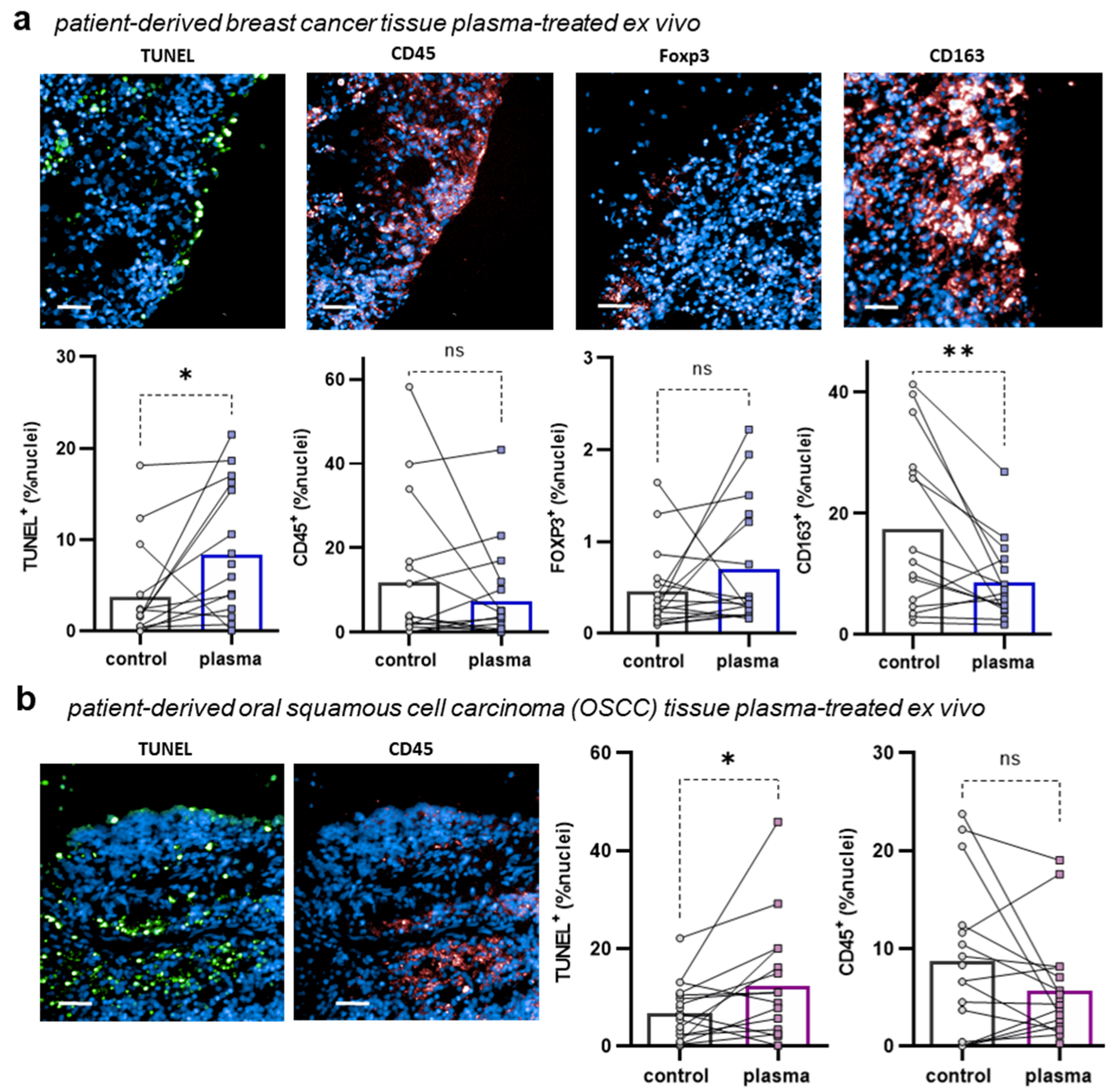
3.2. Argon Plasma Induced Apoptosis in Patient-Derived BC and OSCC Cancer Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, C.H.; Parker, J.S.; Karaca, G.; Wu, J.; Funkhouser, W.K.; Moore, D.; Butterfoss, D.; Xiang, D.; Zanation, A.; Yin, X.; et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 2004, 5, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Cueni, L.N.; Hegyi, I.; Shin, J.W.; Albinger-Hegyi, A.; Gruber, S.; Kunstfeld, R.; Moch, H.; Detmar, M. Tumor lymphangiogenesis and metastasis to lymph nodes induced by cancer cell expression of podoplanin. Am. J. Pathol. 2010, 177, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Srinivasalu, V.K.; Subramaniam, N.; Balasubramanian, D.; Kumar, N.; Philip, A.; Susan, A.; Pushpaja, K.U.; Nair, A.R.; Thankappan, K.; Jose, W.; et al. Concurrent chemoradiotherapy for head and neck cancers in older patients: Outcomes and their determinants. Indian J. Cancer 2019, 56, 261–266. [Google Scholar] [CrossRef]
- Petit, C.; Lacas, B.; Pignon, J.P.; Le, Q.T.; Gregoire, V.; Grau, C.; Hackshaw, A.; Zackrisson, B.; Parmar, M.K.B.; Lee, J.W.; et al. Chemotherapy and radiotherapy in locally advanced head and neck cancer: An individual patient data network meta-analysis. Lancet Oncol. 2021, 22, 727–736. [Google Scholar] [CrossRef]
- Zheng, D.W.; Deng, W.W.; Song, W.F.; Wu, C.C.; Liu, J.; Hong, S.; Zhuang, Z.N.; Cheng, H.; Sun, Z.J.; Zhang, X.Z. Biomaterial-mediated modulation of oral microbiota synergizes with PD-1 blockade in mice with oral squamous cell carcinoma. Nat. Biomed. Eng. 2022, 6, 32–43. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.; Dong, W.; Lin, M.; He, J.; Zhang, X.; Tian, T.; Yang, Y.; Chen, K.; Lei, Q.Y.; et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc. Natl. Acad. Sci. USA 2022, 119, e2114851119. [Google Scholar] [CrossRef]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxidative Med. Cell. Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Clemen, R. Plasma, cancer, immunity. J. Phys. D-Appl. Phys. 2022, 55, 473003. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Toyokuni, S.; Kajiyama, H.; Kikkawa, F.; Hori, M. Cancer Treatments Using Low-Temperature Plasma. Curr. Med. Chem. 2021, 28, 8549–8558. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Bazaka, K.; Thompson, E.W.; Ostrikov, K.K. Cold Atmospheric Plasma: A Promising Controller of Cancer Cell States. Cancers 2020, 12, 3360. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R.; Niessner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical Gas Plasma Jet Technology Targets Murine Melanoma in an Immunogenic Fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Moritz, J.; Helfrich, I.; Boeckmann, L.; Weltmann, K.D.; Emmert, S.; Metelmann, H.R.; Stoffels, I.; von Woedtke, T. Ex Vivo Exposure of Human Melanoma Tissue to Cold Physical Plasma Elicits Apoptosis and Modulates Inflammation. Appl. Sci. 2020, 10, 1971. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D-Appl. Phys. 2018, 51, 23. [Google Scholar] [CrossRef]
- Saadati, F.; da Silva Brito, W.A.; Emmert, S.; Bekeschus, S. Optimized High-Content Imaging Screening Quantifying Micronuclei Formation in Polymer-Treated HaCaT Keratinocytes. Nanomaterials 2022, 12, 4463. [Google Scholar] [CrossRef]
- Lukamowicz, M.; Kirsch-Volders, M.; Suter, W.; Elhajouji, A. In vitro primary human lymphocyte flow cytometry based micronucleus assay: Simultaneous assessment of cell proliferation, apoptosis and MN frequency. Mutagenesis 2011, 26, 763–770. [Google Scholar] [CrossRef]
- Rezaeinezhad, A.; Eslami, P.; Mirmiranpour, H.; Ghomi, H. The effect of cold atmospheric plasma on diabetes-induced enzyme glycation, oxidative stress, and inflammation; in vitro and in vivo. Sci. Rep. 2019, 9, 19958. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.D.; Wende, K. Basic Research in Plasma Medicine—A Throughput Approach from Liquids to Cells. J. Vis. Exp. 2017, 129, e56331. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Christodoulou, A.M.; Tachliabouri, M.; Meropoulis, S.; Christopoulou, M.E.; Karalis, T.T.; Chatzopoulos, A.; Skandalis, S.S. Cold Atmospheric Plasma Attenuates Breast Cancer Cell Growth Through Regulation of Cell Microenvironment Effectors. Front. Oncol. 2021, 11, 826865. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Z.; Wang, Z.; Obenchain, R.; Wen, D.; Li, H.; Wirz, R.E.; Gu, Z. Portable air-fed cold atmospheric plasma device for postsurgical cancer treatment. Sci. Adv. 2021, 7, eabg5686. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.U.; Cho, J.H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K.; et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, S.; Zhang, H.; Kong, X.; Ding, L.; Shen, J.; Lan, Y.; Cheng, C.; Zhu, T.; Xia, W. Selective effects of non-thermal atmospheric plasma on triple-negative breast normal and carcinoma cells through different cell signaling pathways. Sci. Rep. 2017, 7, 7980. [Google Scholar] [CrossRef]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Arndt, S.; Wacker, E.; Li, Y.F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxidative Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- De Morais Gouvêa Lima, G.; Carta, C.F.L.; Borges, A.C.; Nishime, T.M.C.; da Silva, C.A.V.; Caliari, M.V.; Mayer, M.P.A.; Kostov, K.G.; Koga-Ito, C.Y. Cold Atmospheric Pressure Plasma Is Effective against P. gingivalis (HW24D-1) Mature Biofilms and Non-Genotoxic to Oral Cells. Appl. Sci. 2022, 12, 7247. [Google Scholar] [CrossRef]
- Boxhammer, V.; Li, Y.F.; Koritzer, J.; Shimizu, T.; Maisch, T.; Thomas, H.M.; Schlegel, J.; Morfill, G.E.; Zimmermann, J.L. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat. Res. 2013, 753, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Ispirjan, M.; Freund, E.; Kinnen, F.; Moritz, J.; Saadati, F.; Eckroth, J.; Singer, D.; Stope, M.B.; Wende, K.; et al. Gas Plasma Exposure of Glioblastoma Is Cytotoxic and Immunomodulatory in Patient-Derived GBM Tissue. Cancers 2022, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Saadati, F.; Moritz, J.; Berner, J.; Freund, E.; Miebach, L.; Helfrich, I.; Stoffels, I.; Emmert, S.; Bekeschus, S. Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet. Int. J. Mol. Sci. 2021, 22, 11446. [Google Scholar] [CrossRef] [PubMed]
- Gelbrich, N.; Miebach, L.; Berner, J.; Freund, E.; Saadati, F.; Schmidt, A.; Stope, M.; Zimmermann, U.; Burchardt, M.; Bekeschus, S. Medical gas plasma augments bladder cancer cell toxicity in preclinical models and patient-derived tumor tissues. J. Adv. Res. 2023, 47, 209–223. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Zhang, L.; Zhang, J.; Xiao, Y.; Wu, Q.; Wang, Y.; Zhan, Q. Tumor-associated macrophage (TAM)-derived CCL22 induces FAK addiction in esophageal squamous cell carcinoma (ESCC). Cell. Mol. Immunol. 2022, 19, 1054–1066. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Mahdikia, H.; Saadati, F.; Freund, E.; Gaipl, U.S.; Majidzadeh, A.K.; Shokri, B.; Bekeschus, S. Gas plasma irradiation of breast cancers promotes immunogenicity, tumor reduction, and an abscopal effect in vivo. Oncoimmunology 2020, 10, 1859731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadati, F.; Jahanbakhshi, F.; Mahdikia, H.; Abbasvandi, F.; Ghomi, H.; Yazdani, N.; Aghazadeh, K.; Emmert, S.; Bekeschus, S. Cold Physical Plasma Toxicity in Breast and Oral Squamous Carcinoma In Vitro and in Patient-Derived Cancer Tissue Ex Vivo. Appl. Sci. 2023, 13, 6472. https://doi.org/10.3390/app13116472
Saadati F, Jahanbakhshi F, Mahdikia H, Abbasvandi F, Ghomi H, Yazdani N, Aghazadeh K, Emmert S, Bekeschus S. Cold Physical Plasma Toxicity in Breast and Oral Squamous Carcinoma In Vitro and in Patient-Derived Cancer Tissue Ex Vivo. Applied Sciences. 2023; 13(11):6472. https://doi.org/10.3390/app13116472
Chicago/Turabian StyleSaadati, Fariba, Fahimeh Jahanbakhshi, Hamed Mahdikia, Fereshteh Abbasvandi, Hamid Ghomi, Nasrin Yazdani, Keyvan Aghazadeh, Steffen Emmert, and Sander Bekeschus. 2023. "Cold Physical Plasma Toxicity in Breast and Oral Squamous Carcinoma In Vitro and in Patient-Derived Cancer Tissue Ex Vivo" Applied Sciences 13, no. 11: 6472. https://doi.org/10.3390/app13116472





