Antibacterial and In Vitro Bioactivity Studies of Silver-Doped, Cerium-Doped, and Silver–Cerium Co-Doped 80S Mesoporous Bioactive Glass Particles via Spray Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
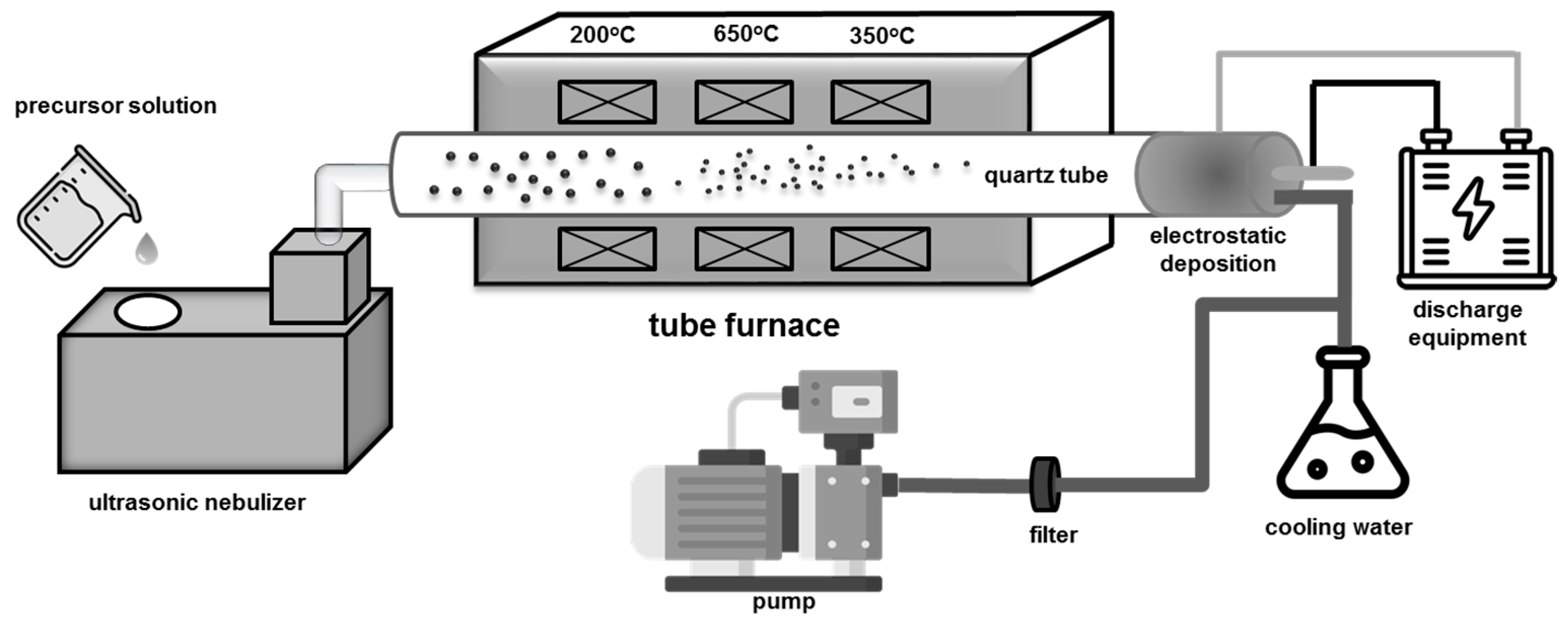
2.1. Synthesis
2.2. Characterization
2.3. Antibacterial Activity
2.4. In Vitro Bioactivity Test
2.5. Cytotoxicity Test
2.6. Statistical Analysis
3. Results
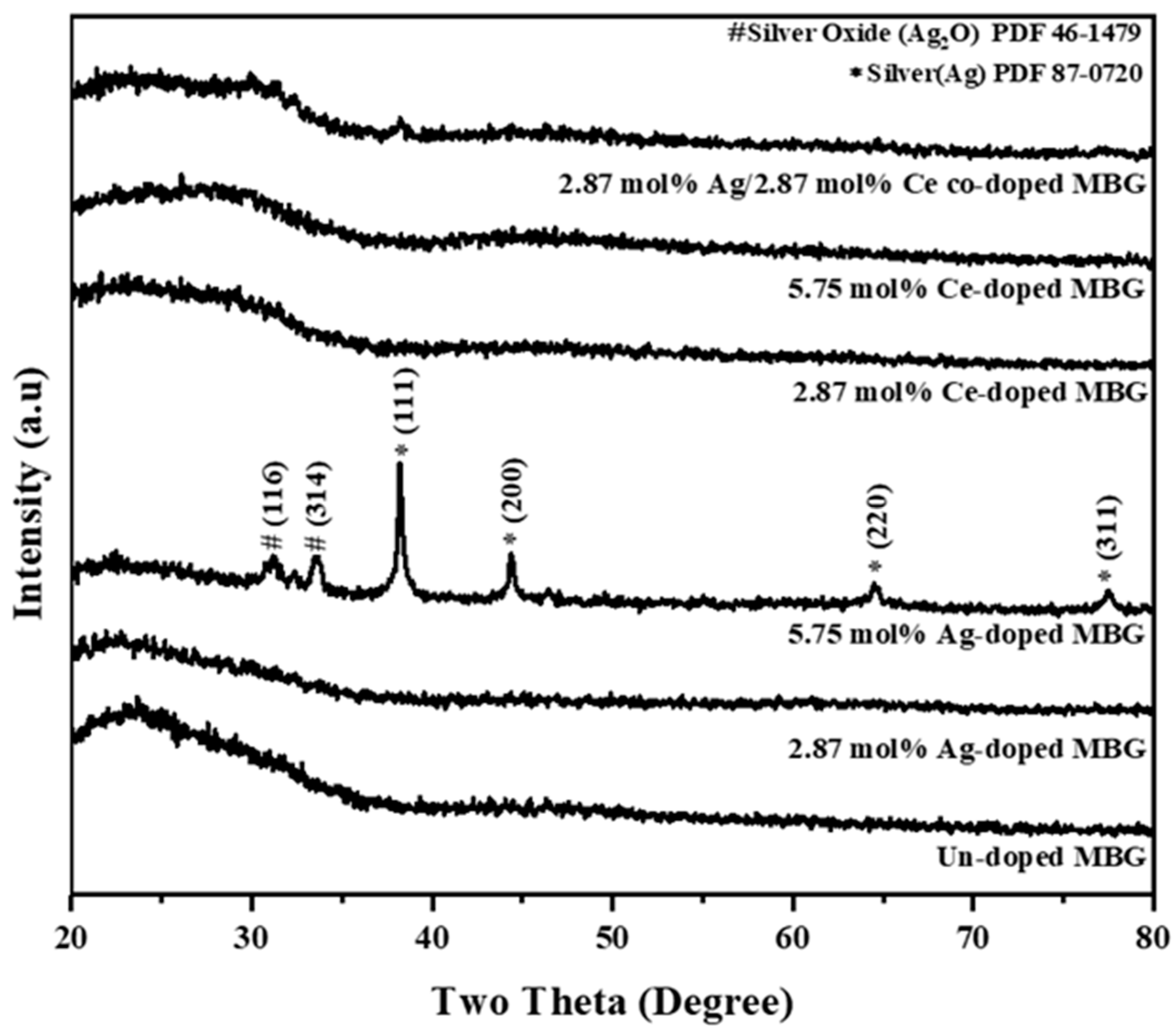
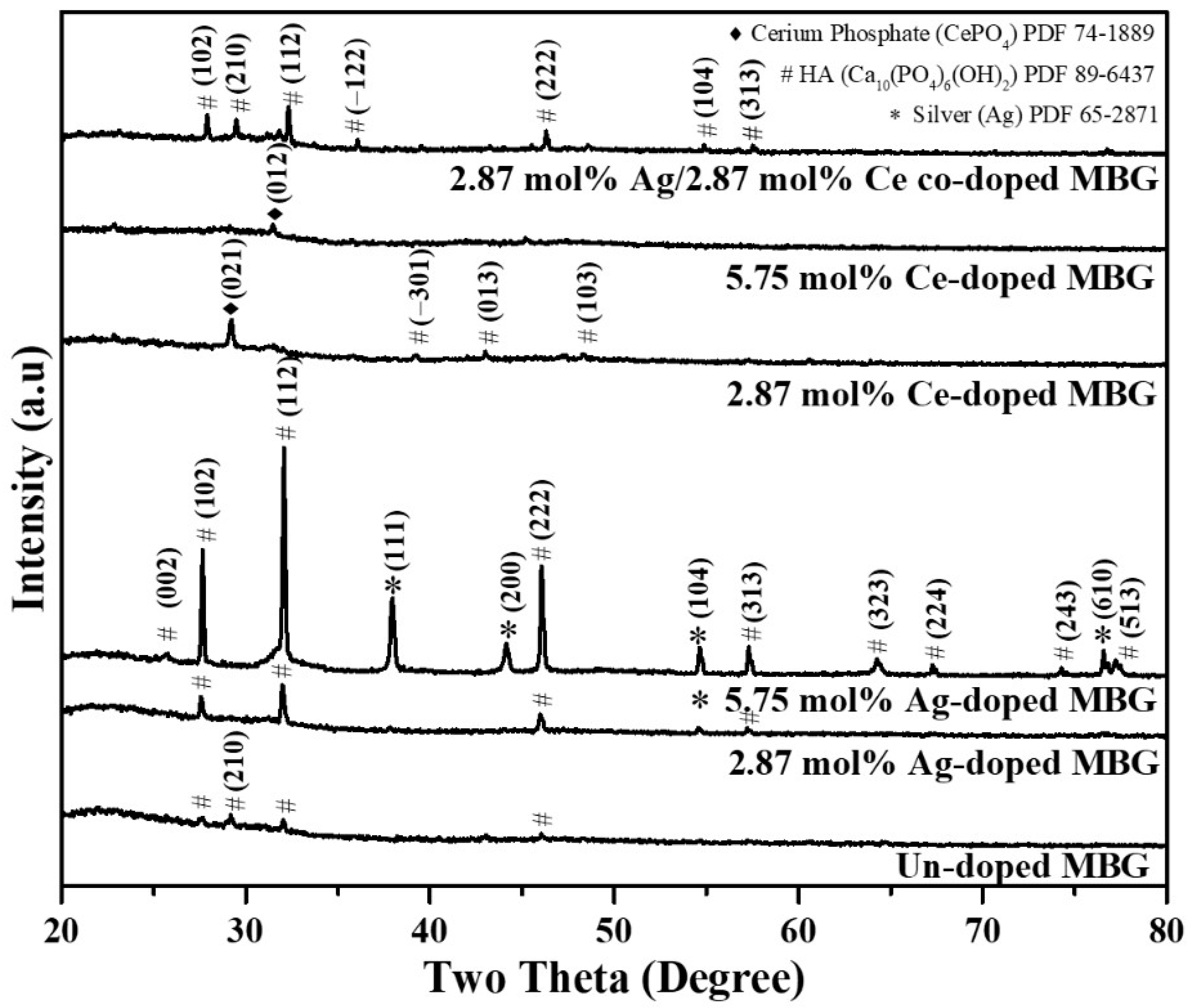
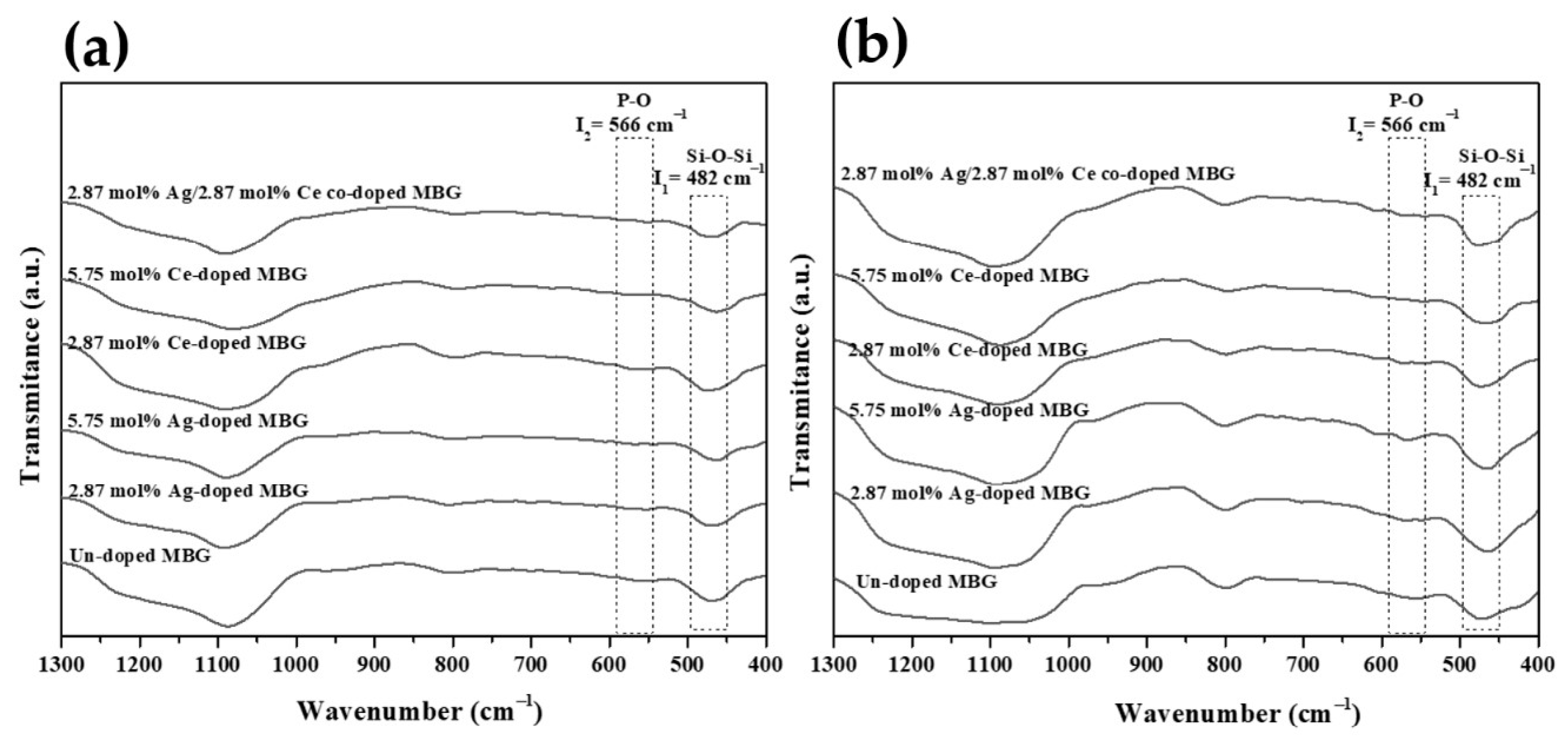
3.1. Phase Composition
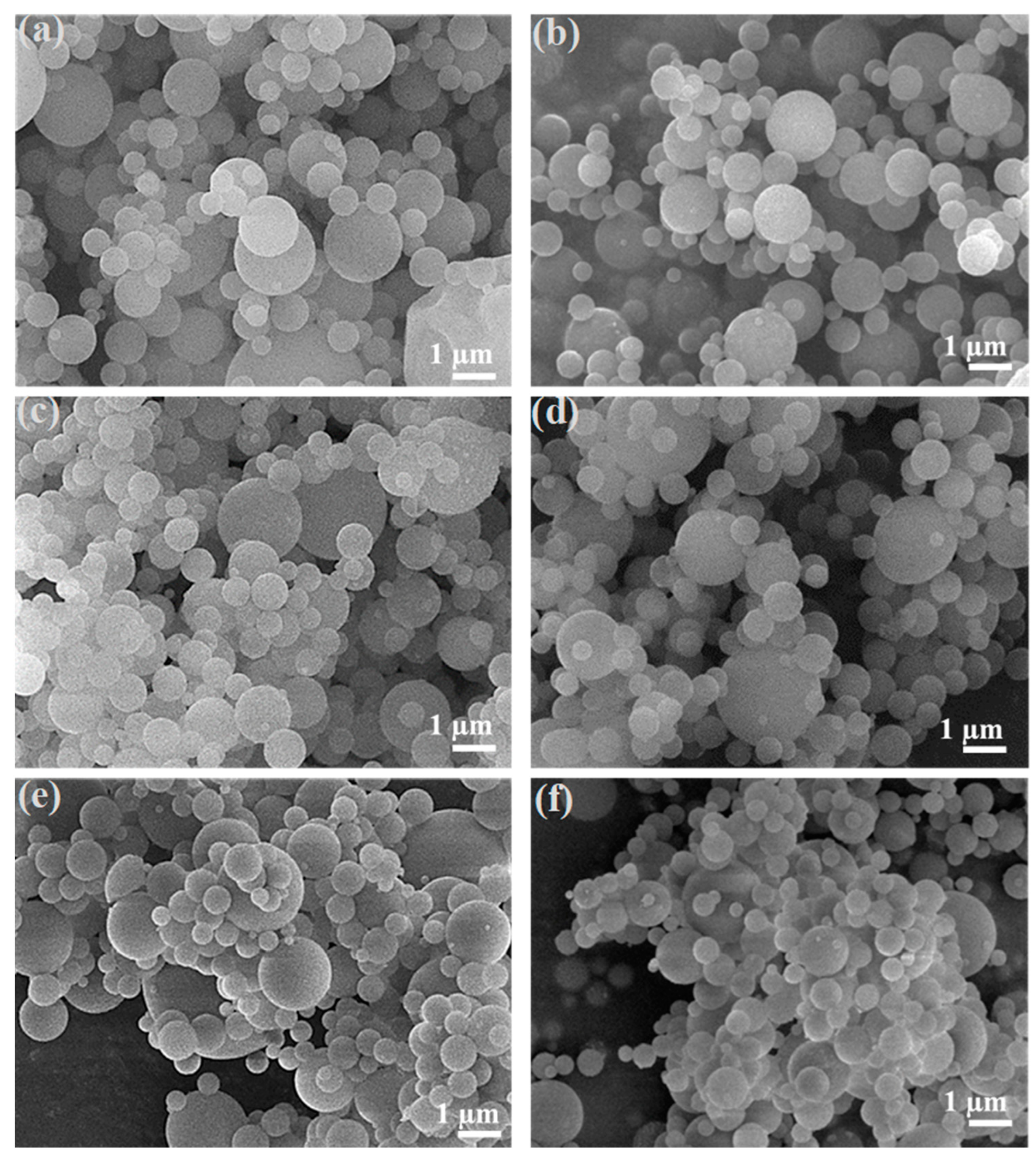
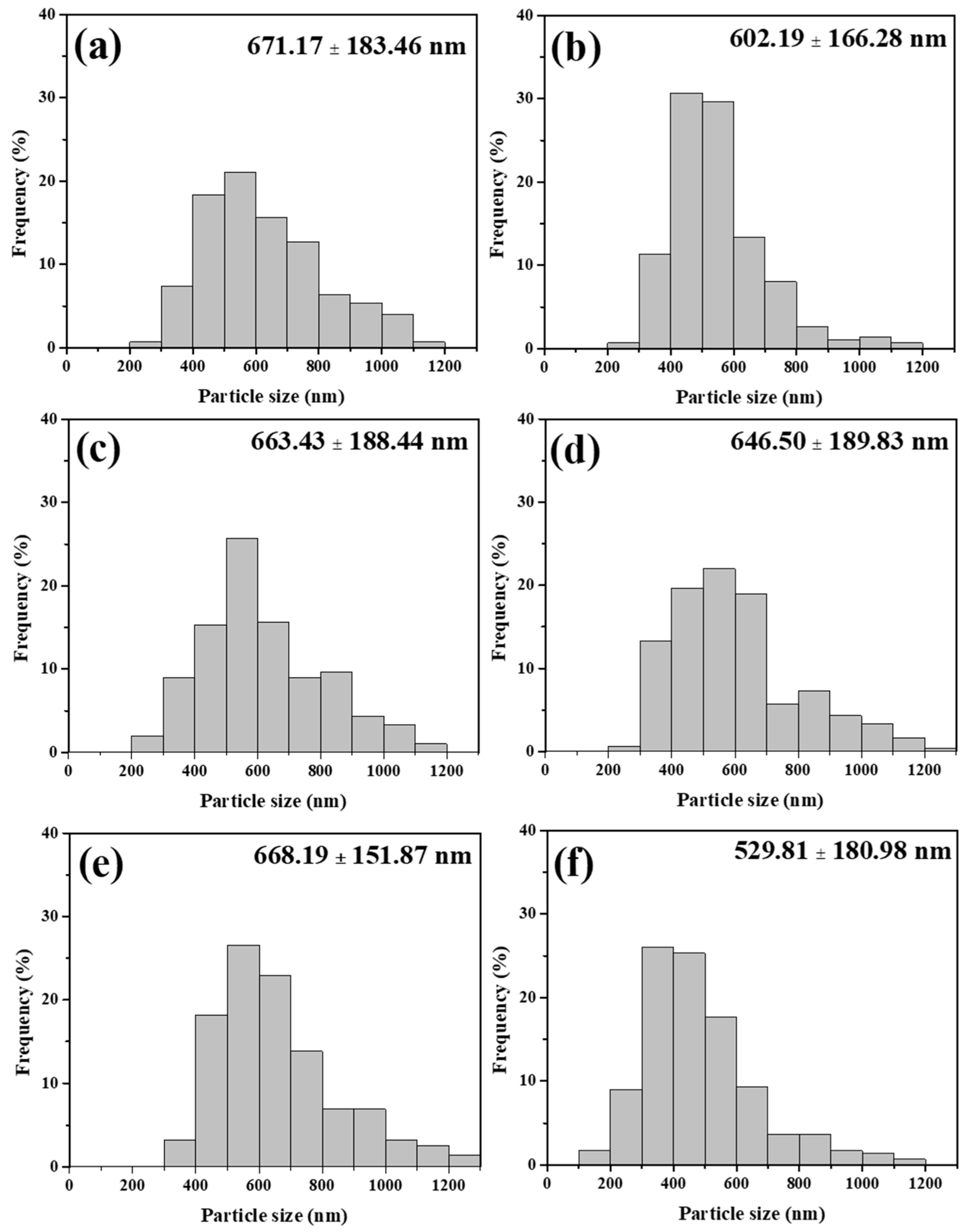
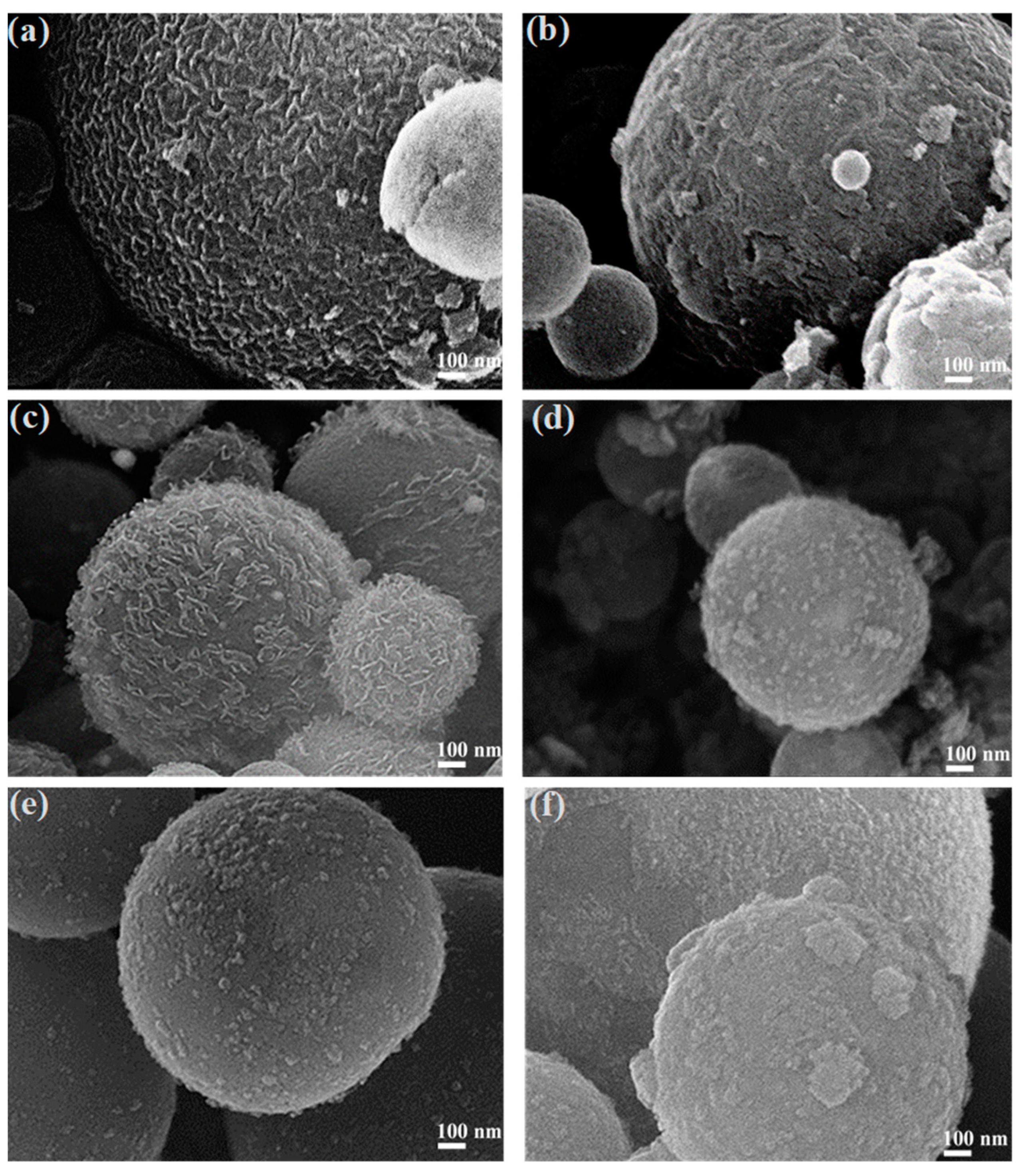
3.2. Morphology and Chemical Composition
3.3. Antibacterial Activities
3.4. In Vitro Bioactivity
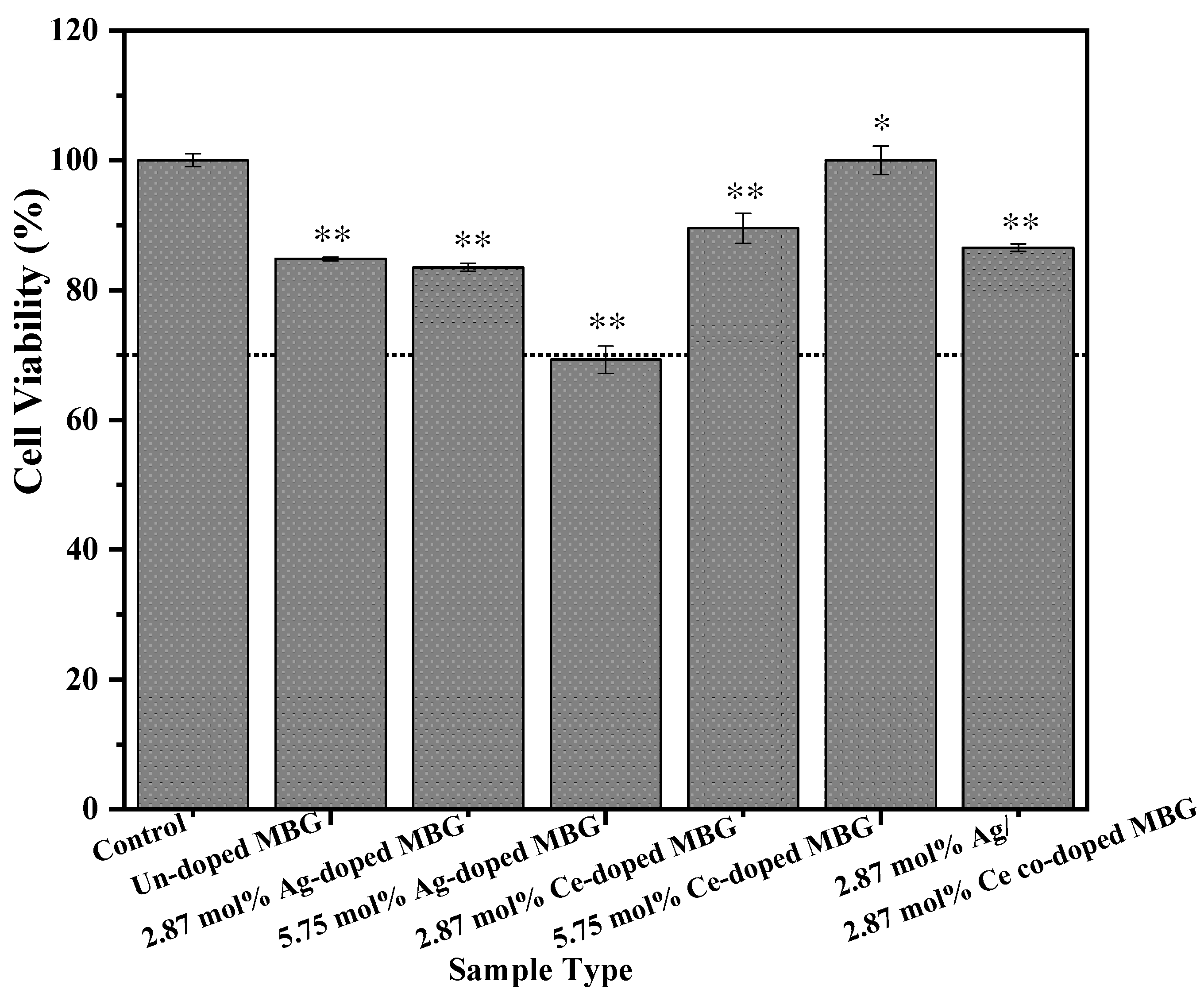
3.5. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef]
- Migneco, C.; Fiume, E.; Verné, E.; Baino, F. A guided walk through the world of mesoporous bioactive glasses (MBGs): Fundamentals, processing, and applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Lin, T.-Y.; Huang, S.-C.; Yang, T.-Y.; Shih, C.-J. Copper-enhanced silver releasing from bimetal-containing bioactive glass (AgCu/80S) elicits antibacterial efficacy against drug-resistant Staphylococcus aureus. J. Non-Cryst. Solids 2022, 584, 121509. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-Y.; Byeon, J.H.; Park, J.-H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Zhao, G.; Stevens, S.E. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 1998, 11, 27–32. [Google Scholar] [CrossRef]
- Matsumoto, N.; Sato, K.; Yoshida, K.; Hashimoto, K.; Toda, Y. Preparation and characterization of β-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater. 2009, 5, 3157–3164. [Google Scholar] [CrossRef]
- O’neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance Chaired by Jim O’Neill. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 4 October 2023).
- Bhattacharjee, B.; Ghosh, S.; Patra, D.; Haldar, J. Advancements in release-active antimicrobial biomaterials: A journey from release to relief. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1745. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Awasthi, R.; Rakholia, V.; Agrawal, S.; Dhingra, L.S.; Nagori, A.; Kaur, H.; Sethi, T. Estimating the impact of health systems factors on antimicrobial resistance in priority pathogens. J. Glob. Antimicrob. Resist. 2022, 30, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Prodan, A.-M.; Costescu, A. Development of cerium-doped hydroxyapatite coatings with antimicrobial properties for biomedical applications. Coatings 2020, 10, 516. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Skariah, S.; Büsselberg, D. Bacteriophage treatment: Critical evaluation of its application on World Health Organization priority pathogens. Viruses 2020, 13, 51. [Google Scholar] [CrossRef]
- Morris, S.; Cerceo, E. Trends, epidemiology, and management of multi-drug resistant gram-negative bacterial infections in the hospitalized setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in surgical site infections: Recent advances and novel prevention and eradication strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A. Antibiotic resistance: One health one world outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Taye, M.B. Biomedical applications of ion-doped bioactive glass: A review. Appl. Nanosci. 2022, 12, 3797–3812. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef]
- Klueh, U.; Wagner, V.; Kelly, S.; Johnson, A.; Bryers, J. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 53, 621–631. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.; Ingle, A.; Gade, A. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Sales, D.A.; Marques, T.M.; Ghosh, A.; Gusmão, S.B.; Vasconcelos, T.L.; Luz-Lima, C.; Ferreira, O.P.; Hollanda, L.M.; Lima, I.S.; Silva-Filho, E.C. Synthesis of silver-cerium titanate nanotubes and their surface properties and antibacterial applications. Mater. Sci. Eng. C 2020, 115, 111051. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, A.; Nerantzaki, M.; Gounari, E.; Duggal, M.; Giannoudis, P.; Jha, A.; Bikiaris, D. Antibacterial properties and regenerative potential of Sr2+ and Ce3+ doped fluorapatites; a potential solution for peri-implantitis. Sci. Rep. 2019, 9, 14469. [Google Scholar] [CrossRef]
- Farag, M.M.; Al-Rashidy, Z.M.; Ahmed, M.M. In vitro drug release behavior of Ce-doped nano-bioactive glass carriers under oxidative stress. J. Mater. Sci. Mater. Med. 2019, 30, 18. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, X.; Zhang, Y.; Zhu, W.; Wang, A.; Xu, M.; Zhuang, S. Rapid hemostasis and excellent antibacterial cerium-containing mesoporous bioactive glass/chitosan composite sponge for hemostatic material. Mater. Today Chem. 2022, 23, 100735. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Pei, P.; Qi, X.; Du, X.; Zhu, M.; Zhao, S.; Zhu, Y. Three-dimensional printing of tricalcium silicate/mesoporous bioactive glass cement scaffolds for bone regeneration. J. Mater. Chem. B 2016, 4, 7452–7463. [Google Scholar] [CrossRef]
- Shen, Q.; Qi, Y.; Kong, Y.; Bao, H.; Wang, Y.; Dong, A.; Wu, H.; Xu, Y. Advances in copper-based biomaterials with antibacterial and osteogenic properties for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 9, 795425. [Google Scholar] [CrossRef]
- Atkinson, I.; Seciu-Grama, A.M.; Petrescu, S.; Culita, D.; Mocioiu, O.C.; Voicescu, M.; Mitran, R.-A.; Lincu, D.; Prelipcean, A.-M.; Craciunescu, O. Cerium-containing mesoporous bioactive glasses (MBGs)-derived scaffolds with drug delivery capability for potential tissue engineering applications. Pharmaceutics 2022, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. A review of bioactive silicate ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef] [PubMed]
- Fada, R.; Farhadi Babadi, N.; Azimi, R.; Karimian, M.; Shahgholi, M. Mechanical properties improvement and bone regeneration of calcium phosphate bone cement, Polymethyl methacrylate and glass ionomer. J. Nanoanal. 2021, 8, 60–79. [Google Scholar]
- Li, H.; Xia, P.; Pan, S.; Qi, Z.; Fu, C.; Yu, Z.; Kong, W.; Chang, Y.; Wang, K.; Wu, D.; et al. The advances of ceria nanoparticles for biomedical applications in orthopaedics. Int. J. Nanomed. 2020, 15, 7199–7214. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Garcia, M.M.; Colilla, M. Biomedical Applications of Mesoporous Ceramics: Drug Delivery, Smart Materials and Bone Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Vallet-Regí, M.; Navarrete, D.A. Nanoceramics in Clinical Use: From Materials to Applications; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Fan, F.-Y.; Chen, M.-S.; Wang, C.-W.; Shih, S.-J.; Chen, C.-Y.; Pan, Y.-N.; Lin, C.-K. Preparation and characterization of silver nanocrystals decorated mesoporous bioactive glass via synchrotron X-ray reduction. J. Non-Cryst. Solids 2016, 450, 128–134. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Tranquillo, E.; Bollino, F. Surface modifications for implants lifetime extension: An overview of sol-gel coatings. Coatings 2020, 10, 589. [Google Scholar] [CrossRef]
- Ardekani, S.R.; Aghdam, A.S.R.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Workie, A.B.; Ningsih, H.S.; Shih, S.-J. An comprehensive review on the spray pyrolysis technique: Historical context, operational factors, classifications, and product applications. J. Anal. Appl. Pyrolysis 2023, 170, 105915. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Shih, S.-J.; Chou, Y.-J.; Panjaitan, L.V.P. Synthesis and characterization of spray pyrolyzed mesoporous bioactive glass. Ceram. Int. 2013, 39, 8773–8779. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.-J.; Lin, Y.-C.; Valentino Posma Panjaitan, L.; Rahayu Meyla Sari, D. The correlation of surfactant concentrations on the properties of mesoporous bioactive glass. Materials 2016, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Choi, J.H.; Lee, S.; Lee, S.W.; Moon, B.K.; Song, J.E.; Khang, G. Pluronic F-127/silk fibroin for enhanced mechanical property and sustained release drug for tissue engineering biomaterial. Materials 2021, 14, 1287. [Google Scholar] [CrossRef]
- Mortazavi, S.; Rahsepar, M.; Hosseinzadeh, S. Modification of mesoporous structure of silver-doped bioactive glass with antibacterial properties for bone tissue applications. Ceram. Int. 2022, 48, 8276–8285. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Ningsih, H.S.; Shih, S.-J. Preparation, characterization and investigation of antibacterial silver-zinc co-doped β-tricalcium phosphate by spray pyrolysis. Ceram. Int. 2020, 46, 16708–16715. [Google Scholar] [CrossRef]
- Shih, S.J.; Tzeng, W.L.; Jatnika, R.; Shih, C.J.; Borisenko, K.B. Control of Ag nanoparticle distribution influencing bioactive and antibacterial properties of Ag-doped mesoporous bioactive glass particles prepared by spray pyrolysis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 899–907. [Google Scholar] [CrossRef]
- Idalia, V.-M.N.; Bernardo, F. Escherichia coli as a model organism and its application in biotechnology. Recent Adv. Physiol. Pathog. Biotechnol. Appl. Tech. Open Rij. Croat 2017, 13, 253–274. [Google Scholar]
- ASTM E3031-15; Standard Test Method for Determination of Antibacterial Activity on Ceramic Surfaces. ASTM international: West Conshohocken, PA, USA, 2015.
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Smeets, R.; Hartjen, P.; Schnettler, R.; Feyerabend, F.; Klein, M.; Wegner, N.; Walther, F.; Stangier, D.; Henningsen, A. Improved in vitro test procedure for full assessment of the cytocompatibility of degradable magnesium based on ISO 10993-5/-12. Int. J. Mol. Sci. 2019, 20, 255. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Hinze, U.; Chichkov, B.; Leibold, W.; Lenarz, T.; Paasche, G. Validation of eGFP fluorescence intensity for testing in vitro cytotoxicity according to ISO 10993-5. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 715–722. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Pang, S.; Liaw, P.K.; Zhang, T. In vitro responses of bone-forming MC3T3-E1 pre-osteoblasts to biodegradable Mg-based bulk metallic glasses. Mater. Sci. Eng. C 2016, 68, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Chang, J. Synthesis and in vitro bioactivity of dicalcium silicate powders. J. Eur. Ceram. Soc. 2004, 24, 93–99. [Google Scholar] [CrossRef]
- Ma, J.; Chen, C.; Wang, D.; Meng, X.; Shi, J. Influence of the sintering temperature on the structural feature and bioactivity of sol–gel derived SiO2–CaO–P2O5 bioglass. Ceram. Int. 2010, 36, 1911–1916. [Google Scholar] [CrossRef]
- Jones, J.R.; Ehrenfried, L.M.; Hench, L.L. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials 2006, 27, 964–973. [Google Scholar] [CrossRef]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Yang, X.; Jiang, T. Are different crystallinity-index-calculating methods of hydroxyapatite efficient and consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]
- Workie, A.B.; Sefene, E.M. Ion-doped mesoporous bioactive glass: Preparation, characterization, and applications using the spray pyrolysis method. RSC Adv. 2022, 12, 1592–1603. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, C.; Yang, M.; Wang, D.; Peng, S.; Tian, Z.; Shuai, C. Copper-doped mesoporous bioactive glass endows magnesium-based scaffold with antibacterial activity and corrosion resistance. Mater. Chem. Front. 2021, 5, 7228–7240. [Google Scholar] [CrossRef]
- Baino, F. Bioactive glasses–when glass science and technology meet regenerative medicine. Ceram. Int. 2018, 44, 14953–14966. [Google Scholar] [CrossRef]
- Kumar, A.; Das, S.; Munusamy, P.; Self, W.; Baer, D.R.; Sayle, D.C.; Seal, S. Behavior of nanoceria in biologically-relevant environments. Environ. Sci. Nano 2014, 1, 516–532. [Google Scholar] [CrossRef]
- Patil, S.N.; Paradeshi, J.S.; Chaudhari, P.B.; Mishra, S.J.; Chaudhari, B.L. Bio-therapeutic potential and cytotoxicity assessment of pectin-mediated synthesized nanostructured cerium oxide. Appl. Biochem. Biotechnol. 2016, 180, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.-F.; Alshemary, A.Z.; Akram, M.; Kadir, M.R.A.; Hussain, R. In-vitro characterization of antibacterial bioactive glass containing ceria. Ceram. Int. 2014, 40, 729–737. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; El-Kheshen, A.A.; El-Faramawy, N.; Ibrahim, M. In vitro bioactivity evaluation, antimicrobial behavior and mechanical properties of cerium-containing phosphate glasses. Mater. Res. Express 2019, 6, 075212. [Google Scholar] [CrossRef]
- Garner, J.; Heppell, P. Cerium nitrate in the management of burns. Burns 2005, 31, 539–547. [Google Scholar] [CrossRef]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.; Pina-Vaz, C.; Rodrigues, A. In vivo antibiofilm effect of cerium, chitosan and hamamelitannin against usual agents of catheter-related bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 126–130. [Google Scholar] [CrossRef]
- Aimei, C.; Qingshan, S.; Ouyang, Y.; Yiben, C. Effect of Ce3+ on membrane permeability of Escherichia coli cell. J. Rare Earths 2012, 30, 947–951. [Google Scholar]
- Sobek, J.M.; Talburt, D.E. Effects of the rare earth cerium on Escherichia coli. J. Bacteriol. 1968, 95, 47–51. [Google Scholar] [CrossRef]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.M. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; Boltes, K.; Fernández-Pinas, F.; Leganés, F.; García-Calvo, E.; Santiago, J.; Rosal, R. Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms. Toxicol. Sci. 2011, 119, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Holtz, R.D.; Lima, B.A.; Souza Filho, A.G.; Brocchi, M.; Alves, O.L. Nanostructured silver vanadate as a promising antibacterial additive to water-based paints. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qu, F.; Xu, H.; Lai, W.; Andrew Wang, Y.; Aguilar, Z.P.; Wei, H. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157: H7. Biometals 2012, 25, 45–53. [Google Scholar] [CrossRef]
- Huang, Y.; Zhai, X.; Ma, T.; Zhang, M.; Pan, H.; Lu, W.W.; Zhao, X.; Sun, T.; Li, Y.; Shen, J. Rare earth-based materials for bone regeneration: Breakthroughs and advantages. Coord. Chem. Rev. 2022, 450, 214236. [Google Scholar] [CrossRef]
- Kung, J.-C.; Chen, Y.-J.; Chiang, Y.-C.; Lee, C.-L.; Yang-Wang, Y.-T.; Hung, C.-C.; Shih, C.-J. Antibacterial activity of silver nanoparticle (AgNP) confined mesoporous structured bioactive powder against Enterococcus faecalis infecting root canal systems. J. Non-Cryst. Solids 2018, 502, 62–70. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.; Petrescu, S.; Seciu, A.; Stefan, L.; Mocioiu, O.; Predoana, L.; Voicescu, M.; Somacescu, S.; Culita, D. Cerium-containing mesoporous bioactive glasses: Material characterization, in vitro bioactivity, biocompatibility and cytotoxicity evaluation. Microporous Mesoporous Mater. 2019, 276, 76–88. [Google Scholar] [CrossRef]
- Leonelli, C.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Tonelli, M. Synthesis and characterization of cerium-doped glasses and in vitro evaluation of bioactivity. J. Non-Cryst. Solids 2003, 316, 198–216. [Google Scholar] [CrossRef]
- Malavasi, G.; Lusvardi, G. Composition and morphology effects on catalase mimetic activity of potential bioactive glasses. Ceram. Int. 2020, 46, 25854–25864. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Vernè, E.; Di Nunzio, S.; Bosetti, M.; Appendino, P.; Brovarone, C.V.; Maina, G.; Cannas, M. Surface characterization of silver-doped bioactive glass. Biomaterials 2005, 26, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, J.; Qu, F.; Jiang, J.; Jiang, P. In vitro hydroxyapatite-forming ability and antimicrobial properties of mesoporous bioactive glasses doped with Ti/Ag. J. Nanomater. 2013, 2013, 24. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Lin, S.-H.; Shih, C.-J.; Chang, S.L.; Shih, S.-J. The effect of Ag dopants on the bioactivity and antibacterial properties of one-step synthesized Ag-containing mesoporous bioactive glasses. J. Nanosci. Nanotechnol. 2016, 16, 10001–10007. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.F.; Rizk, R.A.; Ahmed, M.M. Synthesis, characterization and microbiological response of silver doped bioactive glass nanoparticles. Ceram. Int. 2012, 38, 177–188. [Google Scholar] [CrossRef]
- Shi, C.; Gao, J.; Wang, M.; Fu, J.; Wang, D.; Zhu, Y. Ultra-trace silver-doped hydroxyapatite with non-cytotoxicity and effective antibacterial activity. Mater. Sci. Eng. C 2015, 55, 497–505. [Google Scholar] [CrossRef]
- Naseri, S.; Lepry, W.C.; Maisuria, V.B.; Tufenkji, N.; Nazhat, S.N. Development and characterization of silver-doped sol-gel-derived borate glasses with anti-bacterial activity. J. Non-Cryst. Solids 2019, 505, 438–446. [Google Scholar] [CrossRef]


| Atomic Composition (at %) | |||||
|---|---|---|---|---|---|
| Sample Type | Si | Ca | P | Ag | Ce |
| Nominal | 76.92 | 15.38 | 7.69 | - | - |
| Un-doped MBG | 71.49 ± 0.69 | 14.82 ± 0.64 | 11.63 ± 0.73 | 2.06 ± 0.52 | - |
| 2.87 mol% Ag-doped MBG | 69.52 ± 0.33 | 14.34 ± 0.85 | 10.07 ± 0.85 | 6.07 ± 0.69 | - |
| 5.75 mol% Ag-doped MBG | 71.89 ± 0.41 | 14.01 ± 0.34 | 11.07 ± 0.69 | - | 3.03 ± 0.11 |
| 2.87 mol% Ce-doped MBG | 68.78 ± 0.28 | 13.6 ± 0.86 | 11.79 ± 0.89 | - | 5.82 ± 0.23 |
| 5.75 mol% Ce-doped MBG | 69.07 ± 0.37 | 14.0 ± 0.99 | 11.01 ± 0.29 | 2.9 ± 0.35 | 3.02 ± 0.17 |
| 2.87 mol% Ag/2.87 mol% Ce co-doped MBG | 71.49 ± 0.69 | 14.82 ± 0.64 | 11.63 ± 0.73 | 2.06 ± 0.52 | - |
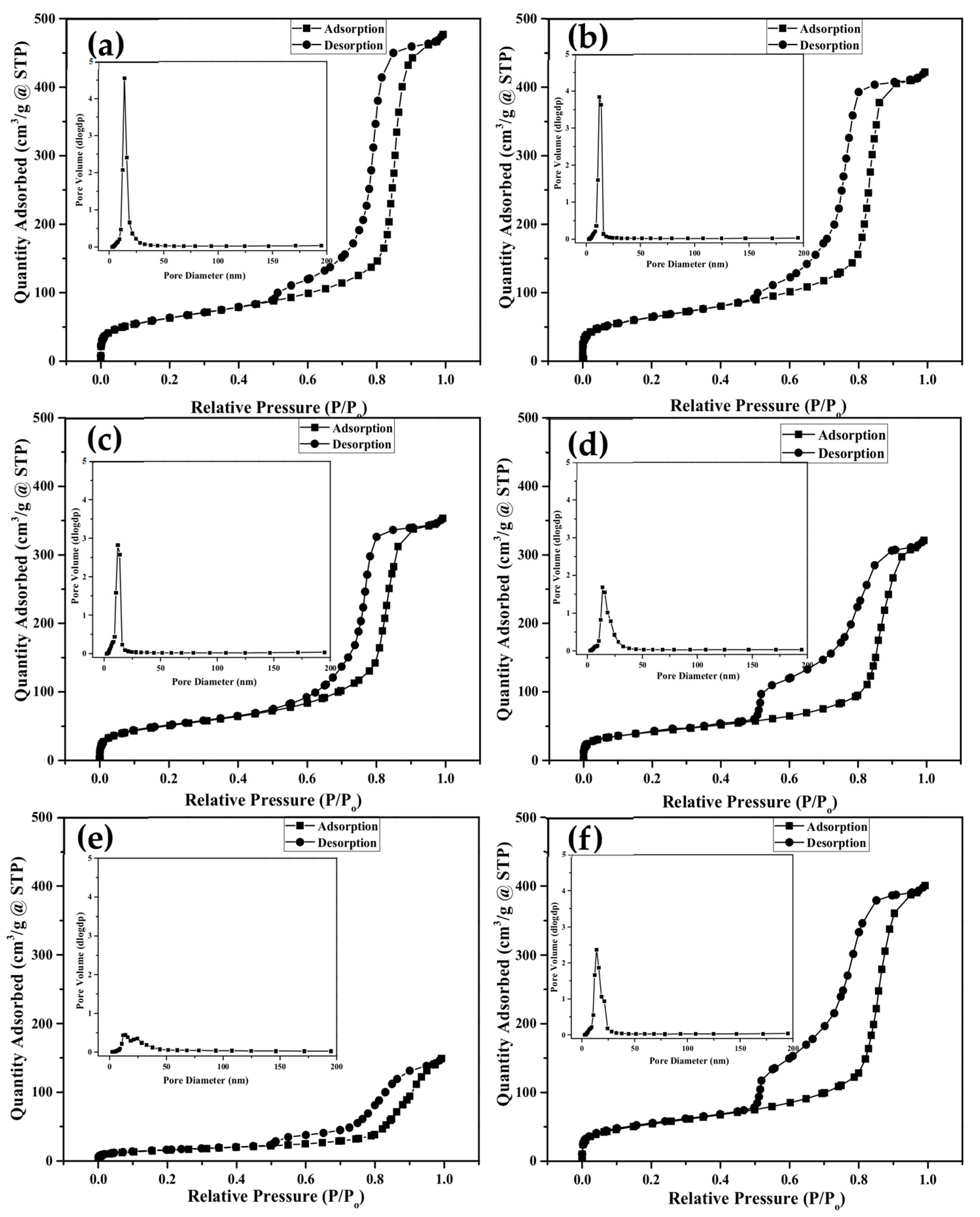
| Sample Type | Specific Surface Area (m2 g−1) | Total Volume (cm3 g−1) | Pore Radius (nm) |
|---|---|---|---|
| Un-doped MBG | 230.68 ± 10.61 | 0.7227 ± 0.018 | 5.09 ± 2.12 |
| 2.87 mol% Ag-doped MBG | 217.78 ± 13.07 | 0.6144 ± 0.052 | 4.67 ± 1.50 |
| 5.75 mol% Ag-doped MBG | 186.01 ± 7.57 | 0.5274 ± 0.026 | 4.82 ± 1.73 |
| 2.87 mol% Ce-doped MBG | 170.12 ± 19.99 | 0.4822 ± 0.018 | 5.45 ± 1.69 |
| 5.75 mol% Ce-doped MBG | 57.39 ± 1.49 | 0.2927 ± 0.090 | 8.16 ± 0.06 |
| 2.87 mol% Ag/2.87 mol% Ce co-doped MBG | 191.93 ± 0.54 | 0.6225 ± 0.006 | 6.38 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taye, M.B.; Ningsih, H.S.; Shih, S.-J. Antibacterial and In Vitro Bioactivity Studies of Silver-Doped, Cerium-Doped, and Silver–Cerium Co-Doped 80S Mesoporous Bioactive Glass Particles via Spray Pyrolysis. Appl. Sci. 2023, 13, 12637. https://doi.org/10.3390/app132312637
Taye MB, Ningsih HS, Shih S-J. Antibacterial and In Vitro Bioactivity Studies of Silver-Doped, Cerium-Doped, and Silver–Cerium Co-Doped 80S Mesoporous Bioactive Glass Particles via Spray Pyrolysis. Applied Sciences. 2023; 13(23):12637. https://doi.org/10.3390/app132312637
Chicago/Turabian StyleTaye, Mannie Belay, Henni Setia Ningsih, and Shao-Ju Shih. 2023. "Antibacterial and In Vitro Bioactivity Studies of Silver-Doped, Cerium-Doped, and Silver–Cerium Co-Doped 80S Mesoporous Bioactive Glass Particles via Spray Pyrolysis" Applied Sciences 13, no. 23: 12637. https://doi.org/10.3390/app132312637





