Unconventional Extraction Methods of Oleaginous Yeast Cell Pretreatment and Disruption
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
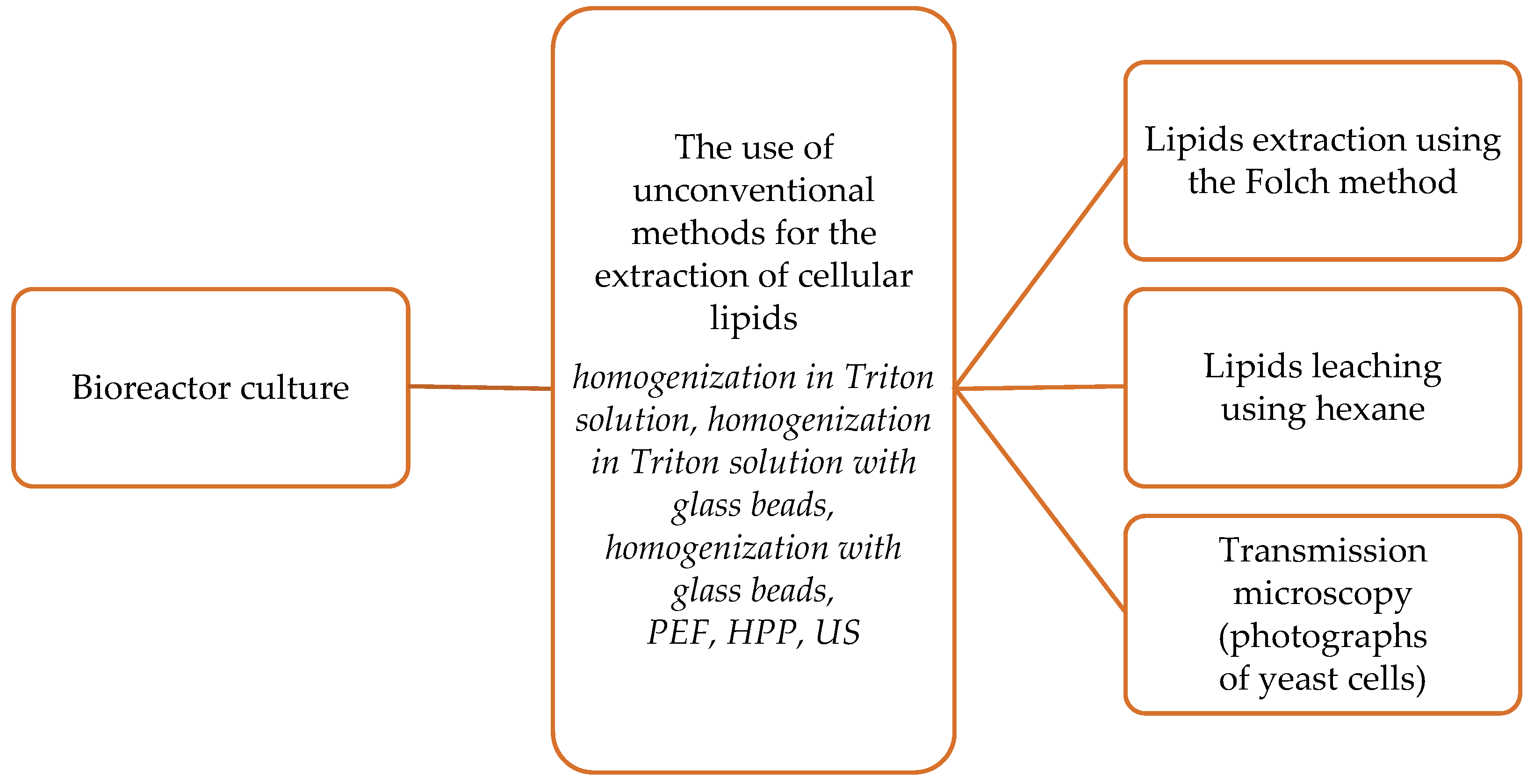
2.2. Experimental Design
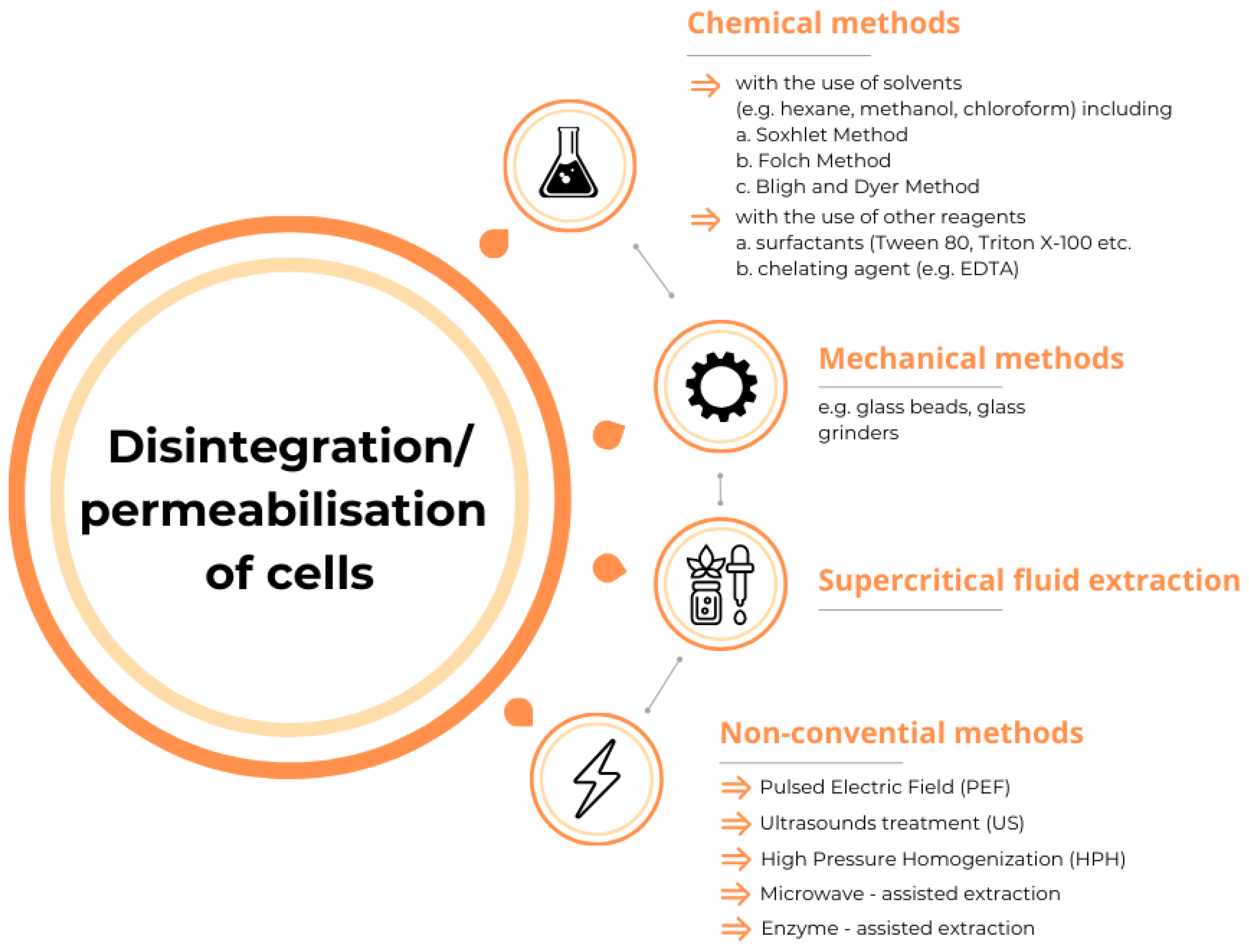
2.2.1. Mechanical and Chemical Methods for Lipid Extraction
2.2.2. Pulsed Electric Field
2.2.3. Ultrasound Treatment
2.2.4. High-Pressure Processing
2.3. Determination of Oil Content
2.3.1. Evaluation of Fat Content in Yeast Cells Using the Folch Method
2.3.2. Leaching of Intracellular Oil from Yeast Cells
2.4. Protein Determination Using the Lowry Method
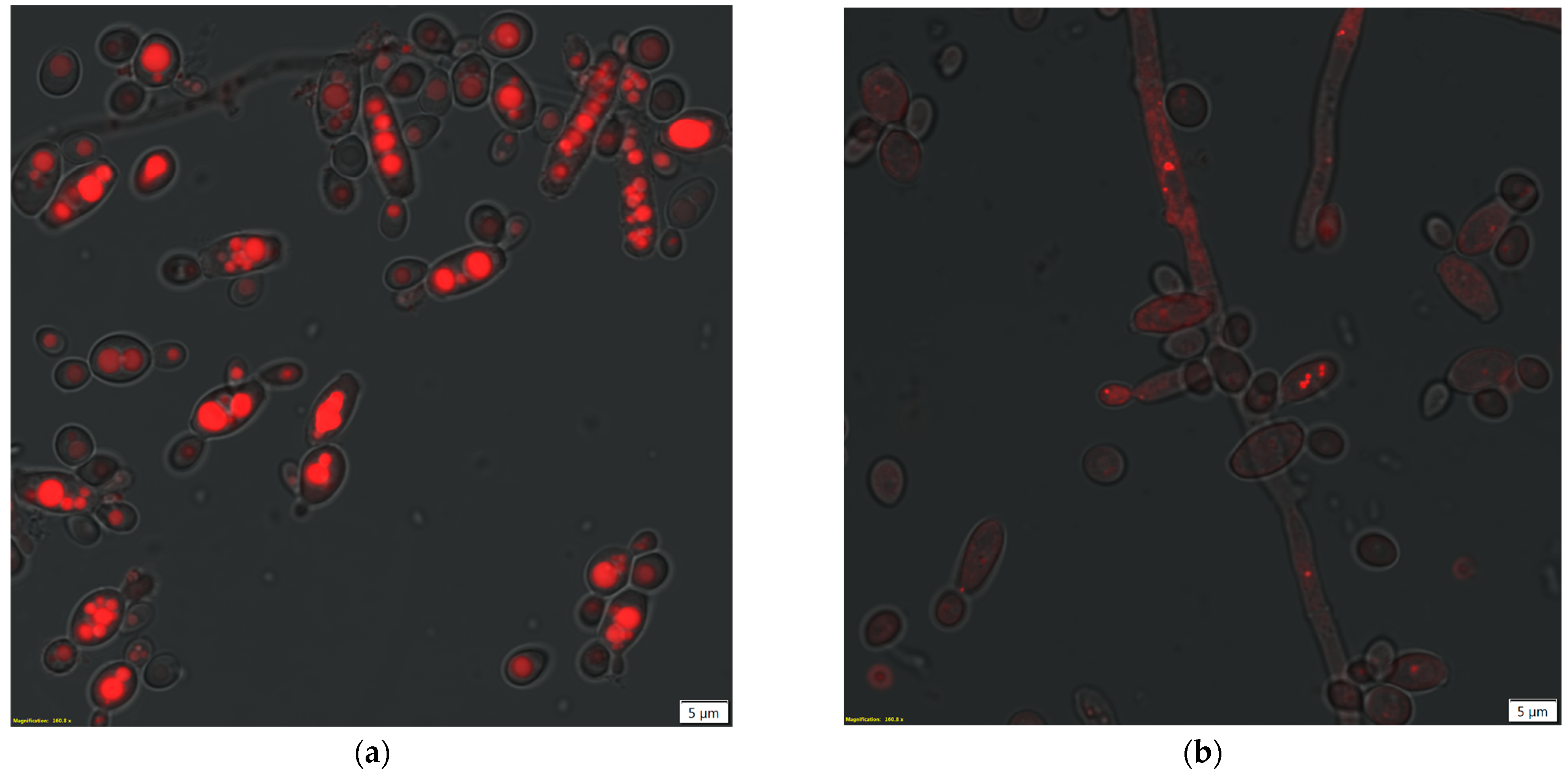
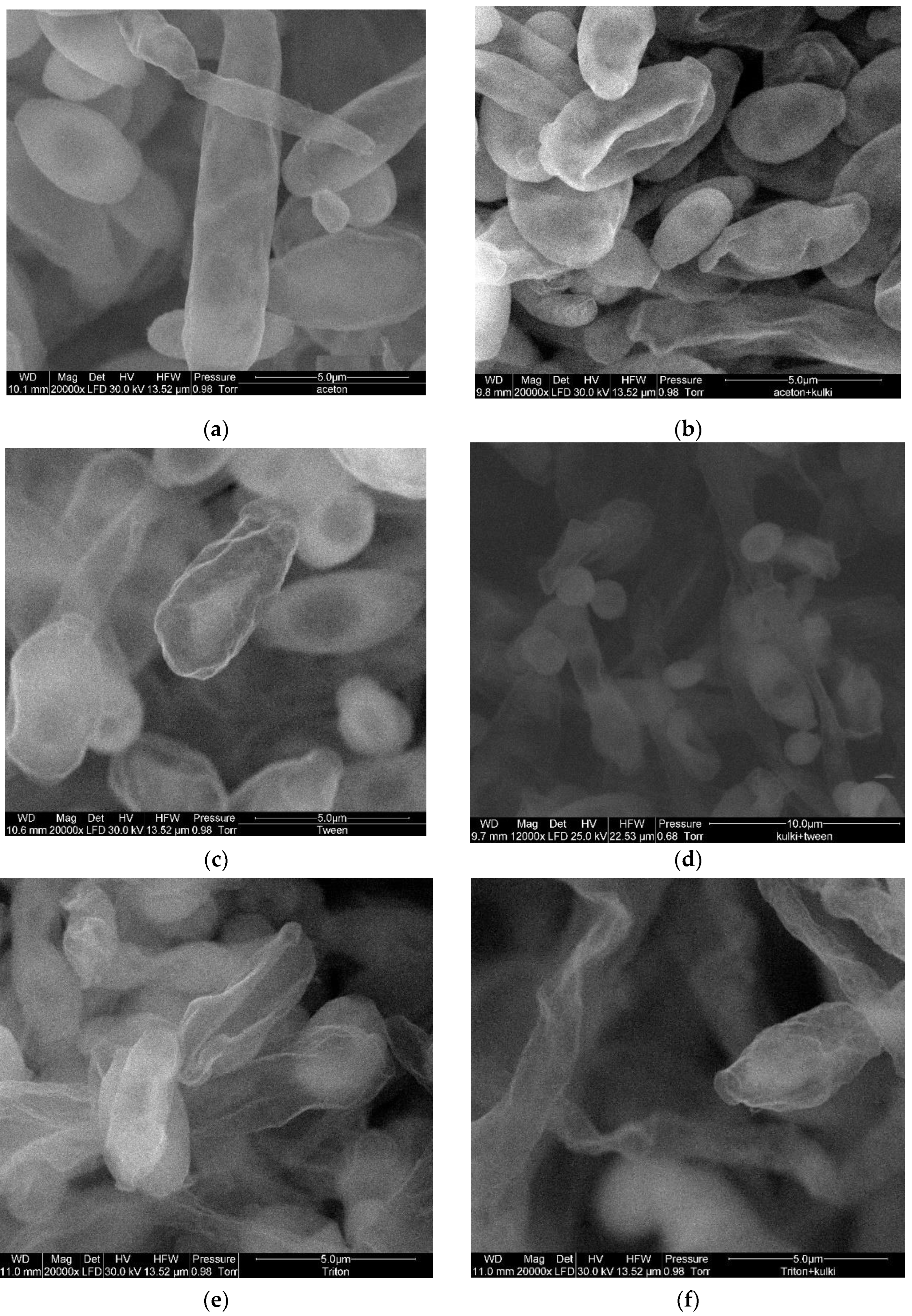
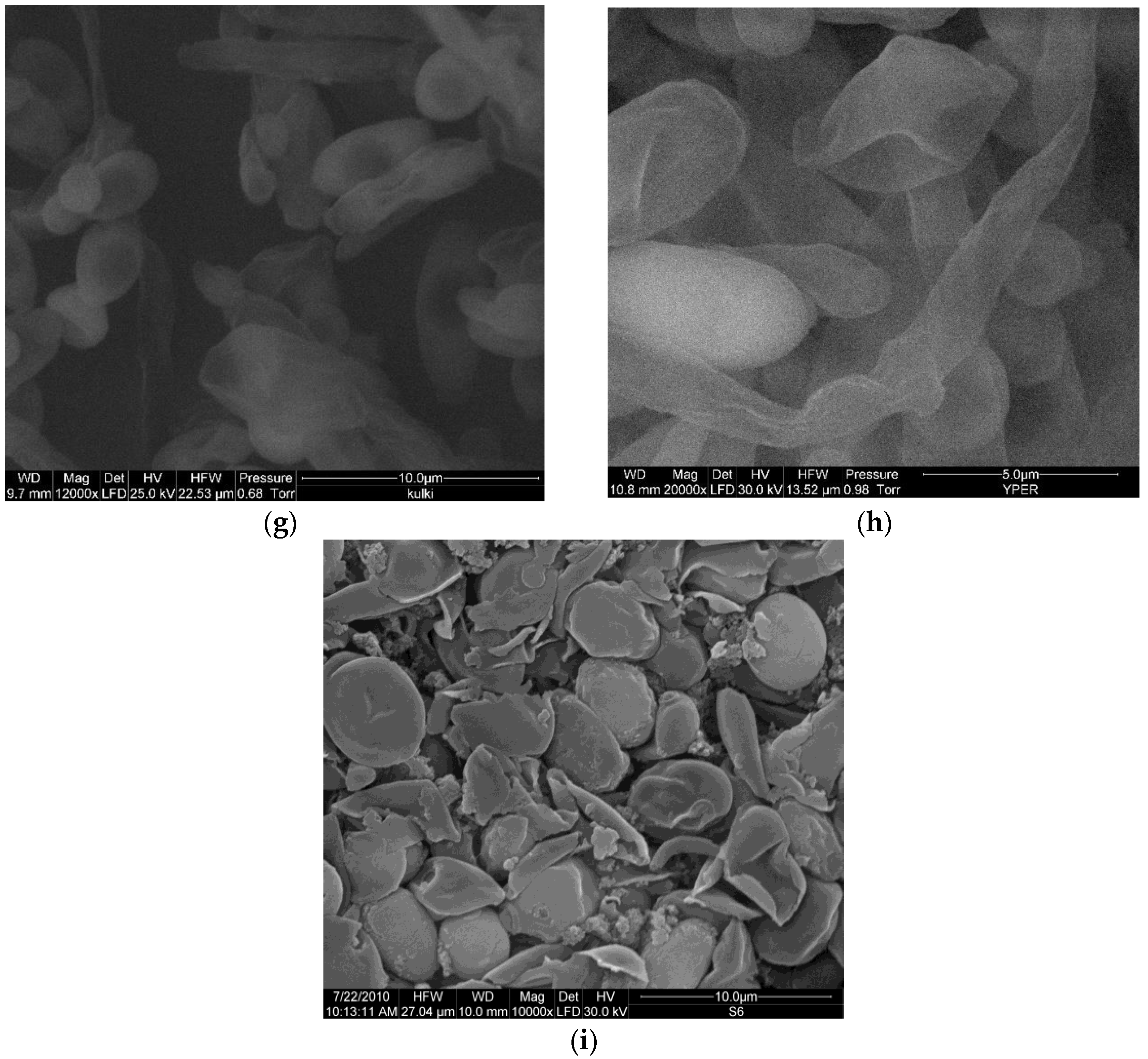
2.5. Microscopy Techniques
2.6. Statistical Analysis
3. Results
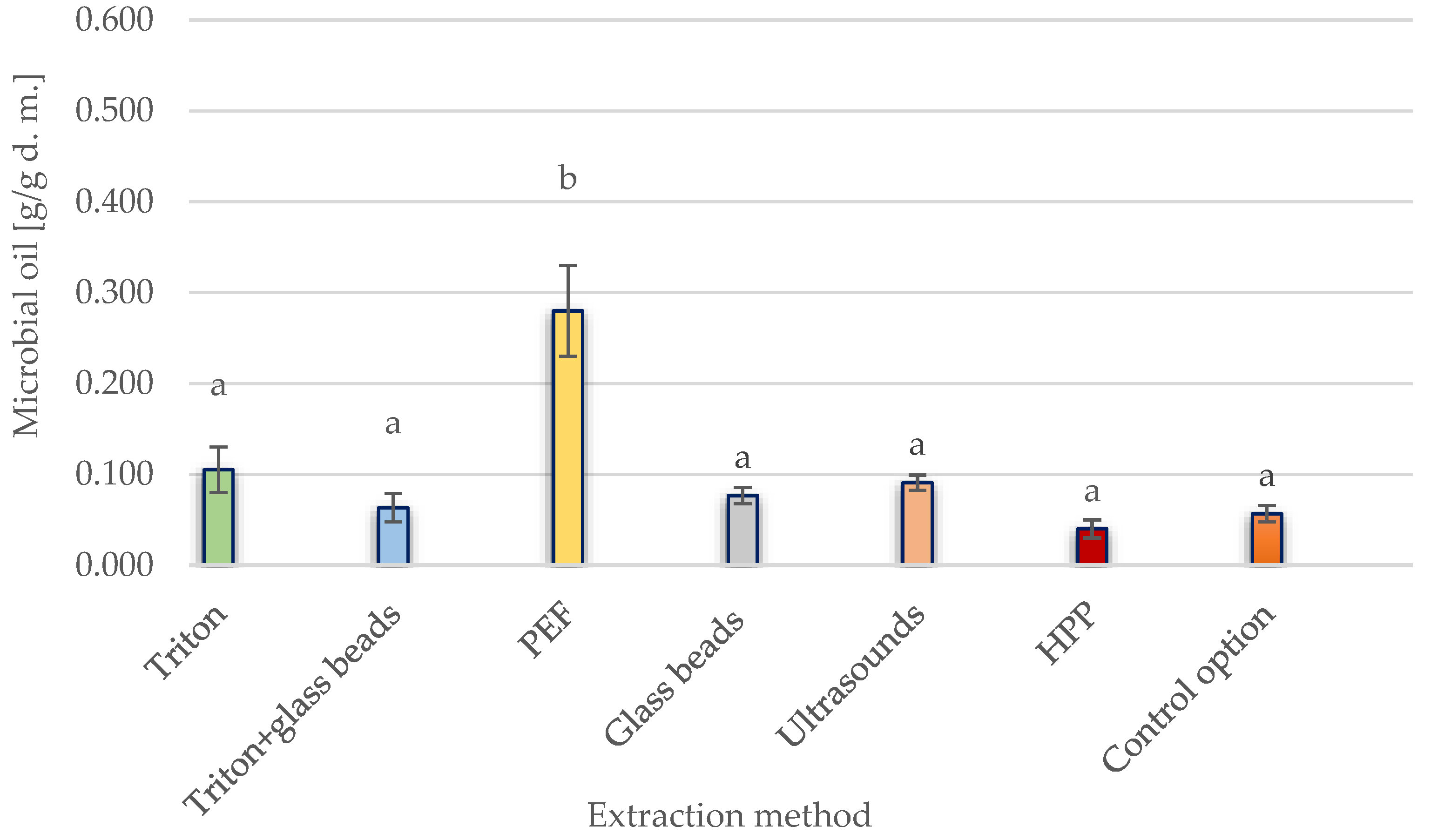
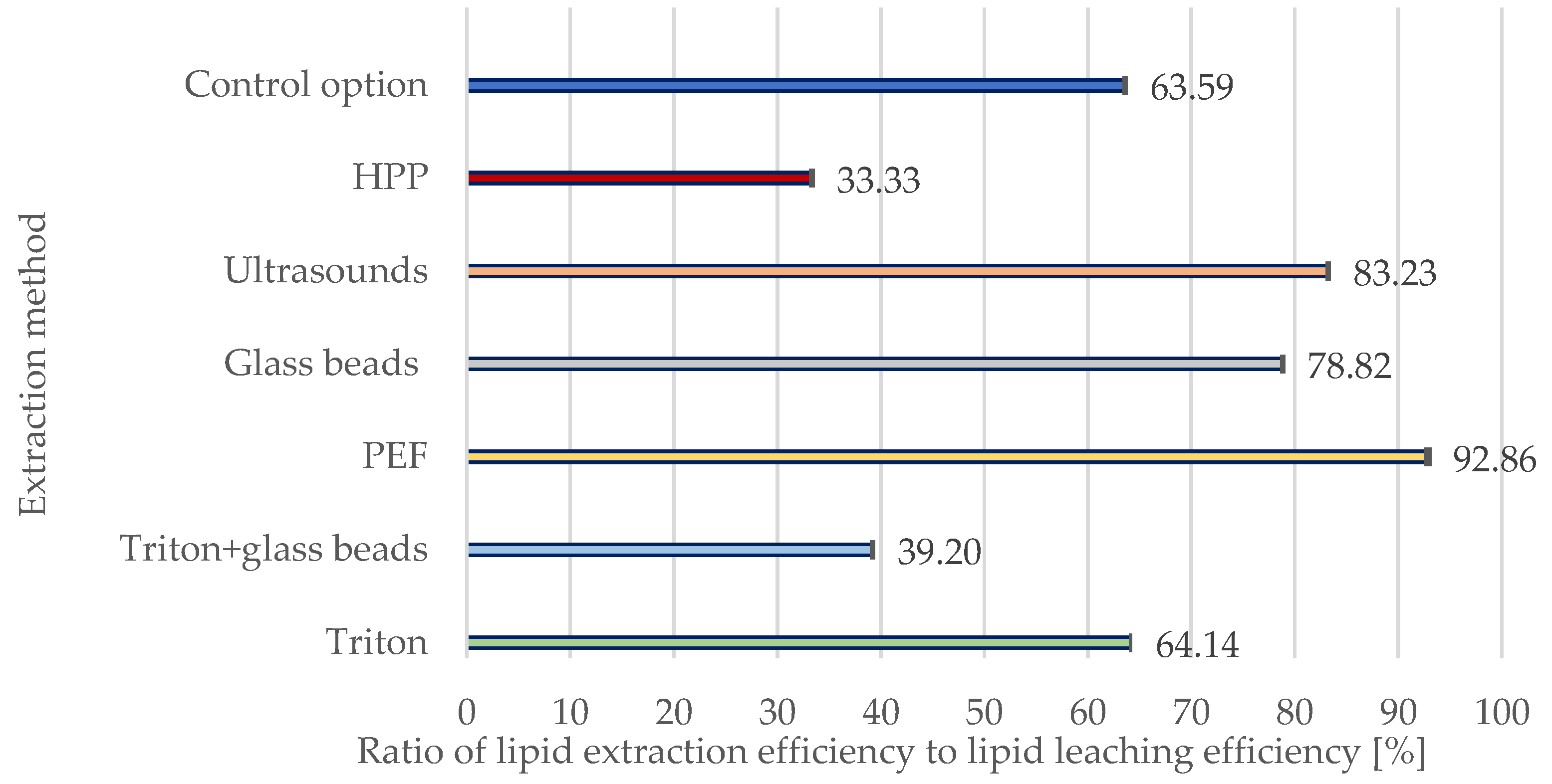
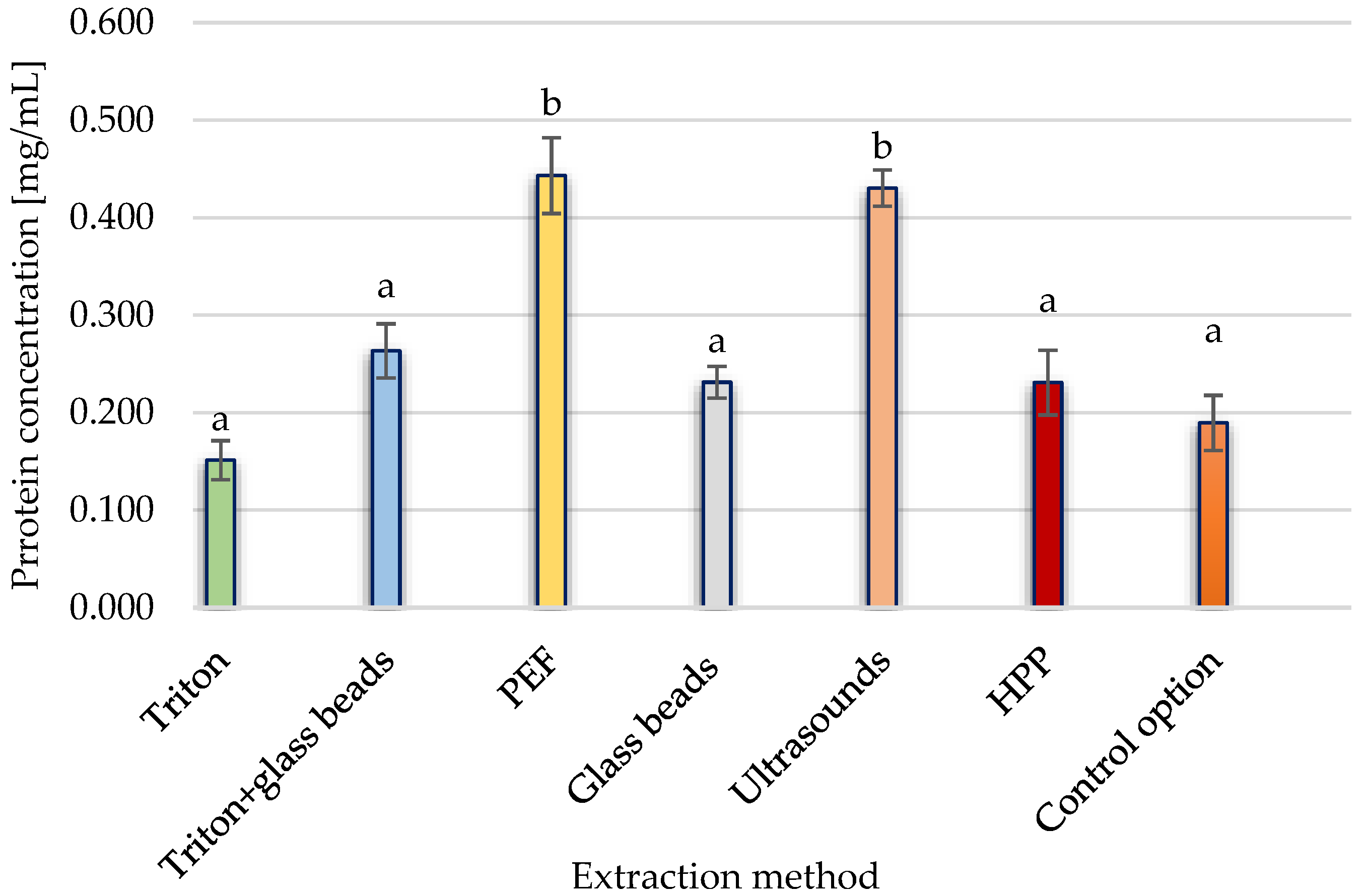
3.1. Effects of Conventional and Unconventional Extracting Methods for Y. lipolytica Yeast Cells
3.1.1. Comparison of Lipid Extraction and Leaching Efficiencies for Yeast Storage Lipids
3.1.2. Protein Release from Yeast Cells after Conventional and Unconventional Treatment
3.2. Impact of Conventional and Unconventional Methods of Cell Permeabilization and/or Disruption on its Morphology
4. Discussion
| Method Applied for Extraction | Microorganism | Experimental Conditions | Lipid Extraction Efficiency [%] | Reference |
|---|---|---|---|---|
| PEF treatment | Saitozyma podzolica yeast | suspension with a conductivity at 20 °C of either 10.14 ± 0.63 or 1.27 ± 0.09 mS/cm; the output impedance of generator (50 Ω); electric field intensity was varied between 1.4 and 4 MV/m | 54.0 | [34] |
| Yarrowia lipolytica yeast | 20 kV/cm of electrical field strength | 29.4 | [35] | |
| Chlorella algae | max. 35 kV, the pulse width of the square wave pulse from 2 to 99 μS at a pulse repetition rate of 1~1 kHz. | increase by 166.7% | [36] | |
| US treatment | Trichosporon sp. | Ultrasonic bath working at 520 kHz with an intensity of 40 W | 43.0 | [40] |
| Naganishia liquefaciens yeast | US-assisted extraction of 0.6 W/mL of US power combined with solvents | 97.1 | [41] | |
| HPP | Yarrowia lipolytica yeast | 298 K, 1500 × 105 Pa with 20 passes | 83.8 | [35] |
| Saitozyma podzolica yeast | 2000 bar, 5 min, with 15 passes | 95.0 * | [42] | |
| Apiotrichum porosum yeast | 53.0 * |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ledesma-Amaro, R.; Nicaud, J.M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Progr. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Khot, M.; Kamat, S.; Zinjarde, S.; Pant, A.; Chopade, B.; Ravi Kumar, A. Single cell oil of oleaginous fungi from the tropical mangrove wetlands as a potential feedstock for biodiesel. Microb. Cell Factories 2012, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowska, K.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Phosphorus and nitrogen limitation as a part of the strategy to stimulate microbial lipid biosynthesis. Appl. Sci. 2021, 11, 11819. [Google Scholar] [CrossRef]
- Radha, P.; Prabhu, K.; Jayakumar, A.; AbilashKarthik, S.; Ramani, K. Biochemical and kinetic evaluation of lipase and biosurfactant assisted ex novo synthesis of microbial oil for biodiesel production by Yarrowia lipolytica utilizing chicken tallow. Process Biochem. 2020, 95, 17–29. [Google Scholar] [CrossRef]
- Mhlongo, S.I.; Ezeokoli, O.T.; Roopnarain, A.; Ndaba, B.; Sekoai, P.T.; Habimana, O.; Pohl, C.H. The potential of single-cell oils derived from filamentous fungi as alternative feedstock sources for biodiesel production. Front. Microbiol. 2021, 12, 637381. [Google Scholar] [CrossRef]
- Madhu, V.E.; Rao, K.R.; Gobinath, R.; Balasubramanian, P. Predictive capability evaluation and optimization of sustainable biodiesel production from oleaginous biomass grown on pulp and paper industrial wastewater. Renew. Energy 2021, 168, 204–215. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sridharan, S.; Sowmya, V.; Yuvaraj, D.; Praveenkumar, R. Microbial oil—A plausible alternate resource for food and fuel application. Biores. Technol. 2017, 233, 423–432. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Zhang, S.; Zhang, W.; Barrow, C.J. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012, 131, 639–644. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar] [CrossRef]
- Jaafar, L.; Zueco, J. Characterization of a glycosylphosphatidylinositol-bound cell-wall protein (GPI-CWP) in Yarrowia lipolytica. Microbiology 2004, 150, 53–60. [Google Scholar] [CrossRef]
- Teparić, R.; Lozančić, M.; Mrša, V. Evolutionary overview of molecular interactions and enzymatic activities in the yeast cell walls. Int. J. Mol. Sci. 2020, 21, 8996. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography—A review. Arabian J. Chem. 2016, 13, 8. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; Luque de Castro, M.D. Soxhlet Extraction. In Handbooks in Separation. Liquid Phase Extraction; Poole, F.C., Ed.; Elsevier: Córdoba, Spain, 2020; pp. 327–354. [Google Scholar]
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch methods for solid–liquid–liquid extraction of lipids from microorganisms. comprehension of solvatation mechanisms and towards substitution with alternative solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.; Bailes, K.L.; Meyer, R.G.; Hopkins, D.L. Effect of modified Soxhlet (Soxtec) and Folch extraction method selection on the total lipid determination of aged beef. J. Food Sci. Technol. 2019, 56, 3957–3961. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Marett, L.; Pryce, J.; Rochfort, S. Comparison of workflows for milk lipid analysis: Phospholipids. Foods 2022, 12, 163. [Google Scholar] [CrossRef]
- Knorr, D. Impact of non-thermal processing on plant metabolites. J. Food Eng. 2003, 56, 131–134. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed electric field (PEF): Avant-garde extraction escalation technology in food industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Costa, D.D.S.V.; Bragagnolo, N. Development and validation of a novel microwave assisted extraction method for fish lipids. Eur. J. Lipid Sci. Technol. 2017, 119, 2000. [Google Scholar] [CrossRef]
- Xu, T.; Wumanjiang, E.; Sun, Z. Preparation of high purity lycopene. Fine Chem. Dalian 2006, 23, 62. [Google Scholar]
- Xia, T.; Shi, S.; Wan, X. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- Shene, C.; Monsalve, M.T.; Vergara, D.; Lienqueo, M.E.; Rubilar, M. High pressure homogenization of Nannochloropsis oculata for the extraction of intracellular components: Effect of process conditions and culture age. Eur. J. Lipid Sci. Technol. 2016, 118, 631–639. [Google Scholar] [CrossRef]
- Samarasinghe, N.; Fernando, S.; Faulkner, W.B. Effect of high pressure homogenization on aqueous phase solvent extraction of lipids from Nannochloris oculata microalgae. J. Energy Natural Resour. 2012, 1. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Pakulska, A.; Derewiaka, D.; Piasecka, I.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Concept of batch and fed-batch cultures of Yarrowia lipolytica as a valuable source of sterols with simultaneous valorization of molasses and post-frying rapeseed oil. Appl. Sci. 2022, 12, 12877. [Google Scholar] [CrossRef]
- Kapturowska, A.; Stolarzewicz, I.; Krzyczkowska, J.; Białecka-Florjańczyk, E. Studies on lipolytic activity of sonicated enzymes from Yarrowia lipolytica. Ultrason Sonochem. 2012, 19, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowska, K.; Musiałek, A.; Zieniuk, B.; Jasińska, K.; Nowak, D.; Fabiszewska, A. Is There Any Possibility to Use Ultrasounds, High-Pressure Homogenization or Pulsed Electric Field in Single Cell Oil Release from Oleaginous Yeast Cells? Biol. Life Sci. Forum 2022, 18, 56. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Eur-lex. Available online: https://eur-lex.europa.eu/eli/reg_impl/2019/0760/oj (accessed on 3 November 2023).
- Madzak, C. Yarrowia lipolytica Strains and Their Biotechnological Applications: How Natural Biodiversity and Metabolic Engineering Could Contribute to Cell Factories Improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Miklavčič, D.; Arczewska, M. Pulsed Electric Field (PEF) Enhances Iron Uptake by the Yeast Saccharomyces cerevisiae. Biomolecules 2021, 11, 850. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Stefanou, N.; Andreou, V.; Taoukis, P. Effect of Pulsed Electric Fields on the Production of Yeast Extract by Autolysis. Innov. Food Sci. Emerg. Technol. 2018, 48, 287–295. [Google Scholar] [CrossRef]
- Gorte, O.; Nazarova, N.; Papachristou, I.; Wüstner, R.; Leber, K.; Syldatk, C.; Ochsenreither, K.; Frey, W.; Silve, A. Pulsed Electric Field treatment promotes lipid extraction on fresh oleaginous yeast Saitozyma podzolica DSM 27192. Front. Bioeng. Biotechnol. 2020, 8, 575379. [Google Scholar] [CrossRef]
- Drévillon, L.; Koubaa, M.; Nicaud, J.-M.; Vorobiev, E. Cell disruption pre-treatments towards an effective recovery of oil from Yarrowia lipolytica oleaginous yeast. Biomass Bioenergy 2019, 128, 105320. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.; Xu, G.; Fu, X. Improving the lipid extraction yield from Chlorella based on the controllable electroporation of cell membrane by Pulsed Electric Field. Bioresour. Technol. 2021, 330, 124933. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, M.; Lin, S.; Liu, J.; Yang, Y.; Jin, Y. optimization of extraction parameters for protein from beer waste brewing yeast treated by Pulsed Electric Fields (PEF). Afr. J. Microbiol. Res. 2012, 6, 4739–4746. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of food and natural products. mechanisms, techniques, combinations, protocols and applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, I.; Brzezińska, R.; Ostrowska-Ligęza, E.; Wiktor, A.; Górska, A. Ultrasound-Assisted Extraction of cranberry seed oil: Food waste valorization approach. Eur. Food Res. Technol. 2023, 249, 2763–2775. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Banerjee, R. Enhanced lipid extraction from oleaginous yeast biomass using ultrasound assisted extraction: A greener and scalable process. Ultrasonics Sonochem. 2019, 52, 25–32. [Google Scholar] [CrossRef]
- Selvakumar, P.; Sivashanmugam, P. Ultrasound assisted oleaginous yeast lipid extraction and garbage lipase catalyzed transesterification for enhanced biodiesel production. Energy Convers. Manag. 2019, 179, 141–151. [Google Scholar] [CrossRef]
- Gorte, O.; Hollenbach, R.; Papachristou, I.; Steinweg, C.; Silve, A.; Frey, W.; Syldatk, C.; Ochsenreither, K. evaluation of downstream processing, extraction, and quantification strategies for Single Cell Oil produced by the oleaginous yeasts Saitozyma podzolica DSM 27192 and Apiotrichum porosum DSM 27194. Front. Bioeng. Biotechnol. 2020, 8, 355. [Google Scholar] [CrossRef]
- Milanesio, J.; Hegel, P.; Medina-Gonzalez, Y.; Camy, S.; Condoret, J.S. Extraction of lipids from Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 2013, 88, 378–387. [Google Scholar] [CrossRef]
- Yook, S.D.; Kim, J.; Woo, H.M.; Um, Y.; Lee, S.M. Efficient lipid extraction from the oleaginous yeast Yarrowia lipolytica using switchable solvents. Renew. Energ. 2019, 132, 61–67. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiszewska, A.; Pakulska, A.; Zieniuk, B.; Wierzchowska, K.; Jasińska, K.; Małajowicz, J.; Nowak, D. Unconventional Extraction Methods of Oleaginous Yeast Cell Pretreatment and Disruption. Appl. Sci. 2023, 13, 13135. https://doi.org/10.3390/app132413135
Fabiszewska A, Pakulska A, Zieniuk B, Wierzchowska K, Jasińska K, Małajowicz J, Nowak D. Unconventional Extraction Methods of Oleaginous Yeast Cell Pretreatment and Disruption. Applied Sciences. 2023; 13(24):13135. https://doi.org/10.3390/app132413135
Chicago/Turabian StyleFabiszewska, Agata, Anna Pakulska, Bartłomiej Zieniuk, Katarzyna Wierzchowska, Karina Jasińska, Jolanta Małajowicz, and Dorota Nowak. 2023. "Unconventional Extraction Methods of Oleaginous Yeast Cell Pretreatment and Disruption" Applied Sciences 13, no. 24: 13135. https://doi.org/10.3390/app132413135





