DNA Damage of Iron-Gold Nanoparticle Heterojunction Irradiated by kV Photon Beams: A Monte Carlo Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Monte Carlo Simulation
2.2. Geant4-DNA Monte Carlo Code
2.3. Simulation Geometry
2.4. Simulation Code
2.5. Realistic DNA Model
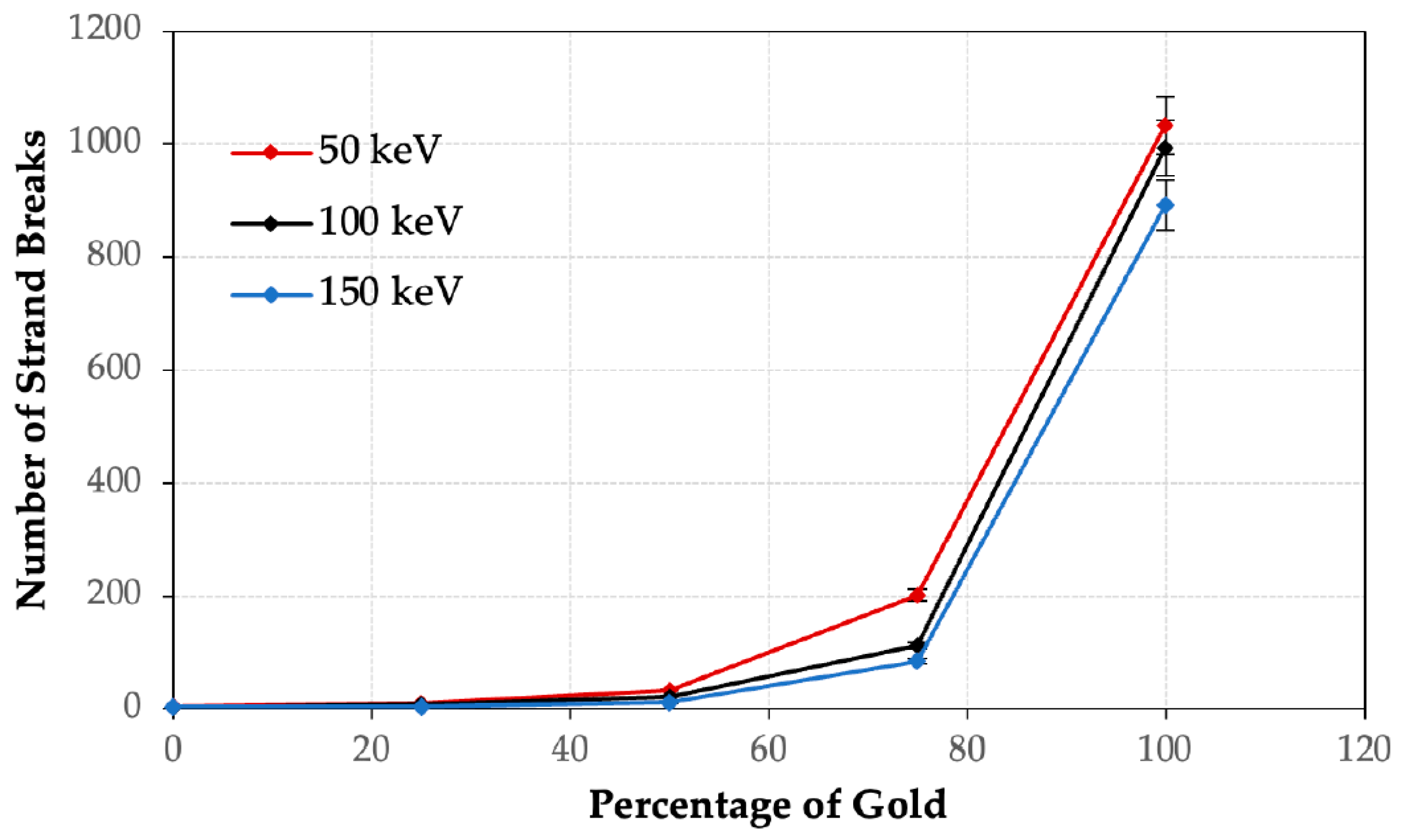
3. Results
4. Discussion
4.1. Dependence of DNA Damage on the Volume of Gold or Iron
4.2. Dependence of DNA Damage on the Photon Beam Energy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 1, 209–249. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society; Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2021; Canadian Cancer Society: Toronto, ON, Canada, 2021; Available online: Cancer.ca/Canadian-Cancer-Statistics-2021-EN (accessed on 5 July 2023).
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Canadian Cancer Statistics Advisory Committee Projected estimates of cancer in Canada in 2020. CMAJ Can. Med. Assoc. J. 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joiner, M.C.; van der Kogel, A.J.; Steel, G.G. Introduction: The significance of radiobiology and radiotherapy for cancer treatment. Basic Clin. Radiobiol. 2009, 13, 1. [Google Scholar]
- Chow, J.C.L. Photon and electron interactions with gold nanoparticles: A Monte Carlo study on gold nanoparticle-enhanced radiotherapy. Nanobiomaterials Med. Imaging 2016, 1, 45–70. [Google Scholar]
- Chow, J.C.L. Magnetic nanoparticles as contrast agents in magnetic resonance imaging and radiosensitizers in radiotherapy. Fundam. Ind. Appl. Magn. Nanoparticles 2022, 1, 291–316. [Google Scholar]
- Abdulle, A.; Chow, J.C.L. Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: A Monte Carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials 2019, 9, 920. [Google Scholar] [CrossRef] [Green Version]
- Albayedh, F.; Chow, J.C.L. Monte Carlo simulation on the imaging contrast enhancement in nanoparticle-enhanced radiotherapy. J. Med. Phys. 2018, 43, 195–199. [Google Scholar] [PubMed]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Moore, J.; Chow, J.C.L. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Recent advances in functionalized nanoparticles in cancer theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wu, A.; Chen, X. Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244 Pt A, 108–121. [Google Scholar] [CrossRef]
- McMillan, T.J.; Tobi, S.; Mateos, S.; Lemon, C. The use of DNA double-strand break quantification in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Chow, J.C.L. Radiation dose enhancement in skin therapy with nanoparticle addition: A Monte Carlo study on kilovoltage photon and megavoltage electron beams. World J. Radiol. 2017, 9, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kroese, D.P.; Brereton, T.; Taimre, T.; Botev, Z.I. Why the Monte Carlo method is so important today. Wiley Interdiscip. Rev. Comput. Stat. 2014, 6, 386–392. [Google Scholar] [CrossRef]
- Rogers, D.W. Fifty years of Monte Carlo simulations for medical physics. Phys. Med. Biol. 2006, 51, R287. [Google Scholar] [CrossRef]
- Santiago, C.A.; Chow, J.C.L. Variations in Gold Nanoparticle Size on DNA Damage: A Monte Carlo Study Based on a Multiple-Particle Model Using Electron Beams. Appl. Sci. 2023, 13, 4916. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.A.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.J.; et al. GEANT4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef] [Green Version]
- Arce, P.; Rato, P.; Canadas, M.; Lagares, J.I. GAMOS: A Geant4-based easy and flexible framework for nuclear medicine applications. In Proceedings of the IEEE Nuclear Science Symposium Conference Record, Dresden, Germany, 19–25 October 2008; pp. 3162–3168. [Google Scholar]
- Incerti, S.; Baldacchino, G.; Bernal, M.; Capra, R.; Champion, C.; Francis, Z.; Gueye, P.; Mantero, A.; Mascialino, B.; Moretto, P.; et al. The geant4-dna project. Int. J. Model. Simul. Sci. Comput. 2010, 1, 157–178. [Google Scholar] [CrossRef]
- Taheri, A.; Khandaker, M.U.; Moradi, F.; Bradley, D.A. A review of recent advances in the modeling of nanoparticle radiosensitization with the Geant4-DNA toolkit. Radiat. Phys. Chem. 2023, 1, 111146. [Google Scholar]
- Pitt-Francis, J.; Whiteley, J. Guide to Scientific Computing in C++; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Jabeen, M.; Chow, J.C.L. Gold Nanoparticle DNA Damage by Photon Beam in a Magnetic Field: A Monte Carlo Study. In special Issue entitled “Application of Nanomaterials in Biomedical Imaging and Cancer Therapy”. Nanomaterials 2021, 11, 1751. [Google Scholar] [CrossRef]
- Chatzipapas, K.P.; Tran, N.H.; Dordevic, M.; Zivkovic, S.; Zein, S.; Shin, W.G.; Sakata, D.; Lampe, N.; Brown, J.M.; Ristic-Fira, A.; et al. Simulation of DNA damage using Geant4-DNA: An overview of the “molecularDNA” example application. Precis. Radiat. Oncol. 2023, 7, 4–14. [Google Scholar] [CrossRef]
- Delage, E.; Pham, Q.T.; Karamitros, M.; Payno, H.; Stepan, V.; Incerti, S.; Maigne, L.; Perrot, Y. PDB4DNA: Implementation of DNA geometry from the Protein Data Bank (PDB) description for Geant4-DNA Monte-Carlo simulations. Comput. Phys. Commun. 2015, 192, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.C.L. Characteristics of secondary electrons from irradiated gold nanoparticle in radiotherapy. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Chapter 10; pp. 1–18. ISBN 978-3-319-13188-7. [Google Scholar]
- Roeske, J.C.; Nuñez, L.; Hoggarth, M.; Labay, E.; Weichselbaum, R.R. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007, 6, 395–401. [Google Scholar] [CrossRef]
- Paunesku, T.; Woloschak, G.E. Future directions of intraoperative radiation therapy: A brief review. Front. Oncol. 2017, 7, 300. [Google Scholar] [CrossRef] [Green Version]
- Kyrgias, G.; Hajiioannou, J.; Tolia, M.; Kouloulias, V.; Lachanas, V.; Skoulakis, C.; Skarlatos, I.; Rapidis, A.; Bizakis, I. Intraoperative radiation therapy (IORT) in head and neck cancer: A systematic review. Medicine 2016, 95, e5035. [Google Scholar] [CrossRef]
- Sedlmayer, F.; Reitsamer, R.; Fussl, C.; Ziegler, I.; Zehentmayr, F.; Deutschmann, H.; Kopp, P.; Fastner, G. Boost IORT in breast cancer: Body of evidence. Int. J. Breast Cancer 2014, 2014, 472516. [Google Scholar]


| Percentage of Gold | Percentage of Iron |
|---|---|
| 0% | 100% |
| 25% | 75% |
| 50% | 50% |
| 75% | 25% |
| 100% | 0% |
| Percentage of Gold to Iron | SSBs | DSBs |
|---|---|---|
| (a) | ||
| 0% Au, 100% Fe | 4 | 0 |
| 25% Au, 75% Fe | 9 | 1 |
| 50% Au, 50% Fe | 30 | 4 |
| 75% Au, 25% Fe | 201 | 1 |
| 100% Au, 0% Fe | 1011 | 22 |
| (b) | ||
| 0% Au, 100% Fe | 2 | 0 |
| 25% Au, 75% Fe | 8 | 1 |
| 50% Au, 50% Fe | 22 | 0 |
| 75% Au, 25% Fe | 109 | 3 |
| 100% Au, 0% Fe | 982 | 11 |
| (c) | ||
| 0% Au, 100% Fe | 4 | 0 |
| 25% Au, 75% Fe | 4 | 0 |
| 50% Au, 50% Fe | 11 | 1 |
| 75% Au, 25% Fe | 83 | 2 |
| 100% Au, 0% Fe | 885 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, J.C.L.; Santiago, C.A. DNA Damage of Iron-Gold Nanoparticle Heterojunction Irradiated by kV Photon Beams: A Monte Carlo Study. Appl. Sci. 2023, 13, 8942. https://doi.org/10.3390/app13158942
Chow JCL, Santiago CA. DNA Damage of Iron-Gold Nanoparticle Heterojunction Irradiated by kV Photon Beams: A Monte Carlo Study. Applied Sciences. 2023; 13(15):8942. https://doi.org/10.3390/app13158942
Chicago/Turabian StyleChow, James C. L., and Christine A. Santiago. 2023. "DNA Damage of Iron-Gold Nanoparticle Heterojunction Irradiated by kV Photon Beams: A Monte Carlo Study" Applied Sciences 13, no. 15: 8942. https://doi.org/10.3390/app13158942






