Effects of Freeze-Drying on Sensory Characteristics and Nutrient Composition in Black Currant and Sea Buckthorn Berries
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Material
2.2. Freeze-Drying
2.3. Sensory Assessment
2.4. Determination of Moisture
2.5. Determination of Vitamins
2.6. Determination of Organic Acids
2.7. Determination of Soluble Sugars
2.8. Statistical Analysis
3. Results
3.1. Sensory Evaluation and Moisture Content
3.2. Vitamins
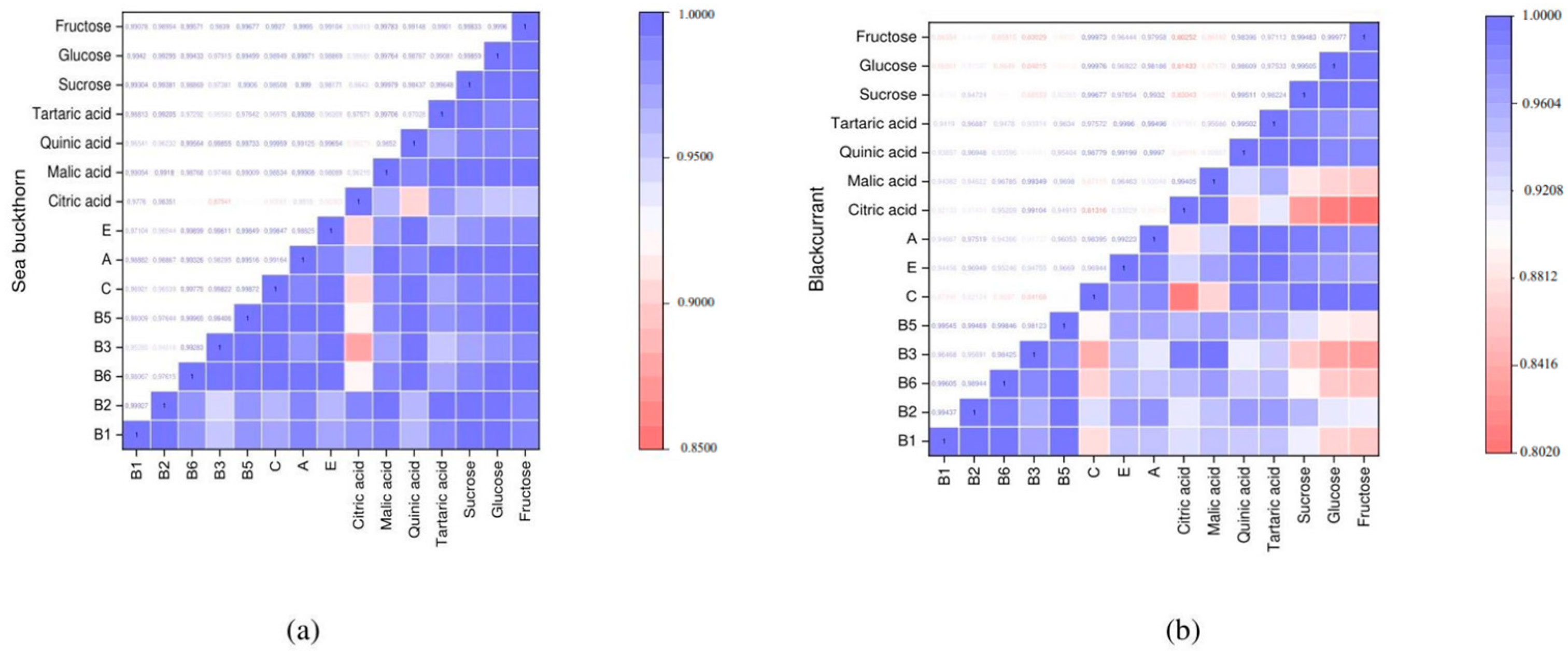
3.3. Organic Acids and Sugars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Urso, G.; Piacente, S.; Pizza, C.; Montoro, P. Metabolomics of healthy berry fruits. Curr. Med. Chem. 2018, 25, 4888–4902. [Google Scholar] [CrossRef] [PubMed]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Silicka, I.; Dembovska, I.; Teirumnieka, Ē.; Dembovskis, I. Analysis of hiking food processing technologies on the market. J. Reg. Econ. Soc. Dev. 2020, 12, 171. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Niro, C.M.; Bresolin, J.D.; Soares, V.F.; Ferreira, M.D.; Sivieri, K.; Azeredo, H.M. Dehydrated strawberries for probiotic delivery: Influence of dehydration and probiotic incorporation methods. LWT 2021, 144, 111105. [Google Scholar] [CrossRef]
- Rafalska, A.; Abramowicz, K.; Krauze, M. Sea buckthorn (Hippophae rhamnoides L.) as a plant for universal application. World Sci. News 2017, 72, 123–140. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef]
- Cortez, R.E.; Gonzalez de Mejia, E. Blackcurrants (Ribes nigrum): A review on chemistry, processing, and health benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Burčová, Z. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A Rev. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Cyboran, S.; Bonarska-Kujawa, D.; Pruchnik, H.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Phenolic content and biological activity of extracts of blackcurrant fruit and leaves. Food Res. Int. 2014, 65, 47–58. [Google Scholar] [CrossRef]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.O.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-oxidant and anti-enzymatic activities of sea buckthorn (Hippophaë rhamnoides L.) fruits modulated by chemical components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Archaina, D.; Sosa, N.; Rivero, R.; Schebor, C. Freeze-dried candies from blackcurrant (Ribes nigrum L.) and yoghurt. Physicochemical and sensorial characterization. LWT 2019, 100, 444–449. [Google Scholar] [CrossRef]
- Hummer, K.E.; Barney, D.L. Currants. HortTechnology 2002, 12, 377–387. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. Ascorbic acid, anthocyanins, organic acids and mineral content of some black and red currant cultivars. Fruits 2011, 66, 353–362. [Google Scholar] [CrossRef]
- Rachtan-Janicka, J.; Ponder, A.; Hallmann, E. The effect of organic and conventional cultivations on antioxidants content in blackcurrant (Ribes nigrum L.) species. Appl. Sci. 2021, 11, 5113. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Beveridge, T.; Li, T.S.; Oomah, B.D.; Smith, A. Sea buckthorn products: Manufacture and composition. J. Agric. Food Chem. 1999, 47, 3480–3488. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Stobdan, T.; Yadav, A.; Mishra, G.P.; Chaurasia, O.P.; Srivastava, R.B. Seabuckthorn: The Super Plant; Defence Institute of High Altitude Research, Defence Research and Development Organisation: Leh, India, 2011. [Google Scholar]
- Pallavee, K.; Ashwani, M. Sea buckthorn juice: Nutritional therapeutic properties and economic considerations. Int. J. Pharmacogn. Phytochem. Res 2017, 9, 880–884. [Google Scholar] [CrossRef][Green Version]
- Tang, X.; Kälviäinen, N.; Tuorila, H. Sensory and hedonic characteristics of juice of sea buckthorn (Hippophae rhamnoides L.) origins and hybrids. LWT-Food Sci. Technol. 2001, 34, 102–110. [Google Scholar] [CrossRef]
- Bordonaba, J.G.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria× ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Stampar, F.; Veberic, R. Parameters of inner quality of the apple scab resistant and susceptible apple cultivars (Malus domestica Borkh.). Sci. Hortic. 2007, 114, 37–44. [Google Scholar] [CrossRef]
- Niesteruk, A.; Lewandowska, H.; Golub, Z.; Swisłocka, R.; Lewandowski, W. Let’s get interested with sea buckthorn. Preparations of sea buckthorn as food additives and assessment of their market in Poland. Kosmos 2013, 4, 571–581. [Google Scholar]
- Mikulic-Petkovsek, M.; Slatnar, A.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Chemical profile of black currant fruit modified by different degree of infection with black currant leaf spot. Sci. Hortic. 2013, 150, 399–409. [Google Scholar] [CrossRef]
- Bordonaba, J.G.; Terry, L.A. Biochemical profiling and chemometric analysis of seventeen UK-grown black currant cultivars. J. Agric. Food Chem. 2008, 56, 7422–7430. [Google Scholar] [CrossRef]
- Mörsel, J.T.; Zubarev, Y.; Eagle, D. (Eds.) Seabuckthorn. Research for a Promising Crop: A look at Recent Developments in Cultivation, Breeding, Technology, Health and Environment; Books in Demand: Norderstedt, Germany, 2014. [Google Scholar]
- Raffo, A.; Paoletti, F.; Antonelli, M. Changes in sugar, organic acid, flavonol and carotenoid composition during ripening of berries of three seabuckthorn (Hippophae rhamnoides L.) cultivars. Eur. Food Res. Technol. 2004, 219, 360–368. [Google Scholar] [CrossRef]
- Qi, Y.; Yu, F.; Wang, X.; Wan, N.; Yang, M.; Wu, Z.; Li, Y. Drying of wolfberry fruit juice using low-intensity pulsed ultrasound. LWT 2021, 141, 110953. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Pavkov, I.; Stamenković, Z.; Radojčin, M.; Krstan, K.; Bursić, V.; Bikić, S.; Mitrevski, V. Osmotic and convective drying of strawberries: Effects of experimental parameters on the drying kinetics, color and rehydration. J. Process. Energy Agric. 2018, 22, 58–64. [Google Scholar] [CrossRef]
- Sagar, V.R.; Kumar, P.S. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lahsasni, S.; Kouhila, M.; Mahrouz, M.; Jaouhari, J.T. Drying kinetics of prickly pear fruit (Opuntia ficus indica). J. Food Eng. 2004, 61, 173–179. [Google Scholar] [CrossRef]
- Zielinska, M.; Zielinska, D.; Markowski, M. The effect of microwave-vacuum pre-treatment on the drying kinetics, color and the content of bioactive compounds in osmo-microwave-vacuum dried cranberries (Vaccinium macrocarpon). Food Bioprocess Technol. 2018, 11, 585–602. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Cardador-Martínez, A.; Tejada-Ortigoza, V.; Soria-Mejía, M.C.; Balderas-León, I.; Alonzo-Macías, M. Antioxidant content of frozen, convective air-dried, freeze-dried, and swell-dried chokecherries (Prunus virginiana L.). Molecules 2020, 25, 1190. [Google Scholar] [CrossRef]
- Bustos, M.C.; Rocha-Parra, D.; Sampedro, I.; de Pascual-Teresa, S.; León, A.E. The influence of different air-drying conditions on bioactive compounds and antioxidant activity of berries. J. Agric. Food Chem. 2018, 66, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Figiel, A.; Michalska, A. Overall quality of fruits and vegetables products affected by the drying processes with the assistance of vacuum-microwaves. Int. J. Mol. Sci. 2016, 18, 71. [Google Scholar] [CrossRef]
- Issis, Q.F.; Antonio, V.G.; Elsa, U.; Valeria, V.; Nicole, C. Vacuum drying application to maqui (Aristotelia chilensis [Mol] Stuntz) berry: Weibull distribution for process modelling and quality parameters. J. Food Sci. Technol. 2019, 56, 1899–1908. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different drying methods on chemical composition and antioxidant activity in chokeberries. LWT-Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Sette, P.; Franceschinis, L.; Schebor, C.; Salvatori, D. Fruit snacks from raspberries: Influence of drying parameters on colour degradation and bioactive potential. Int. J. Food Sci. Technol. 2017, 52, 313–328. [Google Scholar] [CrossRef]
- Nemzer, B.; Vargas, L.; Xia, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, Z.; Pavkov, I.; Radojčin, M.; TepićHorecki, A.; Kešelj, K.; BursaćKovačević, D.; Putnik, P. Convective drying of fresh and frozen raspberries and change of their physical and nutritive properties. Foods 2019, 8, 251. [Google Scholar] [CrossRef]
- Mejia-Meza, E.I.; Yanez, J.A.; Davies, N.M.; Rasco, B.; Younce, F.; Remsberg, C.M.; Clary, C. Improving nutritional value of dried blueberries (Vaccinium corymbosum L.) combining microwave-vacuum, hot-air drying and freeze-drying technologies. Int. J. Food Eng. 2008, 4, 5–6. [Google Scholar] [CrossRef]
- Araya-Farias, M.; Makhlouf, J.; Ratti, C. Drying of seabuckthorn (Hippophae rhamnoides L.) berry: Impact dehydration methods on kinetics and quality. Dry. Technol. 2011, 29, 351–359. [Google Scholar] [CrossRef]
- ISO 8589: 2007; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- da Silva, G.D.; Barros, Z.M.P.; de Medeiros, R.A.B.; de Carvalho, C.B.O.; Brandão, S.C.R.; Azoubel, P.M. Pretreatments for melon drying implementing ultrasound and vacuum. LWT 2016, 74, 114–119. [Google Scholar] [CrossRef]
- Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2016.
- Paunović, V.; Nikolic, M.; Miletić, R.; Mašković, P. Vitamin and mineral content in black currant (Ribes nigrum L.) fruits as affected by soil management system. Acta Sci. Pol. Hortorum Cultus. 2017, 16, 137–138. [Google Scholar] [CrossRef]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of phenolic compounds, ascorbic acid and vitamin E to antioxidant activity of currant (Ribes L.) and gooseberry (Ribes uva-crispa L.) fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, S.; Wang, Q.; Zhao, Z.K. Determination of organic acids in the presence of inorganic anions by ion chromatography with suppressed conductivity detection. J. Chromatogr. A 2008, 1192, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.H.; Sugiura, A. Changes in sugar composition in relation to invertase activity in the growth and ripening of persimmon (Diospyros kaki) fruits. J. Jpn. Soc. Hortic. Sci. 1990, 59, 281–287. [Google Scholar] [CrossRef][Green Version]
- Shishehgarha, F.; Makhlouf, J.; Ratti, C. Freeze-drying characteristics of strawberries. Dry. Technol. 2002, 20, 131–145. [Google Scholar] [CrossRef]
- Wang, J.; Law, C.L.; Mujumdar, A.S.; Xiao, H.W. The degradation mechanism and kinetics of vitamin C in fruits and vegetables during thermal processing. In Fundamentals & Applications (Part III); Nema, P.K., Kaur, B.P., Mujumdar, A.S., Eds.; New India Publishing Agency: New-Delhi, India, 2017; pp. 227–253. [Google Scholar]
- Gümüşay, Ö.A.; Borazan, A.A.; Ercal, N.; Demirkol, O. Drying effects on the antioxidant properties of tomatoes and ginger. Food Chem. 2015, 173, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Zhu, L.; Wang, J.; Yu, X.; Li, M.; Yang, W.; Yang, X. Drying sea buckthorn berries (Hippophae rhamnoides L.): Effects of different drying methods on drying kinetics, physicochemical properties, and microstructure. Front. Nutr. 2023, 10, 1106009. [Google Scholar] [CrossRef] [PubMed]
- Hawlader, M.N.A.; Perera, C.O.; Tian, M.; Yeo, K.L. Drying of guava and papaya: Impact of different drying methods. Dry. Technol. 2006, 24, 77–87. [Google Scholar] [CrossRef]
- Lentzou, D.; Templalexis, C.; Xanthopoulos, G. Effect of air drying on quality characteristics and mass transfer kinetics of osmotically dehydrated sea buckthorn by stevia. Food Res. 2020, 4, 1140–1150. [Google Scholar] [CrossRef]
- Famiani, F.; Baldicchi, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Soluble sugar and organic acid contents and the occurrence and potential role of phosphoenolpyruvate carboxykinase (PEPCK) in gooseberry (Ribes grossularia L.). J. Hortic. Sci. Biotechnol. 2009, 84, 249–254. [Google Scholar] [CrossRef]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Basson, C.E.; Groenewald, J.H.; Kossmann, J.; Cronjé, C.; Bauer, R. Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: Invertase is the main sucrose hydrolysing enzyme. Food Chem. 2010, 121, 1156–1162. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, R.; Liu, C.; Wu, Y.; Duan, X.; Hu, J.; Wang, H. Dynamic changes in volatile flavor compounds, amino acids, organic acids, and soluble sugars in lemon juice vesicles during freeze-drying and hot-air drying. Foods 2022, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
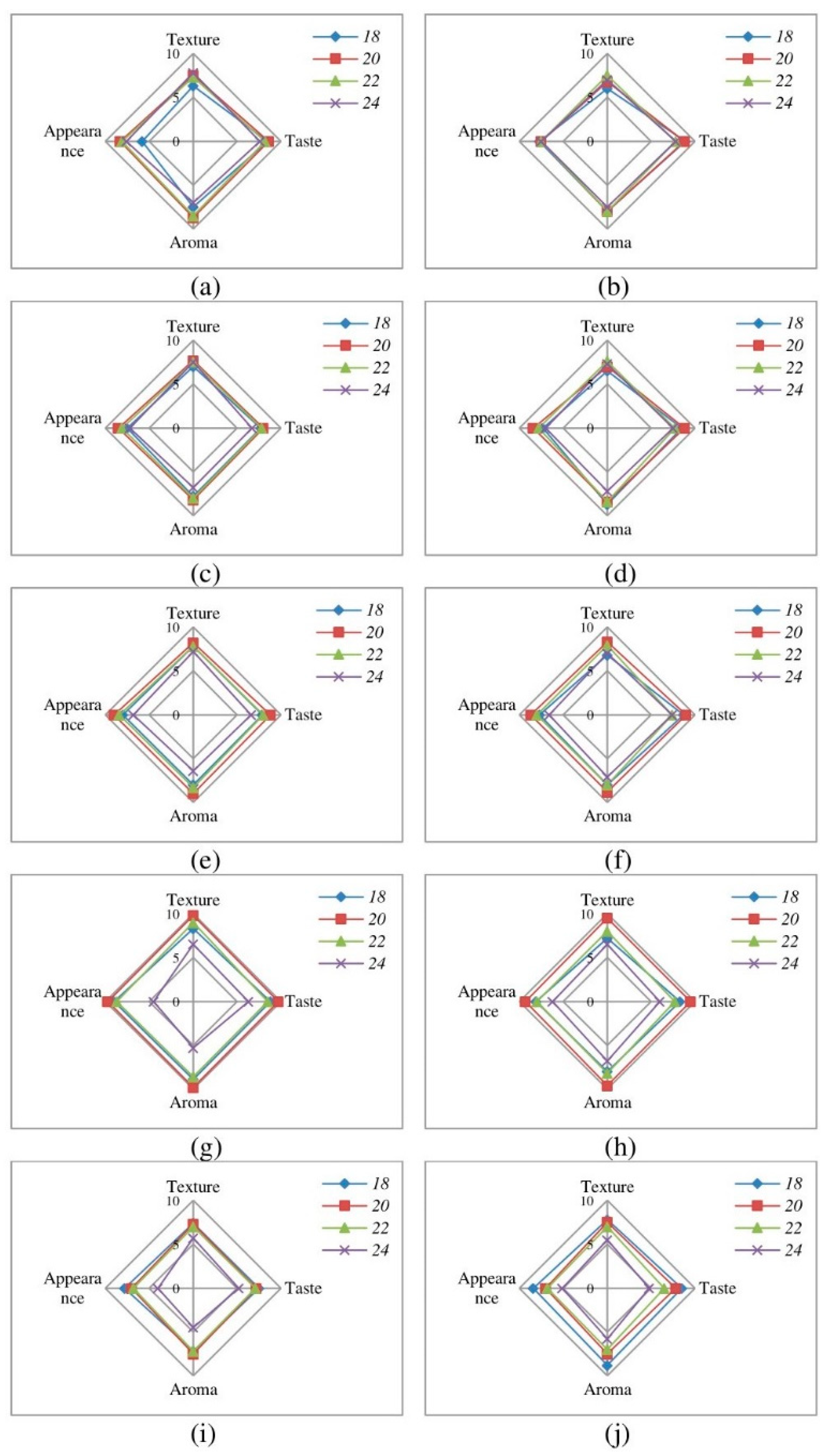

| Name of Berry | Moisture Content of Fresh Berry, % | Time, h | Temperature of Shelves, °C | ||||
|---|---|---|---|---|---|---|---|
| 35 | 40 | 45 | 50 | 55 | |||
| Black currant | 84.00 ± 0.73 | 18 | 72.30 ± 0.13 | 57.23 ± 0.16 | 49.17 ± 0.09 | 28.70 ± 0.07 | 18.10 ± 0.13 |
| 20 | 61.43 ± 0.24 | 53.23 ± 0.18 | 43.30 ± 0.20 | 16.27 ± 0.22 | 12.40 ± 0.07 | ||
| 22 | 42.10 ± 0.13 | 35.03 ± 0.16 | 33.20 ± 0.13 | 10.90 ± 0.07 | 7.93 ± 0.18 | ||
| 24 | 40.73 ± 0.42 | 33.13 ± 0.24 | 27.63 ± 0.24 | 9.40 ± 0.20 | 5.07 ± 0.18 | ||
| Sea buckthorn | 85.00 ± 0.13 | 18 | 83.27 ± 0.16 | 71.07 ± 0.09 | 62.37 ± 0.16 | 36.50 ± 0.20 | 9.30 ± 0.13 |
| 20 | 74.40 ± 0.07 | 64.33 ± 0.16 | 43.10 ± 0.07 | 17.20 ± 0.07 | 7.10 ± 0.07 | ||
| 22 | 68.77 ± 0.16 | 56.47 ± 0.22 | 35.43 ± 0.16 | 12.23 ± 0.18 | 4.97 ± 0.04 | ||
| 24 | 53.90 ± 0.13 | 47.90 ± 0.31 | 29.80 ± 0.18 | 10.50 ± 0.24 | 3.50 ± 0.20 | ||
| Name | B1 | B2 | B6 | B3 | B5 | C | E | A |
|---|---|---|---|---|---|---|---|---|
| Black currant | ||||||||
| Fresh | 0.0710 ± 0.0006 a | 0.0540 ± 0.0012 a | 0.0273 ± 0.0007 a | 0.3367 ± 0.0120 c | 0.3467 ± 0.0088 a | 204.00 ± 0.0012 a | 73.670 ± 0.0088 a | 168.30 ± 0.0009 d |
| 18 h | 0.0667 ± 0.0012 a | 0.0433 ± 0.0009 b | 0.0210 ± 0.0006 b | 0.2867 ± 0.0088 d | 0.2633 ± 0.0088 b | 163.30 ± 0.0015 c | 57.670 ± 0.0088 b | 137.70 ± 0.0007 e |
| 20 h | 0.0630 ± 0.0270 a,b | 0.0370 ± 0.0012 c | 0.0167 ± 0.0019 b,c | 0.2700 ± 0.0115 d | 0.2167 ± 0.0088 c | 161.70 ± 0.0009 c | 56.330 ± 0.0088 b | 131.70 ± 0.0015 f |
| 22 h | 0.0613 ± 0.0009 a,b | 0.0343 ± 0.0009 c | 0.0130 ± 0.0012 c | 0.2233 ± 0.0120 e | 0.1767 ± 0.0088 d | 161.00 ± 0.0012 c,d | 51.330 ± 0.0033 c | 127.70 ± 0.0009 f |
| Sea buckthorn | ||||||||
| Fresh | 0.0350 ± 0.0006 b,c | 0.0557 ± 0.0009 a | 0.0280 ± 0.0006 a | 0.4727 ± 0.0018 a | 0.1497 ± 0.0015 e | 196.30 ± 0.0009 b | 25.270 ± 0.0020 d | 229.30 ± 0.0018 a |
| 18 h | 0.0260 ± 0.0012 c | 0.0477 ± 0.0009 b | 0.0207 ± 0.0009 b | 0.3780 ± 0.0015 b | 0.1213 ± 0.0020 f | 157.30 ± 0.0032 c,d,e | 20.330 ± 0.0029 e | 186.70 ± 0.0023 b |
| 20 h | 0.0230 ± 0.0023 c | 0.0453 ± 0.0023 b | 0.0196 ± 0.0018 b | 0.3767 ± 0.0029 b | 0.1180 ± 0.0006 f | 155.00 ± 0.0012 d,e | 19.700 ± 0.0023 e | 182.70 ± 0.0020 b |
| 22 h | 0.0210 ± 0.0023 c | 0.0430 ± 0.0026 b | 0.0193 ± 0.0020 b | 0.3743 ± 0.0023 b | 0.1160 ± 0.0036 f | 152.70 ± 0.0038 e | 19.700 ± 0.0042 e | 174.00 ± 0.0032 c |
| Name | Citric Acid | Malic Acid | Quinic Acid | Tartary Acid | Sucrose | Glucose | Fructose |
|---|---|---|---|---|---|---|---|
| Black currant | |||||||
| Fresh | 3181.0 ± 113.503 a | 515.23 ± 72.797 d | 1500.3 ± 0.176 b | 63.560 ± 0.185 a | 1.8500 ± 0.029 a | 16.600 ± 0.058 a | 25.667 ± 0.067 a |
| 18 h | 2685.7 ± 94.669 b | 421.40 ± 60.142 d,e | 1445.2 ± 0.057 c | 58.000 ± 0.149 b | 1.4700 ± 0.012 b | 13.777 ± 0.015 b | 21.257 ± 0.018 b |
| 20 h | 2657.2 ± 76.302 b | 413.83 ± 51.842 d,e | 1436.2 ± 0.145 c | 57.500 ± 0.153 b | 1.4167 ± 0.009 c | 13.743 ± 0.019 b | 21.167 ± 0.015 b,c |
| 22 h | 2101.4 ± 103.308 c | 337.23 ± 73.627 e | 1429.4 ± 0.152 c | 56.000 ± 0.150 c | 1.4133 ± 0.020 c | 13.650 ± 0.021 c | 21.137 ± 0.023 c |
| Sea buckthorn | |||||||
| Fresh | 133.33 ± 55.925 d | 3542.2 ± 98.919 a | 1764.5 ± 16.533 a | 4.9600 ± 0.233 d | 0.2100 ± 0.020 d | 2.1533 ± 0.018 e | 1.8067 ± 0.032 e |
| 18 h | 110.00 ± 21.071 d | 3182.7 ± 73.609 b | 1446.7 ± 27.832 c | 3.7300 ± 0.348 e | 0.1300 ± 0.026 e | 1.7667 ± 0.029 e | 1.5067 ± 0.046 e |
| 20 h | 100.20 ± 45.527 d | 3149.2 ± 49.883 b,c | 1439.7 ± 24.390 c | 3.6300 ± 0.296 e | 0.1200 ± 0.010 e | 1.7000 ± 0.017 e,f | 1.4667 ± 0.035 e |
| 22 h | 85.300 ± 32.247 d | 3054.3 ± 42.975 c | 1413.9 ± 11.585 c | 3.1600 ± 0.348 e | 0.1000 ± 0.016 e | 1.6333 ± 0.026 f | 1.4167 ± 0.041 e |
| Name | Fresh (per 100 g) | 18 h Freeze-Dried (per 100 g) | 18 h Recovery (%) | 20 h Freeze-Dried (per 100 g) | 20 h Recovery (%) | 22 h Freeze-Dried (per 100 g) | 22 h Recovery (%) |
|---|---|---|---|---|---|---|---|
| Lactate | 0.07 | 0.066 | 93.94 | 0.062 | 88.73 | 0.06 | 85.71 |
| Acetate | 0.05 | 0.04 | 80.00 | 0.035 | 70.00 | 0.03 | 60.00 |
| Propionate | 0.03 | 0.023 | 76.67 | 0.018 | 60.00 | 0.015 | 50.00 |
| Formate | 0.34 | 0.289 | 85.00 | 0.272 | 80.00 | 0.238 | 70.00 |
| Pyruvate | 0.35 | 0.266 | 76.00 | 0.219 | 62.57 | 0.175 | 50.00 |
| Succinate | 0.10 | 0.085 | 85.00 | 0.08 | 80.00 | 0.075 | 75.00 |
| Malate | 0.25 | 0.20 | 80.00 | 0.1875 | 75.00 | 0.175 | 70.00 |
| Oxalate | 204.00 | 163.30 | 80.05 | 161.70 | 79.26 | 160.00 | 78.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamanova, S.; Temirova, I.; Aldiyeva, A.; Yermekov, Y.; Toimbayeva, D.; Murat, L.; Muratkhan, M.; Khamitova, D.; Tultabayeva, T.; Bulashev, B.; et al. Effects of Freeze-Drying on Sensory Characteristics and Nutrient Composition in Black Currant and Sea Buckthorn Berries. Appl. Sci. 2023, 13, 12709. https://doi.org/10.3390/app132312709
Kamanova S, Temirova I, Aldiyeva A, Yermekov Y, Toimbayeva D, Murat L, Muratkhan M, Khamitova D, Tultabayeva T, Bulashev B, et al. Effects of Freeze-Drying on Sensory Characteristics and Nutrient Composition in Black Currant and Sea Buckthorn Berries. Applied Sciences. 2023; 13(23):12709. https://doi.org/10.3390/app132312709
Chicago/Turabian StyleKamanova, Svetlana, Indira Temirova, Akmaral Aldiyeva, Yernaz Yermekov, Dana Toimbayeva, Linara Murat, Marat Muratkhan, Dina Khamitova, Tamara Tultabayeva, Berdibek Bulashev, and et al. 2023. "Effects of Freeze-Drying on Sensory Characteristics and Nutrient Composition in Black Currant and Sea Buckthorn Berries" Applied Sciences 13, no. 23: 12709. https://doi.org/10.3390/app132312709
APA StyleKamanova, S., Temirova, I., Aldiyeva, A., Yermekov, Y., Toimbayeva, D., Murat, L., Muratkhan, M., Khamitova, D., Tultabayeva, T., Bulashev, B., & Ospankulova, G. (2023). Effects of Freeze-Drying on Sensory Characteristics and Nutrient Composition in Black Currant and Sea Buckthorn Berries. Applied Sciences, 13(23), 12709. https://doi.org/10.3390/app132312709







