Energetic Effects in Methyl- and Methoxy-Substituted Indanones: A Synergistic Experimental and Computational Study
Abstract
:1. Introduction
2. Materials and Methods
- (i)
- The combustion experiments of 2MI, 3MI and 4MI were performed with a static-bomb calorimeter having a twin valve bomb with an internal volume of 0.290 dm3, with a detailed description in the literature [12].
- (ii)
3. Results
3.1. Combustion Energies and Enthalpies of Indanones
3.2. Sublimation or Vaporization Enthalpies of Indanones
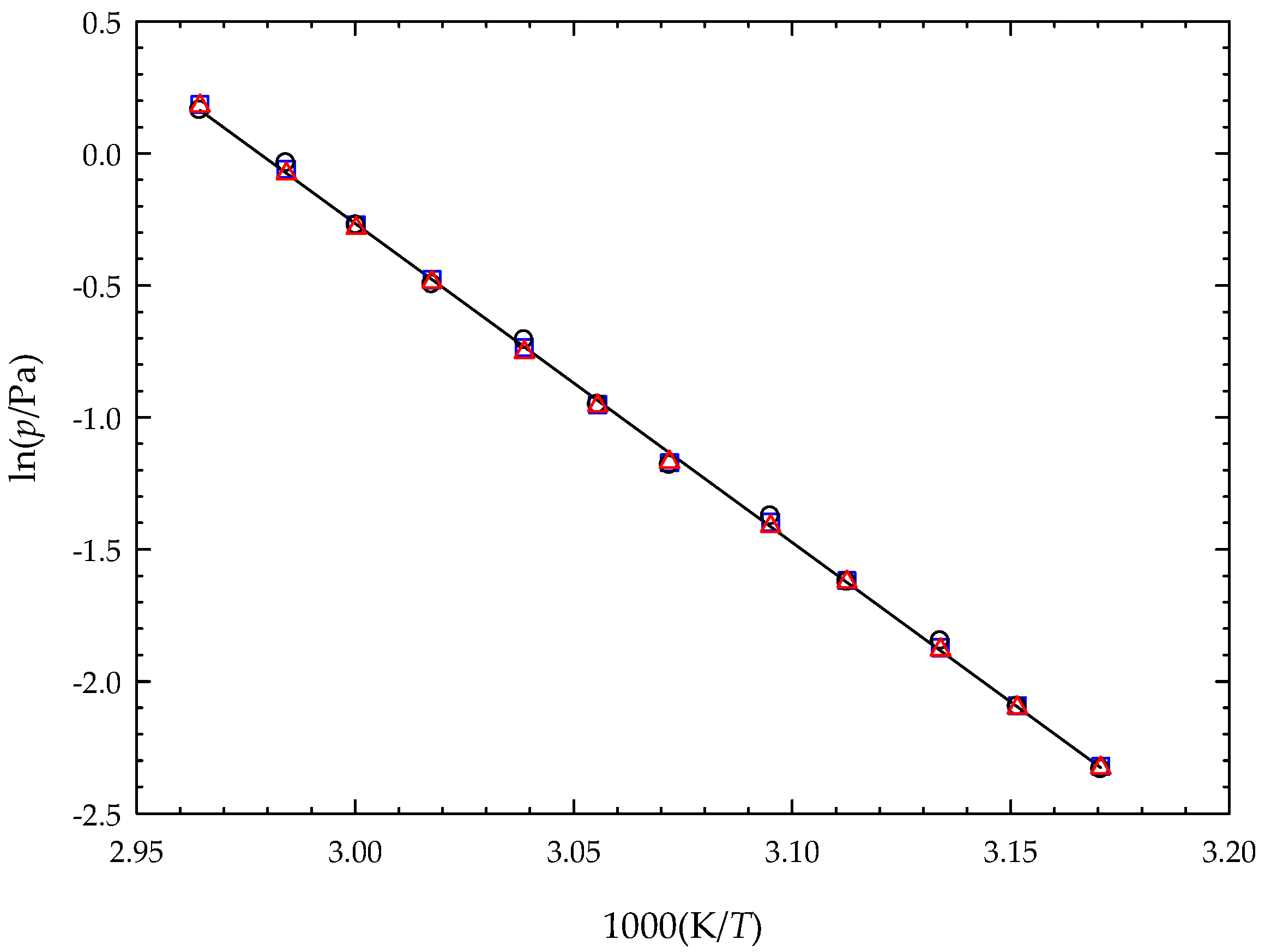
3.3. Vapor Pressures and Sublimation Enthalpy of 5MI
3.4. Gas-Phase Enthalpies of Formation of Indanones
3.5. Energetic Effects Associated with the Substitution of H in Indanone by CH3 or by OCH3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.C. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power, 2nd ed.; John Wily & Sons Ltd.: Ames, IA, USA, 2019. [Google Scholar]
- Serrano-Ruiz, J.C. Advanced Biofuels: Using Catalytic Routes for the Conversion of Biomass Platform Molecules, 1st ed.; Apple Academic Press: Oakville, ON, Canada, 2015. [Google Scholar]
- Freitas, V.L.S.; Lima, A.C.M.O.; Sapei, E.; Ribeiro da Silva, M.D.M.C. Comprehensive thermophysical and thermochemical studies of vanillyl alcohol. J. Chem. Thermodyn. 2016, 102, 287–292. [Google Scholar] [CrossRef]
- Rocha, I.M.; Galvão, T.L.P.; Sapei, E.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Levoglucosan: A calorimetric, thermodynamic, spectroscopic, and computational investigation. J. Chem. Eng. Data 2013, 58, 1813–1821. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Schliesser, J.; Mittal, A.; Decker, S.R.; Santos, A.F.L.O.M.; Freitas, V.L.S.; Urbas, A.; Lang, B.E.; Heiss, C.; Ribeiro da Silva, M.D.M.C.; et al. A thermodynamic investigation of the cellulose allomorphs: Cellulose (am), cellulose Iβ (cr), cellulose II (cr), and cellulose III (cr). J. Chem. Thermodyn. 2015, 81, 184–226. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Lobo Ferreira, A.I.; Shi, Q.; Woodfield, B.F. Thermochemistry of α-D-xylose (cr). J. Chem. Thermodyn. 2013, 58, 20–28. [Google Scholar]
- Kohl, T.; Sapei, E.; Rocha, I.M.; Galvão, T.L.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Modeling of fast pyrolysis of wood for prediction of bio-oil composition. In Proceedings of the Asia Pacific Confederation of Chemical Engineering Congress 2015: APCChE 2015, Incorporating CHEMECA, Melbourne, Australia, 26 September–1 October 2015; Engineers Australia: Melbourne, Australia, 2015; p. 2410. [Google Scholar]
- Silva, A.L.R.; Ribeiro da Silva, M.D.M.C. Energetic and structural studies of two biomass-derived compounds: 6- and 7-hydroxy-1-indanones. Appl. Sci. 2020, 10, 8512. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Moura, C.; Ribeiro da Silva, M.D.M.C. Energetic vs structural study of two biomass degradation derivatives: 2-cyclopentenone and 3-methyl-2-cyclopentenone. J. Chem. Thermodyn. 2019, 132, 390–396. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Lima, A.C.M.O.; Ribeiro da Silva, M.D.M.C. Energetic characterization of indanone derivatives involved in biomass degradation. J. Therm. Anal. Calorim. 2018, 134, 1267–1276. [Google Scholar] [CrossRef]
- Matos, M.A.R.; Miranda, M.S.; Monte, M.J.S.; Santos, L.M.N.B.F.; Morais, V.M.F.; Chickos, J.S.; Umnahanant, P.; Liebman, J.F. Calorimetric and computational study of indanones. J. Phys. Chem. A 2007, 111, 11153–11159. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.D.M.C.; Santos, L.M.N.B.F.; Silva, A.L.R.; Fernandez, O.; Acree, W.E., Jr. Energetics of 6-methoxyquinoline and 6-methoxyquinoline N-oxide: The dissociation enthalpy of the (N–O) bond. J. Chem. Thermodyn. 2003, 35, 1093–1100. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Pilcher, G. The construction, calibration and use of a new high-precision static-bomb calorimeter. Rev. Port. Quím. 1984, 26, 163–172. [Google Scholar]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Pilcher, G. Enthalpies of combustion of 1,2-dihydroxybenzene and of six alkylsubstituted 1,2-dihydroxybenzenes. J. Chem. Thermodyn. 1984, 16, 1149–1155. [Google Scholar] [CrossRef]
- Hubbard, W.N.; Scott, D.W.; Waddington, G. Standard states and corrections for combustions in a bomb at constant volume. In Experimental Thermochemistry. Measurement of Heats of Reaction, 1st ed.; Rossini, F.D., Ed.; Interscience Publishers, Inc.: New York, NY, USA, 1956; Volume 1, pp. 75–128. [Google Scholar]
- Adedeji, F.A.; Brown, D.L.S.; Connor, J.A.; Leung, W.L.; Paz-Andrade, I.M.; Skinner, H.A. Thermochemistry of arene chromium tricarbonyls and the strenghts of arene-chromium bonds. J. Organomet. Chem. 1975, 97, 221–228. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Matos, M.A.R.M.; Amaral, L.M.P.F. Thermochemical study of 2-, 4-, 6-, and 8-methylquinoline. J. Chem. Thermodyn. 1995, 27, 565–574. [Google Scholar] [CrossRef]
- Santos, L.M.N.B.F.; Schröder, B.; Fernandes, O.O.P.; Ribeiro da Silva, M.A.V. Measurement of enthalpies of sublimation by drop method in a Calvet type calorimeter: Design and test of a new system. Thermochim. Acta 2004, 415, 15–20. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Santos, L.M.N.B.F. The design, construction, and testing of a new Knudsen effusion apparatus. J. Chem. Thermodyn. 2006, 38, 778–787. [Google Scholar] [CrossRef]
- Baboul, A.G.; Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-3 theory using density functional geometries and zero-point energies. J Chem Phys. 1999, 110, 7650–7657. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Rossini, F.D. Assignment of uncertainties to thermochemical data. In Experimental Thermochemistry. Measurement of Heats of Reaction, 1st ed.; Rossini, F.D., Ed.; Interscience Publishers, Inc.: New York, NY, USA, 1956; Volume 1, pp. 297–325. [Google Scholar]
- Olofson, G. Assignment of uncertainties. In Combustion Calorimetry, 1st ed.; Sunner, S., Mansson, M., Eds.; Pergamon Press: Oxford, UK, 1979; Volume 1, pp. 137–159. [Google Scholar]
- Cox, J.D.; Wagman, D.D.; Medvedev, V.A. CODATA Key Values for Thermodynamics; Hemisphere: New York, NY, USA, 1979. [Google Scholar]
- Merrick, J.P.; Moran, D.; Radom, L. An evaluation of harmonic vibrational frequency scale factors. J. Phys. Chem. A 2007, 111, 11683–11700. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Almeida, A.R.R.P.; Matos, M.A.R. Thermodynamic study on the sublimation of five aminomethoxybenzoic acids. J. Chem. Eng. Data 2010, 55, 419–423. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G.; Liebman, J.F. Heat capacity corrections to a standard state: A comparison of new and some literature methods for organic liquids and solids. Struct. Chem. 1993, 4, 271–278. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C. Effects of methoxy and formyl substituents on the energetics and reactivity of α-naphthalenes: A calorimetric and computational study. Chemosphere 2014, 107, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.V.; Temprado, M.; Chickos, J.S.; Nagano, Y. Critically evaluated thermochemical properties of polycyclic aromatic hydrocarbons. J. Phys. Chem. Ref. Data 2008, 37, 1855–1996. [Google Scholar] [CrossRef]
- Pedley, J.B. Thermochemical Data and Structures of Organic Compounds; Thermodynamics Research Center: College Station, TX, USA, 1994. [Google Scholar]
- Amaral, L.M.P.F.; Ribeiro da Silva, M.A.V. Thermochemistry of some methoxypyridines. J. Chem. Thermodyn. 2012, 48, 65–69. [Google Scholar] [CrossRef]
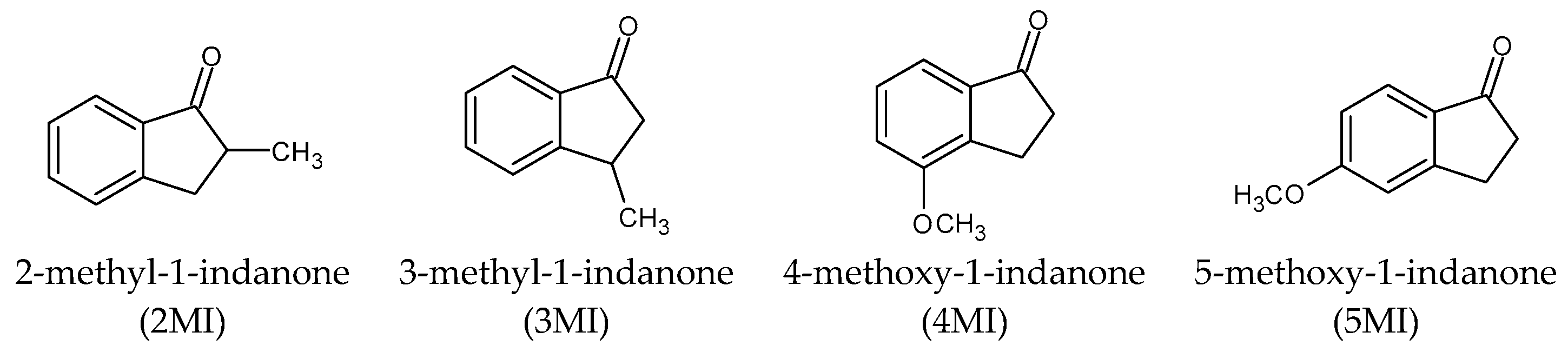


| Compound | Cas No. | Source | Purification Method | Final Mass Fraction Purity |
|---|---|---|---|---|
| 2MI | 17496-14-9 | Sigma-Aldrich a, 99% | Fraction distillation (T = 377 K; p = 0.6 kPa) | 0.9997 b (0.9993 ± 0.0007) c |
| 3MI | 6072-57-7 | Sigma-Aldrich a, 99% | — | 0.9991 b (0.9928 ± 0.0007) c |
| 4MI | 13336-31-7 | Sigma-Aldrich a, 99% | Sublimation in vacuum | 0.9995 b (1.0004 ± 0.0002) c |
| 5MI | 5111-70-6 | Sigma-Aldrich, 98% | Sublimation in vacuum | 0.9999 a (0.9999 ± 0.0004) b |
| 2MI (l) | 3MI (l) | 4MI (cr) | 5MI (cr) | |
|---|---|---|---|---|
| m(cpd)/g | 0.38190 | 0.38534 | 0.42886 | 0.61473 |
| m(fuse)/g | 0.00244 | 0.00329 | 0.00240 | 0.00282 |
| m(n-hexadec.)/g | — | — | 0.10468 | — |
| m(melinex)/g | 0.05616 | 0.04789 | — | — |
| Ti/K | 298.1513 | 298.1504 | 298.1511 | 298.1509 |
| Tf/K | 299.1886 | 299.1866 | 299.3734 | 299.4208 |
| ΔTad/K | 0.95723 | 0.95602 | 1.14400 | 1.19537 |
| εi/J·K−1 | 14.10 | 13.20 | 15.03 | 16.17 |
| εf/J·K−1 | 15.14 | 14.20 | 15.97 | 17.05 |
| (εcal)corr/J·K−1 | 15,537.91 | 15,543.35 | — | — |
| Δm(H2O)/g | −2.1 | −0.8 | 0.0 | 0.0 |
| −ΔU(IBP)/J | 14,887.29 | 15,543.35 | 18,324.77 | 19,148.57 |
| ΔU(HNO3)/J | 1.36 | 2.44 | 0.65 | 0.48 |
| ΔU(ign)/J | 0.56 | 0.61 | 0.72 | 0.83 |
| ΔUΣ/J | 8.35 | 8.34 | 9.13 | 11.48 |
| −ΔU(n-hexadec.)/J | — | — | 4934.25 | — |
| −ΔU(melinex)/J | 1286.16 | 1096.78 | — | — |
| −ΔU(fuse)/J | 39.63 | 53.43 | 38.98 | 45.80 |
| −/(J·g─1) | 35,485.18 | 35,583.46 | 31,109.22 | 31,098.55 |
| Compound | ||||
|---|---|---|---|---|
| 2MI (l) | −35,495.53 ± 6.4 | −5188.9 ± 2.6 | −5193.9 ± 2.6 | −170.4 ± 2.9 |
| 3MI (l) | −35,587.25 ± 6.5 | −5202.3 ± 2.6 | −5207.3 ± 2.6 | −157.0 ± 3.0 |
| 4MI (cr) | −31,104.44 ± 4.3 | −5044.7 ± 2.1 | −5048.4 ± 2.1 | −315.9 ± 2.4 |
| 5MI (cr) | −31,097.79 ± 2.8 | −5043.6 ± 1.6 | −5047.3 ± 1.6 | −317.0 ± 2.1 |
| Compound | No. Exp. | Texp/K | |||
|---|---|---|---|---|---|
| 2MI | 6 | 345.08 ± 0.02 | 73.21 ± 0.35 | 8.20 ± 0.01 | 65.0 ± 1.8 |
| 3MI | 6 | 355.08 ± 0.03 | 74.19 ± 0.43 | 10.12 ± 0.01 | 64.1 ± 1.8 |
| 4MI | 6 | 365.69 ± 0.05 | 111.55 ± 0.57 | 13.25 ± 0.01 | 98.3 ± 2.4 |
| 5MI | 7 | 365.75 ± 0.04 | 112.72 ± 1.06 | 13.27 ± 0.01 | 99.4 ± 3.1 |
| Effusion Orifices | a | b | <T>/K | p(<T>)/Pa | ||
|---|---|---|---|---|---|---|
| Serie A | 36.03 ± 0.11 | 12,099 ± 34 | 0.353 | 100.6 ± 0.3 | ||
| Serie B | 35.97 ± 0.17 | 12,077 ± 55 | 0.355 | 100.4 ± 0.5 | ||
| Serie C | 35.95 ± 0.09 | 12,074 ± 29 | 0.351 | 100.4 ± 0.2 | ||
| Global | 35.98 ± 0.12 | 12,083 ± 39 | 326.36 | 0.352 | 100.5 ± 0.3 | 307.8 ± 1.0 |
| 178.22 | −32.3 | 101.4 ± 0.3 | 206.3 ± 1.0 | 39.9 ± 0.5 |
| Compound | |||
|---|---|---|---|
| 2MI | −170.4 ± 2.9 | 65.0 ± 1.8 | −105.4 ± 3.5 |
| 3MI | −157.0 ± 3.0 | 64.1 ± 1.8 | −92.9 ± 3.5 |
| 4MI | −315.9 ± 2.4 | 98.3 ± 2.4 | −217.6 ± 3.4 |
| 5MI | −317.0 ± 2.1 | 99.4 ± 3.1 b 101.4 ± 0.3 c | −215.6 ± 2.1 d |
| Compound | Experimental Value | Computed G3(MP2)//B3LYP Gas-Phase Enthalpies of Formation | Δ a |
|---|---|---|---|
| 2MI | −105.4 ± 3.5 | −94.4 ± 4.2 | +10.2 |
| 3MI | −92.9 ± 3.5 | −94.3 ± 4.2 | −1.4 |
| 4MI | −217.6 ± 3.4 | −219.1 ± 3.3 | −1.5 |
| 5MI | −215.6 ± 2.1 | −220.3 ± 3.3 | −4.7 |
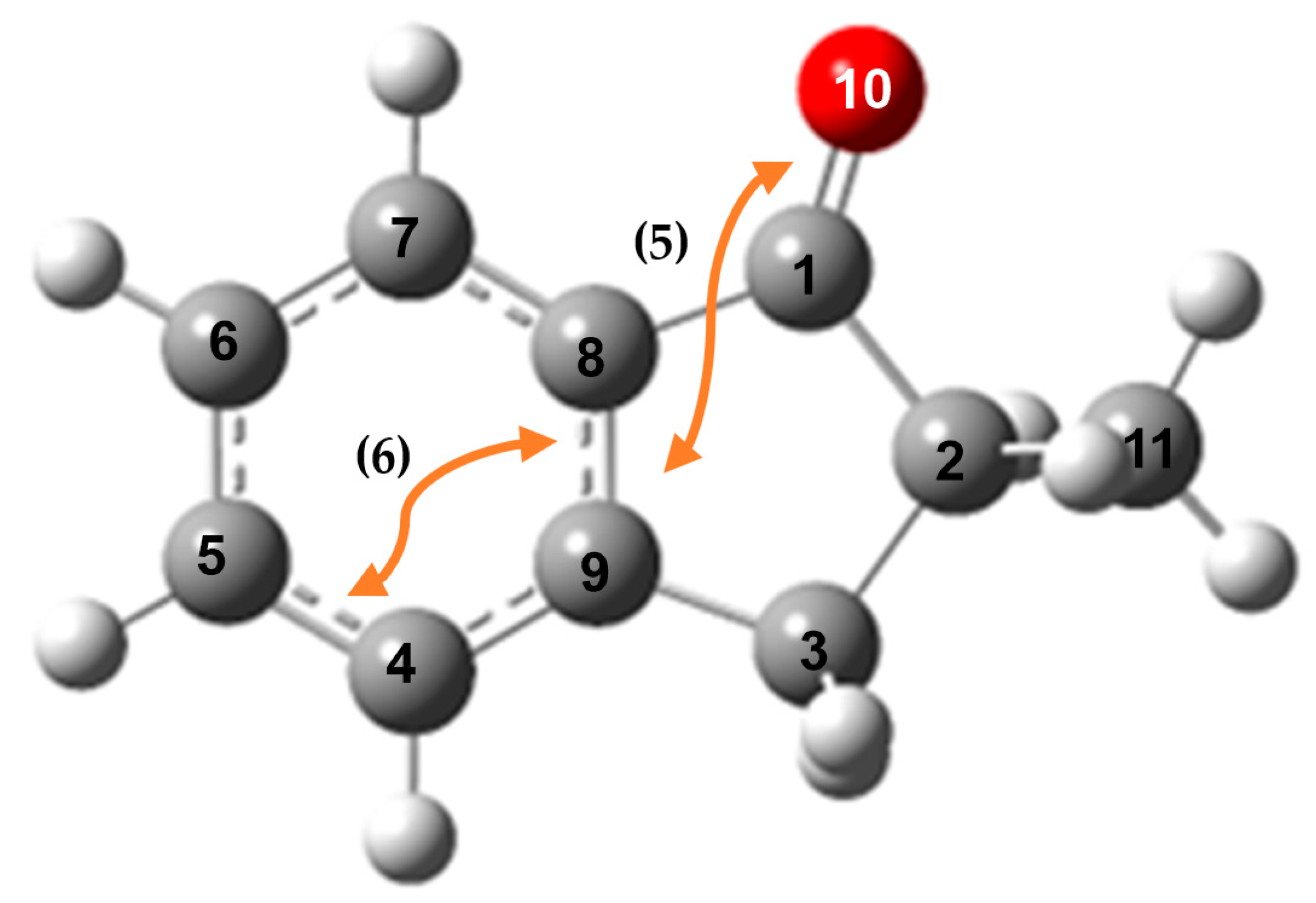
| Electronic Interactions | Donor (i) | Acceptor (j) | E (kJ·mol−1) | |
|---|---|---|---|---|
| 2MI | 3MI | |||
| (1) | 95.1 | 95.2 | ||
| (2) | 71.2 | 71.2 | ||
| (3) | 77.5 | 77.6 | ||
| (4) | 90.3 | 90.0 | ||
| (5) | 589.1 | 544.4 | ||
| (6) | 1006.1 | 945.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.L.R.; León, G.P.; Ribeiro da Silva, M.D.M.C. Energetic Effects in Methyl- and Methoxy-Substituted Indanones: A Synergistic Experimental and Computational Study. Appl. Sci. 2023, 13, 10262. https://doi.org/10.3390/app131810262
Silva ALR, León GP, Ribeiro da Silva MDMC. Energetic Effects in Methyl- and Methoxy-Substituted Indanones: A Synergistic Experimental and Computational Study. Applied Sciences. 2023; 13(18):10262. https://doi.org/10.3390/app131810262
Chicago/Turabian StyleSilva, Ana L. R., Gastón P. León, and Maria D. M. C. Ribeiro da Silva. 2023. "Energetic Effects in Methyl- and Methoxy-Substituted Indanones: A Synergistic Experimental and Computational Study" Applied Sciences 13, no. 18: 10262. https://doi.org/10.3390/app131810262






