The Use of Mulch and Shading Improves the Survival of Sclerophyllous Species Established in Island Plots in Central Chile
Abstract
1. Introduction
2. Materials and Methods
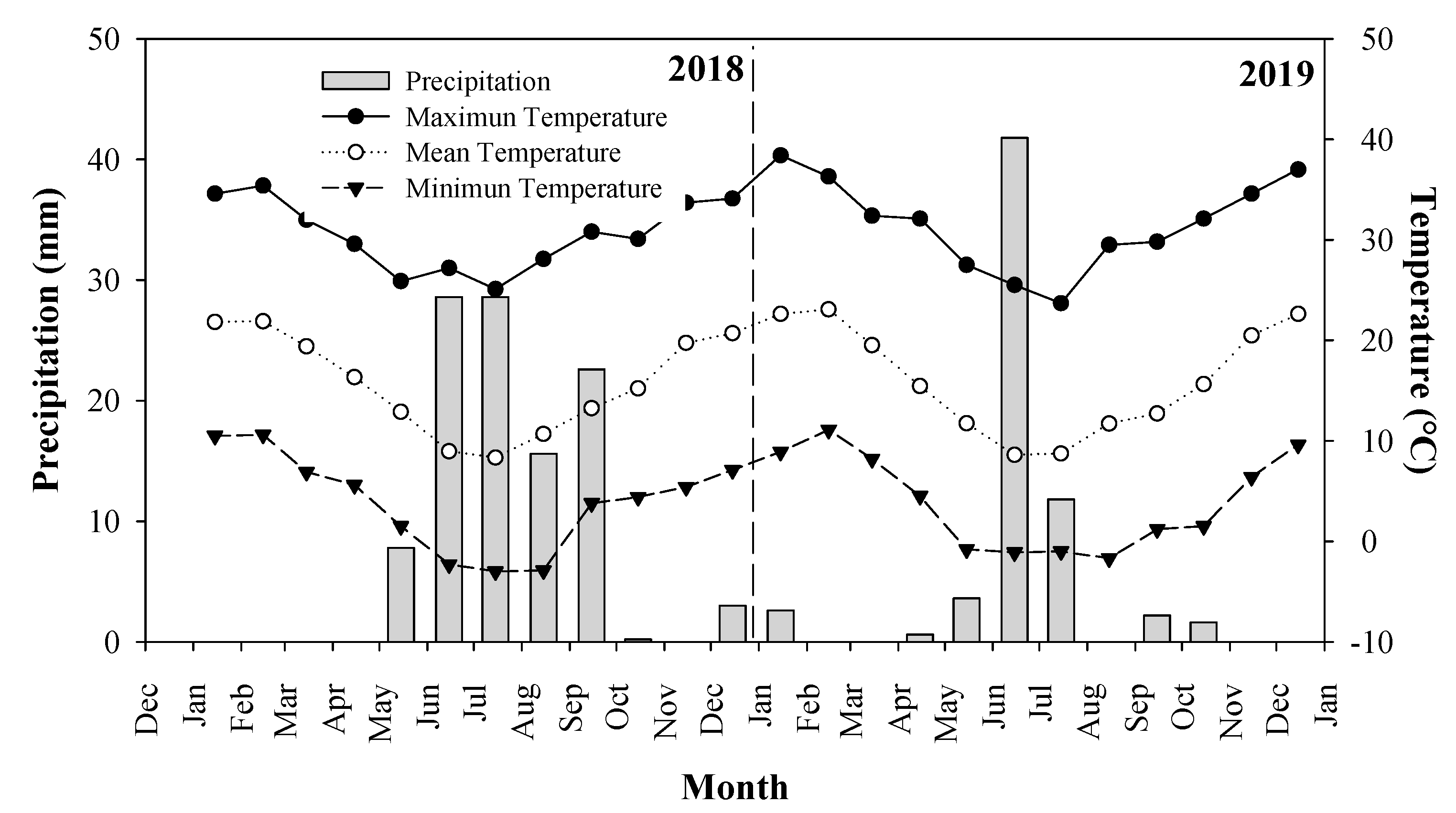
2.1. Study Site
2.2. Experiment Installation
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van de Wouw, P.; Echeverría, C.; Rey-Benayas, J.M.; Holmgren, M. Persistent Acacia savannas replace Mediterranean sclerophyllous forests in South America. For. Ecol. Manag. 2011, 262, 1100–1108. [Google Scholar] [CrossRef]
- Bannister, J.R.; Vidal, O.J.; Teneb, E.; Sandoval, V. Latitudinal patterns and regionalization of plant diversity along a 4270-km gradient in continental Chile. Austral Ecol. 2012, 37, 500–509. [Google Scholar] [CrossRef]
- Root-Bernstein, M.; Jaksic, F. The Chilean Espinal: Restoration for a Sustainable Silvopastoral System. Restor. Ecol. 2013, 21, 409–414. [Google Scholar] [CrossRef]
- Heilmayr, R.; Echeverría, C.; Fuentes, R.; Lambin, E.F. A plantation-dominated forest transition in Chile. Appl. Geogr. 2016, 75, 71–82. [Google Scholar] [CrossRef]
- Benavidez-Silva, C.; Jensen, M.; Pliscoff, P. Future Scenarios for Land Use in Chile: Identifying Drivers of Change and Impacts over Protected Area System. Land 2021, 10, 408. [Google Scholar] [CrossRef]
- Fuentealba, A.; Duran, L.; Morales, N.S. The impact of forest science in Chile: History, contribution, and challenges. Can. J. For. Res. 2021, 51, 753–765. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Fischer, C.; Colinas, C. Climate Change Effects on Mediterranean Forests and Preventive Measures. New For. 2006, 33, 29–40. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Alvarez-Garreton, C.; Barichivich, J.; Boisier, J.P.; Christie, D.; Galleguillos, M.; LeQuesne, C.; McPhee, J.; Zambrano-Bigiarini, M. The 2010–2015 megadrought in central Chile: Impacts on regional hydroclimate and vegetation. Hydrol. Earth Syst. Sci. 2017, 21, 6307–6327. [Google Scholar] [CrossRef]
- Acevedo, M.; Álvarez, C.; Cartes, E.; Dumroese, R.K.; González, M. Production and establishment techniques for the restoration of Nothofagus alessandrii, an endangered keystone species in a Mediterranean forest. New For. 2019, 51, 159–174. [Google Scholar] [CrossRef]
- Hoyos-Santillan, J.; Miranda, A.; Lara, A.; Sepulveda-Jauregui, A.; Zamorano-Elgueta, C.; Gómez-González, S.; Vásquez-Lavín, F.; Garreaud, R.D.; Rojas, M. Diversifying Chile’s climate action away from industrial plantations. Environ. Sci. Policy 2021, 124, 85–89. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Climatol. 2019, 40, 1–19. [Google Scholar] [CrossRef]
- Acevedo, M.; Álvarez-Maldini, C.; Dumroese, R.K.; Bannister, J.R.; Cartes, E.; González, M. Native Plant Production in Chile. Is It Possible to Achieve Restoration Goals by 2035? Land 2021, 10, 71. [Google Scholar] [CrossRef]
- Palma, A.C.; Laurance, S.G.W. A review of the use of direct seeding and seedling plantings in restoration: What do we know and where should we go? Appl. Veg. Sci. 2015, 18, 561–568. [Google Scholar] [CrossRef]
- Becerra, P.I.; Smith-Ramírez, C.; Armesto, J.J. Altitudinal and interannual variation in seedling survival of tree species in central Chile: Implications for sclerophyllous forest restoration. Bosque 2016, 37, 539–547. [Google Scholar] [CrossRef]
- Reis, A.; Campanha, F.; Bazzo de Espindola, M.; Kochntopp, N.; Lopez de Souza, L. Restoration of damaged land areas: Using nucleation to improve successional processes. Nat. Conser. 2003, 1, 85–92. [Google Scholar]
- Saha, S.; Kuehne, C.; Bauhus, J. Lessons learned from oak cluster planting trials in central Europe. Can. J. For. Res. 2017, 47, 139–148. [Google Scholar] [CrossRef]
- Méndez, M.; García, D.; Maestre, F.T.; Escudero, A. More Ecology is Needed to Restore Mediterranean Ecosystems: A Reply to Valladares and Gianoli. Restor. Ecol. 2008, 16, 210–216. [Google Scholar] [CrossRef]
- Bannister, J.R.; Vargas-Gaete, R.; Ovalle, J.F.; Acevedo, M.; Fuentes-Ramirez, A.; Donoso, P.J.; Promis, A.; Smith-Ramírez, C. Major bottlenecks for the restoration of natural forests in Chile. Restor. Ecol. 2018, 26, 1039–1044. [Google Scholar] [CrossRef]
- Young, T.P.; Petersen, D.A.; Clary, J.J. The ecology of restoration: Historical links, emerging issues and unexplored realms. Ecol. Lett. 2005, 8, 662–673. [Google Scholar] [CrossRef]
- Bechara, F.C.; Dickens, S.J.; Farrer, E.C.; Larios, L.; Spotswood, E.N.; Mariotte, P.; Suding, K.N. Neotropical rainforest restoration: Comparing passive, plantation and nucleation approaches. Biodivers. Conserv. 2016, 25, 2021–2034. [Google Scholar] [CrossRef]
- Holl, K.D.; Zahawi, R.A.; Cole, R.J.; Ostertag, R.; Cordell, S. Planting Seedlings in Tree Islands Versus Plantations as a Large-Scale Tropical Forest Restoration Strategy. Restor. Ecol. 2011, 19, 470–479. [Google Scholar] [CrossRef]
- Padilla, F.M.; Miranda, J.d.D.; Ortega, R.; Hervás, M.; Sánchez, J.; Pugnaire, F.I. Does shelter enhance early seedling survival in dry environments? A test with eight Mediterranean species. Appl. Veg. Sci. 2011, 14, 31–39. [Google Scholar] [CrossRef]
- Quiroz, I.A.; Espinoza, S.E.; Yáñez, M.A.; Magni, D.C.R. The use of shelters improves growth and survival of the endangered Nothofagus alessandrii after 5 years in a Mediterranean drought-prone site. Ecol. Eng. 2021, 164, 106220. [Google Scholar] [CrossRef]
- Puértolas, J.; Oliet, J.A.; Jacobs, D.F.; Benito, L.F.; Peñuelas, J.L. Is light the key factor for success of tube shelters in forest restoration plantings under Mediterranean climates? For. Ecol. Manag. 2010, 260, 610–617. [Google Scholar] [CrossRef]
- Piñeiro, J.; Maestre, F.T.; Bartolomé, L.; Valdecantos, A. Ecotechnology as a tool for restoring degraded drylands: A meta-analysis of field experiments. Ecol. Eng. 2013, 61, 133–144. [Google Scholar] [CrossRef]
- Gutiérrez, B.; Chung, P. Evaluación de Supervivencia y Desarrollo Inicial de 42 Progenies de Peumo (Cryptocarya alba (Mol.) Looser) Establecidas En La Provincia de Arauco, Región Del BíoBío, Chile; Instituto Forestal: San Lorenzo, Paraguay, 2016; pp. 36–44. [Google Scholar]
- Rojas-Arévalo, N.; Ovalle, J.F.; Oliet, J.A.; Piper, F.I.; Valenzuela, P.; Ginocchio, R.; Arellano, E.C. Solid shelter tubes alleviate summer stresses during outplanting in drought-tolerant species of Mediterranean forests. New For. 2021, 55, 555–569. [Google Scholar] [CrossRef]
- Oliet, J.A.; Puértolas, J.; Valenzuela, P.; Vázquez de Castro, A. Light Transmissivity of Tree Shelters Interacts with Site Environment and Species Ecophysiology to Determine Outplanting Performance in Mediterranean Climates. Land 2021, 10, 753. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Zhao, X.; Tian, L.; Wang, Y. Experimental study of mulching effects on water restoration of deep, desiccated soil in a loess hilly region. Environ. Technol. 2021, 43, 1–11. [Google Scholar] [CrossRef]
- Dodson, E.K.; Peterson, D.W. Mulching effects on vegetation recovery following high severity wildfire in north-central Washington State, USA. For. Ecol. Manag. 2010, 260, 1816–1823. [Google Scholar] [CrossRef]
- Coello-Gomez, J.; Fuentes-Boix, C.; Pique, M. Innovative soil conditioning and mulching techniques for forest restoration in Mediterranean conditions. In Proceedings of the International Conference on Reforestation Challenges, Belgrade, Serbia, 3–6 June 2015; pp. 201–210. [Google Scholar]
- Wang, J.; Liu, H.; Wu, X.; Li, C.; Wang, X. Effects of different types of mulches and legumes for the restoration of urban abandoned land in semi-arid northern China. Ecol. Eng. 2017, 102, 55–63. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Tan, C.; Wang, X.; Xia, M. Effects of Film Mulching on Plant Growth and Nutrients in Artificial Soil: A Case Study on High Altitude Slopes. Sustainability 2021, 13, 11026. [Google Scholar] [CrossRef]
- Blanco-García, A.; Sáenz-Romero, C.; Martorell, C.; Alvarado-Sosa, P.; Lindig-Cisneros, R. Nurse-plant and mulching effects on three conifer species in a Mexican temperate forest. Ecol. Eng. 2011, 37, 994–998. [Google Scholar] [CrossRef]
- Santana, V.M.; Alday, J.G.; Baeza, M.J. Mulch application as post-fire rehabilitation treatment does not affect vegetation recovery in ecosystems dominated by obligate seeders. Ecol. Eng. 2014, 71, 80–86. [Google Scholar] [CrossRef]
- Miranda, A.; Lara, A.; Altamirano, A.; Di Bella, C.; González, M.E.; Julio Camarero, J. Forest browning trends in response to drought in a highly threatened mediterranean landscape of South America. Ecol. Indic. 2020, 115, 106401. [Google Scholar] [CrossRef]
- Santibáñez, F. Atlas Agroclimático de Chile. Estado Actual y Tendencias Del Clima. Tomo III: Regiones de Valparaíso, Metropolitana, O’Higgins y Maule; Valus Limitada: Santiago, Chile, 2017; p. 50. [Google Scholar]
- Ovalle, J.F.; Arellano, E.C.; Ginocchio, R. Trade-Offs between Drought Survival and Rooting Strategy of Two South American Mediterranean Tree Species: Implications for Dryland Forests Restoration. Forests 2015, 6, 3733–3747. [Google Scholar] [CrossRef]
- Coutand, C.; Dupraz, C.; Jaouen, G.; Ploquin, S.; Adam, B. Mechanical stimuli regulate the allocation of biomass in trees: Demonstration with young Prunus avium trees. Ann. Bot. 2008, 101, 1421–1432. [Google Scholar] [CrossRef]
- Milla-Moreno, E.; Guy, R.D. Growth response, uptake and mobilization of metals in native plant species on tailings at a Chilean copper mine. Int. J. Phytoremediation 2021, 23, 539–547. [Google Scholar] [CrossRef]
- Becerra, P.I.; Cruz, G.; Ríos, S.; Castelli, G. Importance of irrigation and plant size in the establishment success of different native species in a degraded ecosystem of central Chile. Bosque 2013, 34, 23–24. [Google Scholar] [CrossRef]
- Root-Bernstein, M.; Jaksic, F.M. Ecosystem process interactions between central Chilean habitats. Glob. Ecol. Conserv. 2015, 3, 776–788. [Google Scholar] [CrossRef]
| N | D | H | |||
|---|---|---|---|---|---|
| Mean (mm) | CV (%) | Mean (cm) | CV (%) | ||
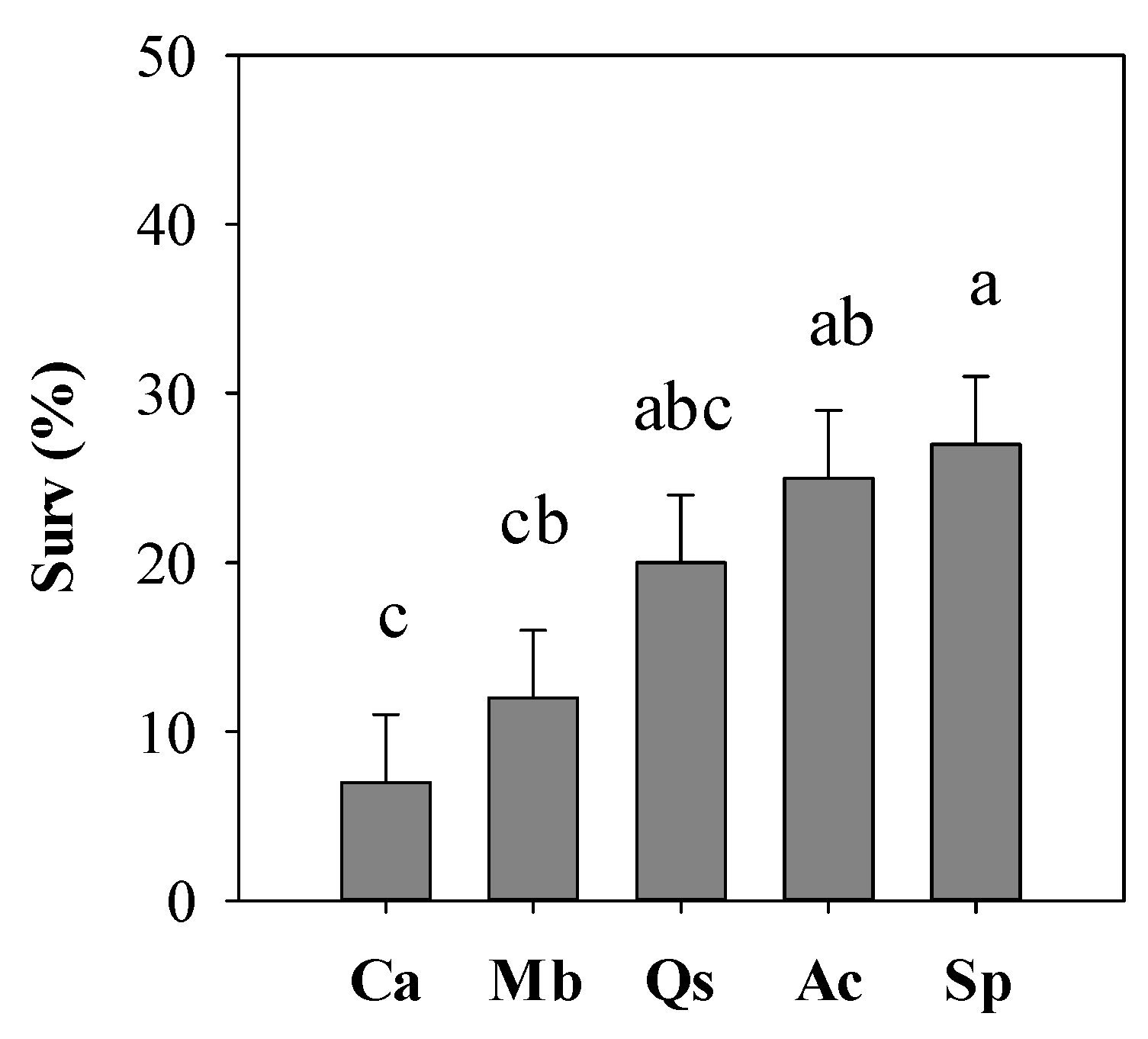
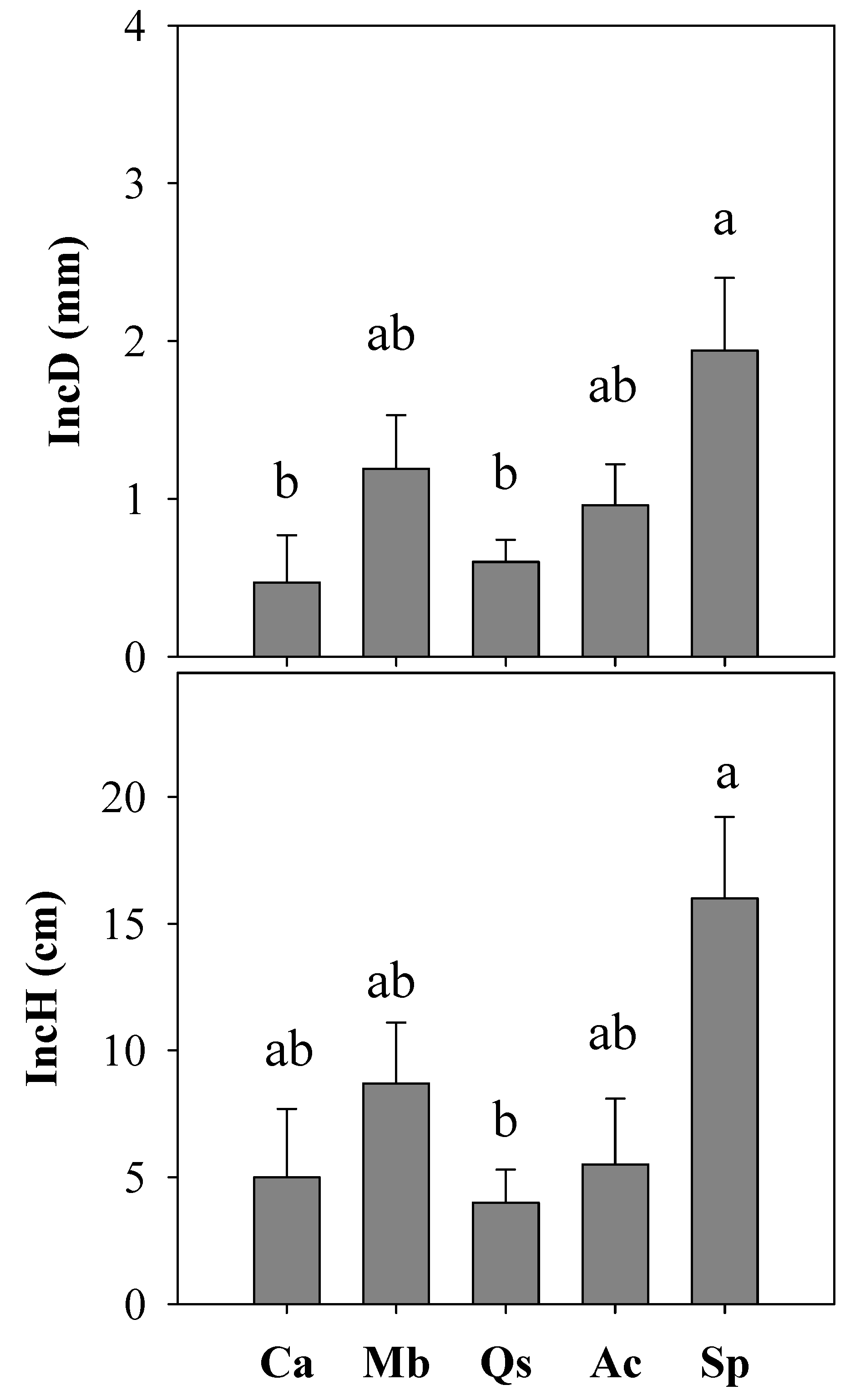
| Ca | 54 | 4.8 a | 18.2 | 15.1 d | 20.8 |
| Mb | 108 | 3.1 c | 20.8 | 23.1 b | 25.4 |
| Qs | 108 | 3.9 b | 16.3 | 19.5 c | 23.7 |
| Ac | 162 | 3.2 c | 18.1 | 27.1 a | 38.1 |
| Sp | 54 | 3.8 b | 13.1 | 23.6 ab | 32.5 |
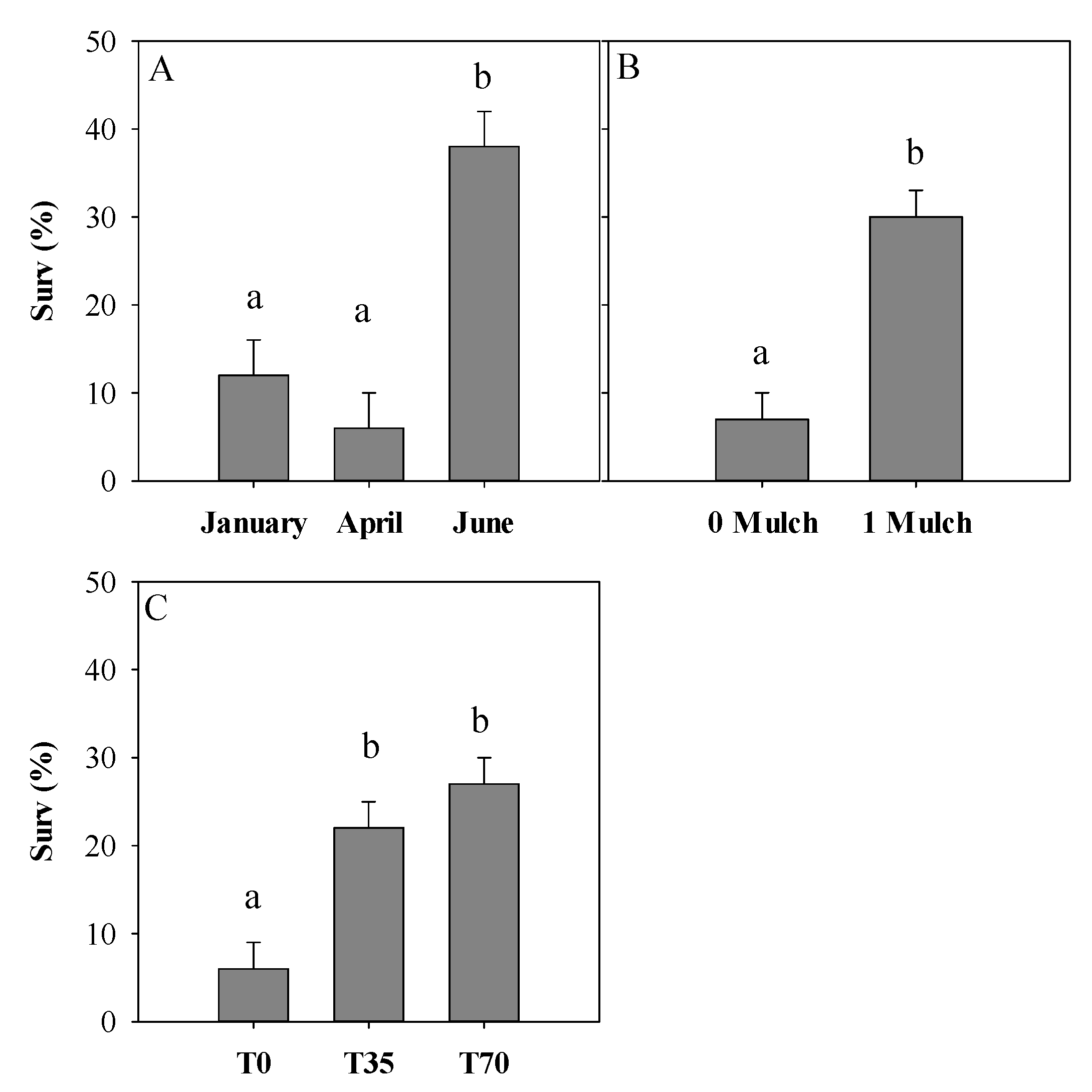
| January | 162 | 3.4 a | 24.8 | 24.2 a | 42.6 |
| April | 162 | 3.6 a | 25.7 | 21.2 a | 30.8 |
| June | 162 | 3.6 a | 21.5 | 22.8 a | 32.5 |
| ANOVA | |||||
| Date (PD) | 0.1501 | 0.2823 | |||
| Species (Sp) | <0.0001 | <0.0001 | |||
| PD × Sp | 0.4881 | 0.2538 | |||
| Source of Variation | Surv | IncD | IncH |
|---|---|---|---|
| Date | 0.0003 | 0.2515 | 0.3763 |
| Mulch | <0.0001 | 0.1734 | 0.1329 |
| Light | 0.0003 | 0.8712 | 0.5673 |
| Date × mulch | 0.3106 | 0.8906 | 0.6049 |
| Date × light | 0.3012 | 0.4164 | 0.5966 |
| Mulch × light | 0.4402 | 0.56037 | 0.9335 |
| Date × mulch × light | 0.9991 | 0.9989 | 0.9986 |
| Species | 0.0085 | 0.0123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Herrera, E.; Bravo, V.; Grez, I.; Vaswani, S.; Toro, N.; Yáñez, M.A.; Espinoza, S.E.; Abarca, B.; Faundez, Á.; Quiroz, I.; et al. The Use of Mulch and Shading Improves the Survival of Sclerophyllous Species Established in Island Plots in Central Chile. Appl. Sci. 2023, 13, 8333. https://doi.org/10.3390/app13148333
Martínez-Herrera E, Bravo V, Grez I, Vaswani S, Toro N, Yáñez MA, Espinoza SE, Abarca B, Faundez Á, Quiroz I, et al. The Use of Mulch and Shading Improves the Survival of Sclerophyllous Species Established in Island Plots in Central Chile. Applied Sciences. 2023; 13(14):8333. https://doi.org/10.3390/app13148333
Chicago/Turabian StyleMartínez-Herrera, Eduardo, Valeria Bravo, Iván Grez, Suraj Vaswani, Nicole Toro, Marco A. Yáñez, Sergio E. Espinoza, Betsabé Abarca, Ángela Faundez, Iván Quiroz, and et al. 2023. "The Use of Mulch and Shading Improves the Survival of Sclerophyllous Species Established in Island Plots in Central Chile" Applied Sciences 13, no. 14: 8333. https://doi.org/10.3390/app13148333
APA StyleMartínez-Herrera, E., Bravo, V., Grez, I., Vaswani, S., Toro, N., Yáñez, M. A., Espinoza, S. E., Abarca, B., Faundez, Á., Quiroz, I., & Magni, C. R. (2023). The Use of Mulch and Shading Improves the Survival of Sclerophyllous Species Established in Island Plots in Central Chile. Applied Sciences, 13(14), 8333. https://doi.org/10.3390/app13148333







