Strength and Microstructure of a Clayey Soil Stabilized with Natural Stone Industry Waste and Lime or Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Industrial Waste
2.3. Lime
2.4. Cement
2.5. Sample Preparation
2.6. Unconfined Compressive Strength (UCS)
2.7. pH
2.8. Mercury Intrusion Porosimetry
2.9. Thermogravimetric Analysis (TGA)
2.10. X-ray Diffraction
2.11. Scanning Electron Microscopy (SEM)
3. Results
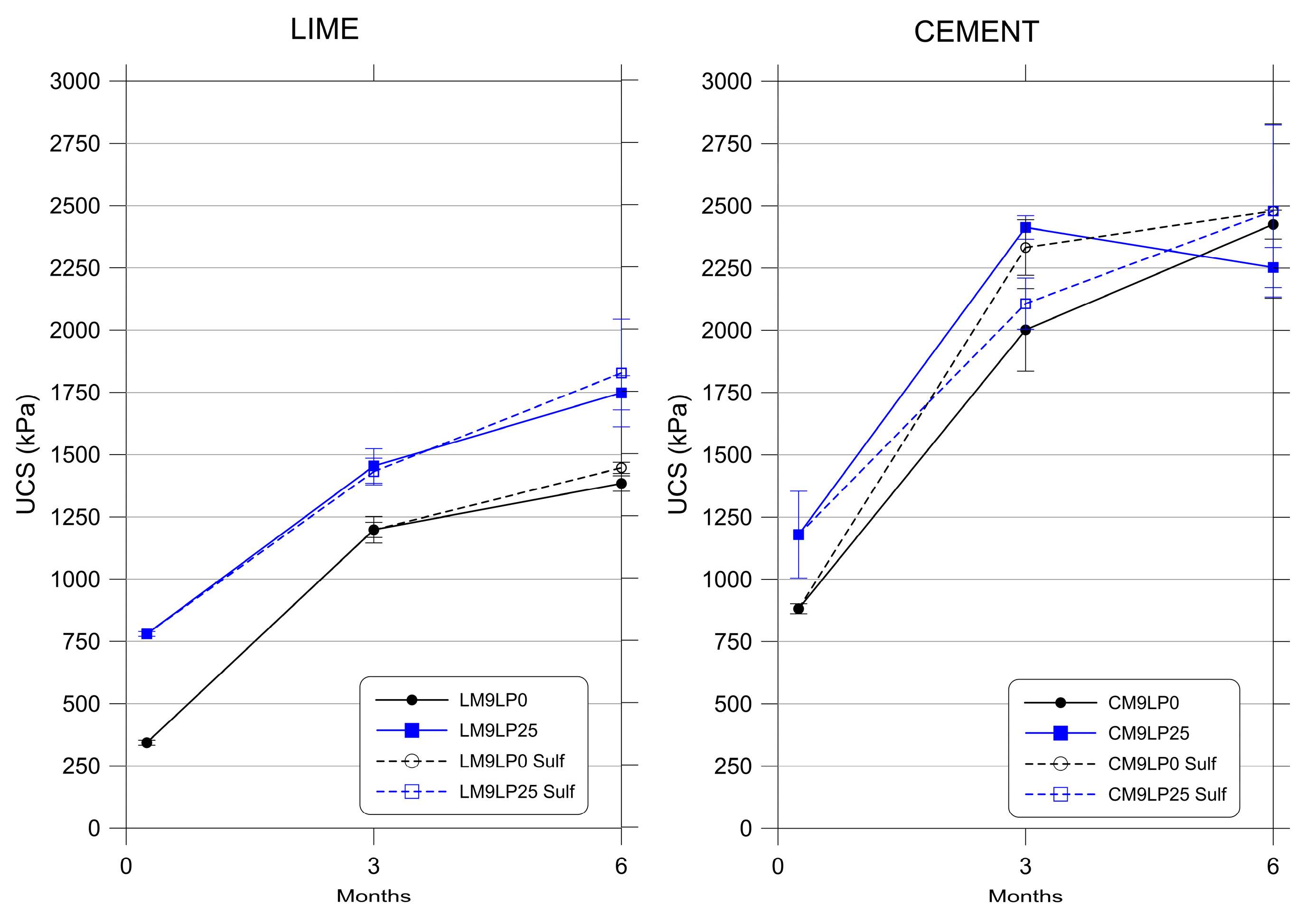
3.1. Unconfined Compressive Strength (UCS)
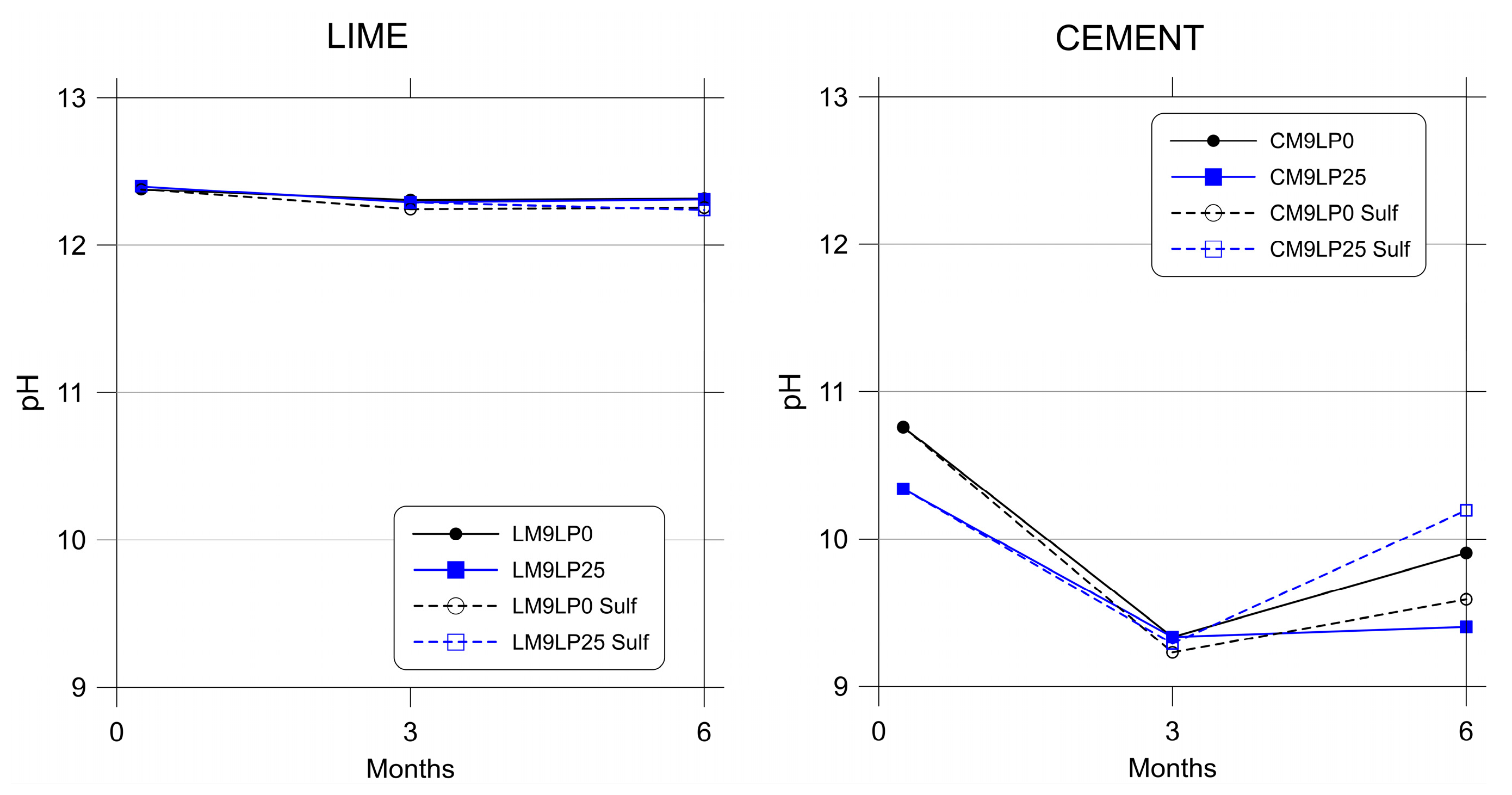
3.2. pH
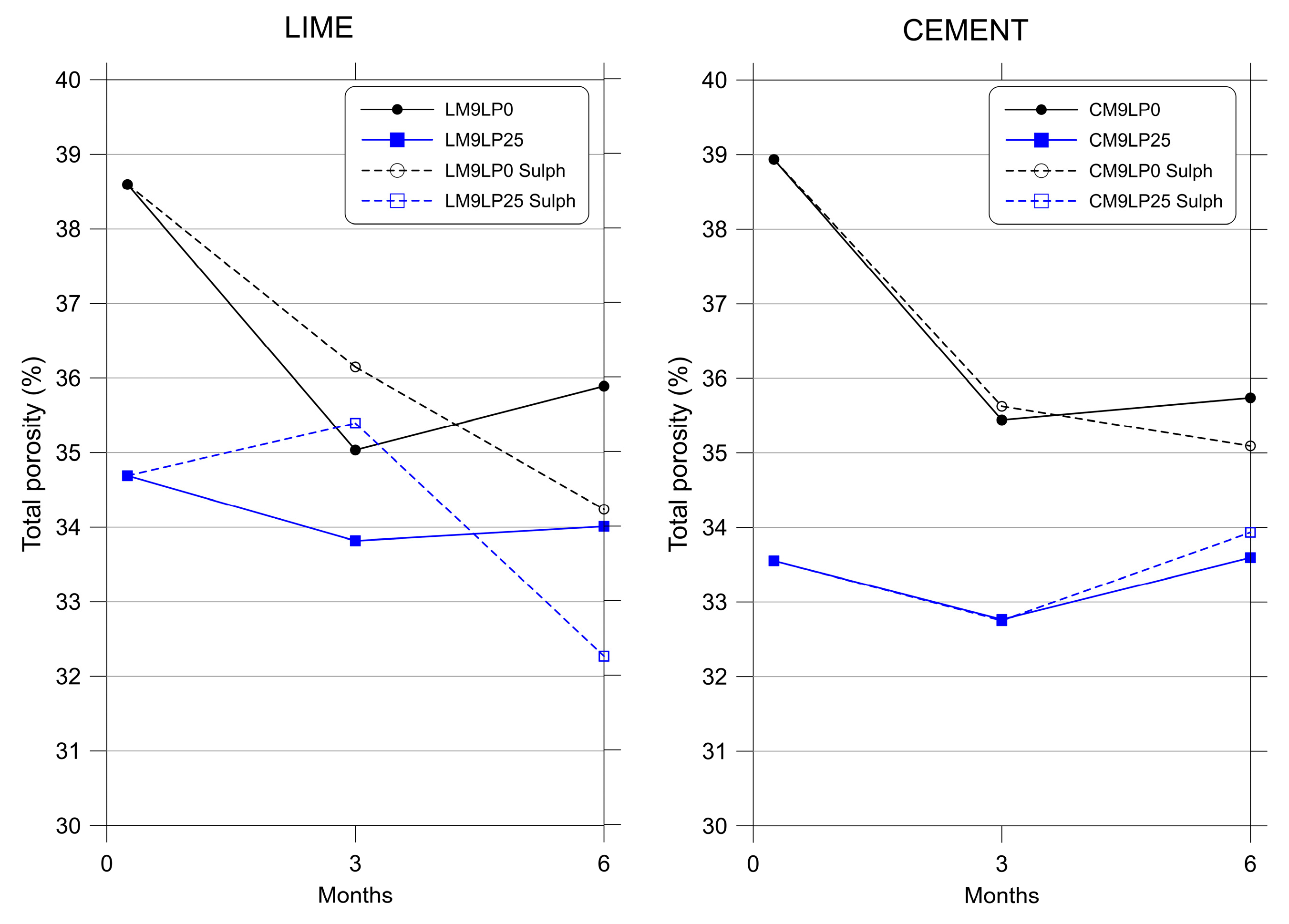
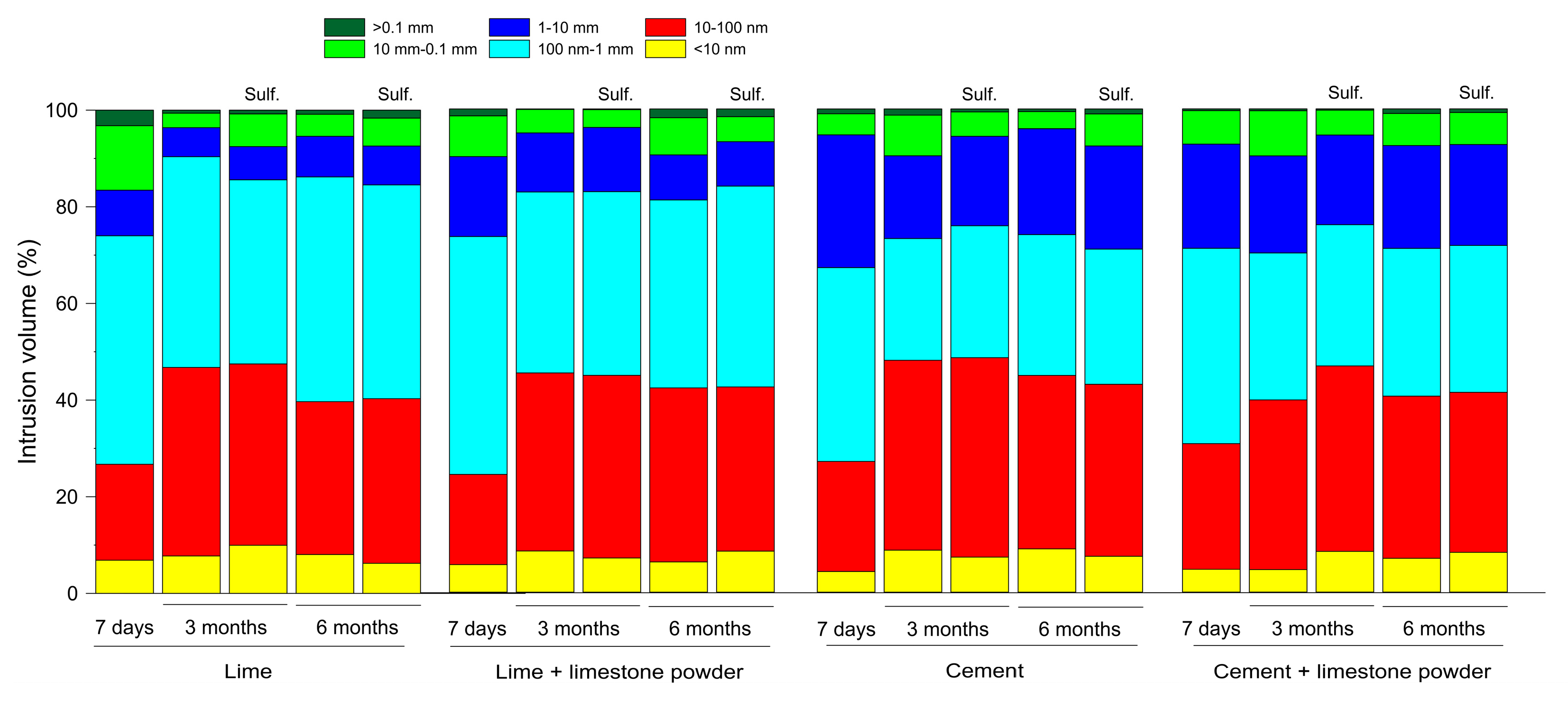
3.3. Mercury Intrusion Porosimetry
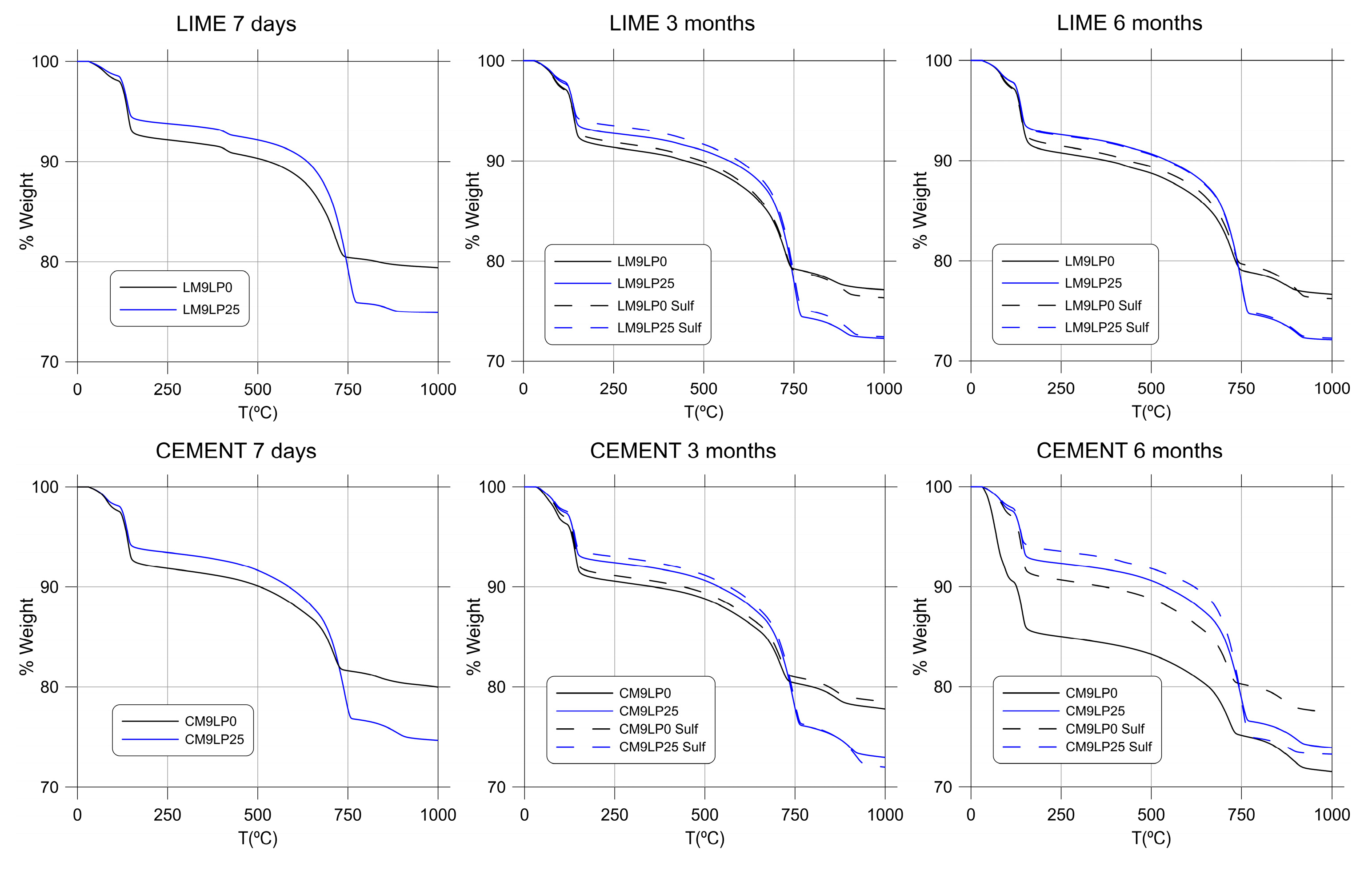
3.4. Thermogravimetric Analysis (TGA)
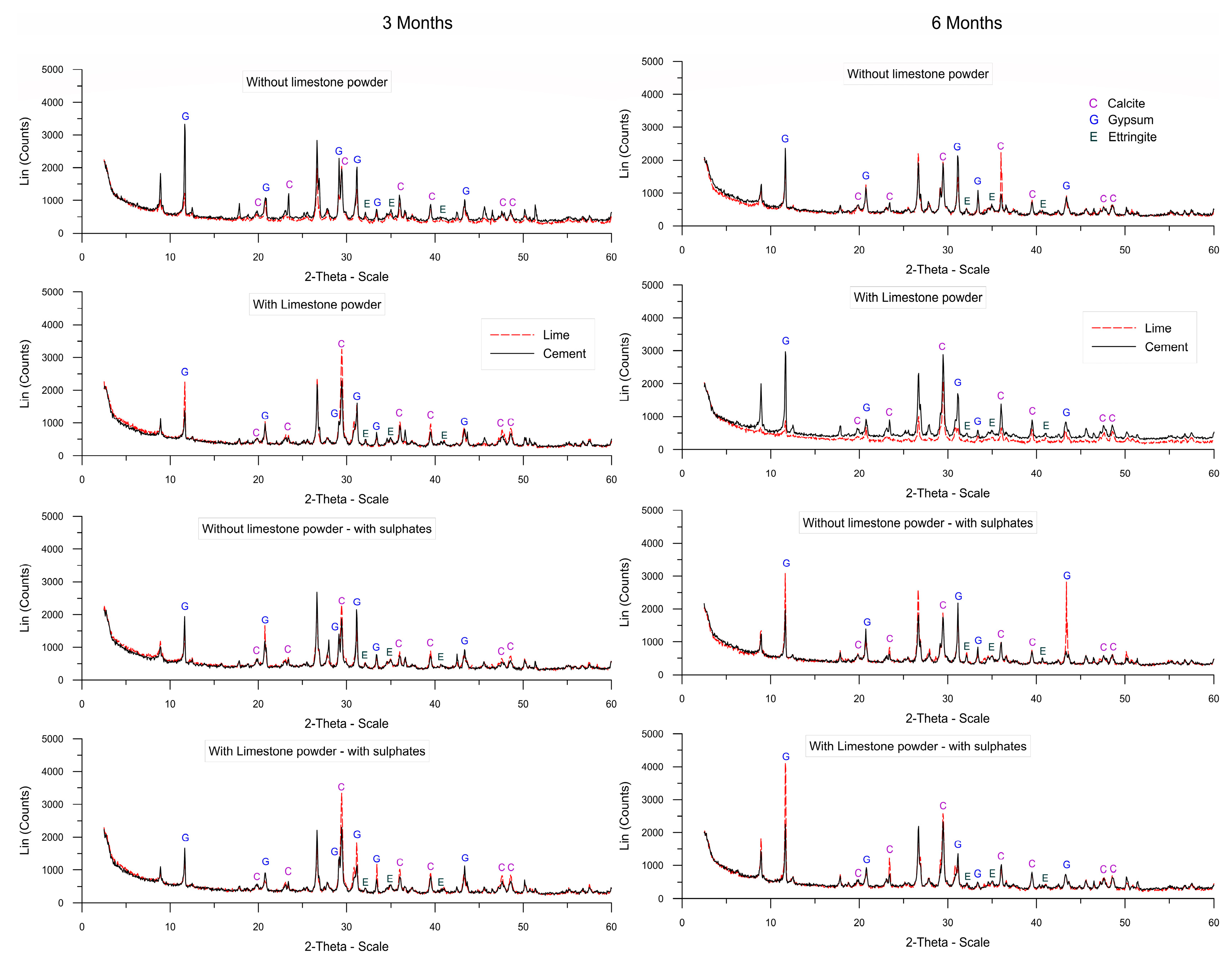
3.5. X-ray Diffraction
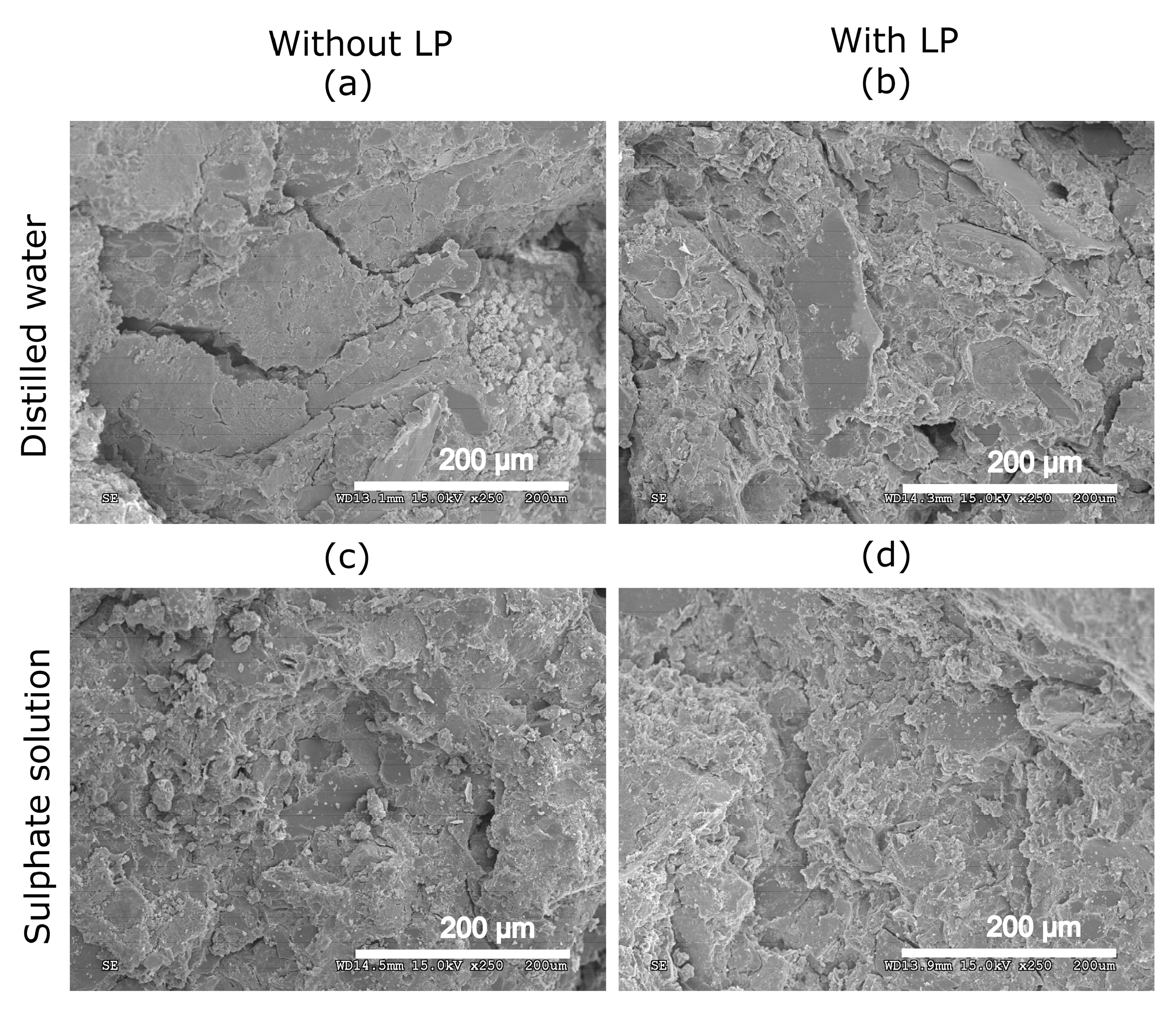
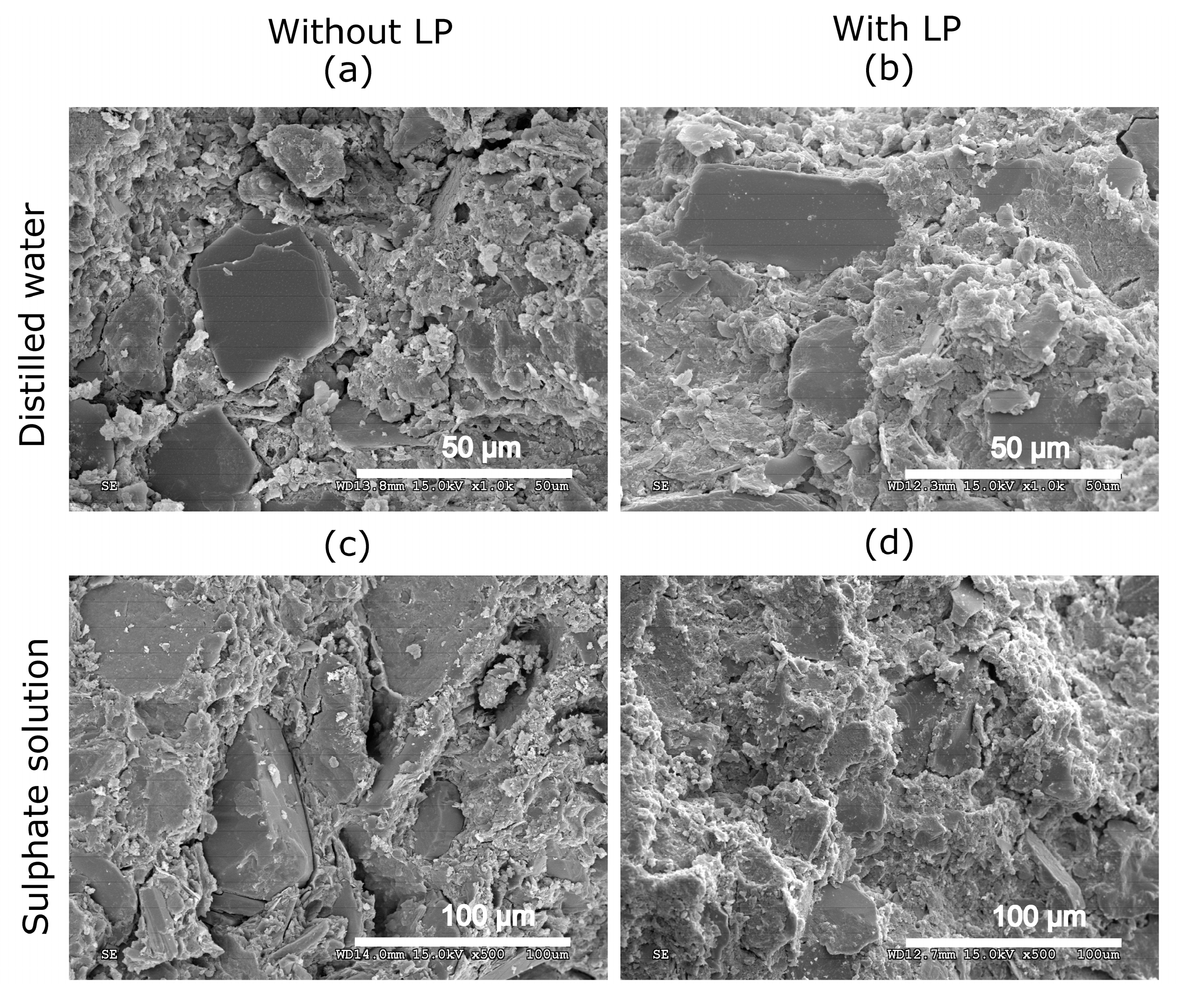
3.6. Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
- A clear positive effect was observed on the unconfined compressive strength of the stabilized soil when adding limestone powder as a ternary element together with lime or cement.
- The strengthening effect of the limestone powder waste was higher when using lime as a commercial binder.
- The addition of limestone powder resulted in a more compact structure with lower values of total porosity.
- Limestone powder waste can be used for soil stabilization in a sulfate aggressive medium, as no harmful effect was observed when samples with cement or lime, with or without limestone powder, were made with a sulfate solution of 3000 mg SO42− per liter up to the maximum age studied (6 months).
- Limestone powder waste from the natural stone industry enhanced the stabilization effect of lime and cement for soil stabilization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mujtaba, H.; Aziz, T.; Farooq, K.; Sivakugan, N.; Das, B.M. Improvement in Engineering Properties of Expansive Soils using Ground Granulated Blast Furnace Slag. J. Geol. Soc. India 2018, 92, 357–362. [Google Scholar] [CrossRef]
- Yadu, L.; Tripathi, R.K. Effects of Granulated Blast Furnace Slag in the Engineering Behaviour of Stabilized Soft Soil. Procedia Eng. 2013, 51, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.K.; Pandey, V.; Murari, K.; Singh, J.P.; Prof, A. Soil stabilisation using ground granulated blast furnace slag. Int. J. Eng. Res. Appl. 2014, 4, 164–171. [Google Scholar]
- Horpibulsuk, S.K.; Rachan, R.L.; Chinkulkijniwat, A.; Raksachon, Y.; Suddeepong, A. Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr. Build. Mater. 2010, 24, 2011–2021. [Google Scholar] [CrossRef]
- Jerin, J.; Ashika, J.; Kurian, J.M.; Francis, J.; James, S.K. Stabilization of expansive soil using fly ash. Int. Res. J. Eng. Technol. 2018, 5, 3075–3078. [Google Scholar]
- Mir, B.A.; Sridharan, A. Physical and Compaction Behaviour of Clay Soil–Fly Ash Mixtures. Geotech. Geol. Eng. 2013, 31, 1059–1072. [Google Scholar] [CrossRef]
- Bağriaçik, B.; Beycioğlu, A.; Topolinski, S.; Akmaz, E.; Sert, S.; Güner, E.D. Assessment of glass fiber-reinforced polyester pipe powder in soil improvement. Front. Struct. Civ. Eng. 2021, 15, 742–753. [Google Scholar] [CrossRef]
- Blayi, R.A.; Sherwani, A.F.H.; Ibrahim, H.H.; Faraj, R.H.; Daraei, A. Strength improvement of expansive soil by utilizing waste glass powder. Case Stud. Constr. Mater. 2020, 13, e00427. [Google Scholar] [CrossRef]
- Rupnow, T.D.; Franklin, B.W.D. Class C fly ash stabilization of recycled asphalt pavement and soil—A case study. In Proceedings of the World of Coal Ash (WOCA) Conference, Nasvhille, TN, USA, 5–7 May 2015; pp. 1–19. [Google Scholar]
- Firoozi, A.A.; Guney Olgun, C.; Firoozi, A.A.; Baghini, M.S. Fundamentals of soil stabilization. Int. J. Geo-Eng. 2017, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Horpibulsuk, S.; Rachan, R.; Raksachon, Y. Role of Fly Ash on Strength and Microstructure Development in Blended Cement Stabilized Silty Clay. Soils Found. 2009, 49, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Wang, C.; Wang, Z.; Li, B.; Liu, Y.-A.; Jiang, C.; Wang, N.; Wang, C.; Li, Z.; Liu, B.; et al. Strength Characteristics and Microstructure of Cement Stabilized Soft Soil Admixed with Silica Fume. Materials 2021, 14, 1929. [Google Scholar] [CrossRef]
- Baldovino, J.J.A.; Izzo, R.L.S.; Rose, J.L.; Domingos, M.D.I. Strength, durability, and microstructure of geopolymers based on recycled-glass powder waste and dolomitic lime for soil stabilization. Constr. Build. Mater. 2021, 271, 121874. [Google Scholar] [CrossRef]
- Jayanghi, P.; Singh, D. Utilization of Sustainable Materials for Soil Stabilization: State-of-the-Art. Adv. Civ. Eng. Mater. 2016, 5, 46–79. [Google Scholar]
- Shi, J.; Liu, B.; Chu, S.H.; Zhang, Y.; Zhang, Z.; Han, K. Recycling air-cooled blast furnace slag in fiber reinforced alkali-activated mortar. Powder Technol. 2022, 407, 117686. [Google Scholar] [CrossRef]
- Shi, J.; Liu, B.; He, Z.; Liu, Y.; Jiang, J.; Xiong, T.; Shi, J. A green ultra-lightweight chemically foamed concrete for building exterior: A feasibility study. J. Clean. Prod. 2021, 288, 125085. [Google Scholar] [CrossRef]
- Global Industry Analysts, Inc. Granite, Marble and Stone—Global Market Trajectory & Analytics 2021; Global Industry Analysts, Inc.: San Jose, CA, USA, 2021. [Google Scholar]
- Sivrikaya, O.; Kıyıldı, K.R.; Karaca, Z. Recycling waste from natural stone processing plants to stabilise clayey soil. Environ. Earth Sci. 2014, 71, 4397–4407. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.; Khan, H.; Shah, A.A. Expansive Soil Stabilization Using Marble Dust and and Bagasse Ash. Int. J. Sci. Res. 2014, 3, 2812–2816. [Google Scholar]
- Jain, A.K.; Jha, A.K. Shivanshi Geotechnical behaviour and micro-analyses of expansive soil amended with marble dust. Soils Found. 2020, 60, 737–751. [Google Scholar] [CrossRef]
- Pastor, J.L.; Tomás, R.; Cano, M.; Riquelme, A.; Gutiérrez, E. Evaluation of the Improvement Effect of Limestone Powder Waste in the Stabilization of Swelling Clayey Soil. Sustainability 2019, 11, 679. [Google Scholar] [CrossRef] [Green Version]
- Saygili, A. Use of Waste Marble Dust for Stabilization of Clayey Soil. Mater. Sci. 2015, 21, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Ogila, W.A.M. The impact of natural ornamental limestone dust on swelling characteristics of high expansive soils. Environ. Earth Sci. 2016, 75, 1493. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Uysal, F.; Yorulmaz, A.; Aydin, K. The Efficiency of Waste Marble Powder in the Stabilization of Fine-Grained Soils in Terms of Volume Changes. Arab. J. Sci. Eng. 2020, 45, 8561–8576. [Google Scholar] [CrossRef]
- Nikolakopoulos, K.; Kavoura, K.; Depountis, N.; Kyriou, A.; Argyropoulos, N.; Koukouvelas, I.; Sabatakakis, N. Preliminary results from active landslide monitoring using multidisciplinary surveys. Eur. J. Remote Sens. 2017, 50, 280–299. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.H.; Hassan, A.M.; Lotfi, H.A. Stabilization of Expansive Sub-grade Soil Using Hydrated Lime and Dolomi-tic-Limestone By-Product (DLP). Geotech. Geol. Eng. 2020, 38, 1605–1617. [Google Scholar] [CrossRef]
- Deboucha, S.; Mamoune, S.M.A.; Sail, Y.; Ziani, H. Effects of Ceramic Waste, Marble Dust, and Cement in Pavement Sub-base Layer. Geotech. Geol. Eng. 2020, 38, 3331–3340. [Google Scholar] [CrossRef]
- Meng, T.; Qiang, Y.; Hu, A.; Xu, C.; Lin, L. Effect of compound nano-CaCO3 addition on strength development and microstructure of cement-stabilized soil in the marine environment. Constr. Build. Mater. 2017, 151, 775–781. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Ouyang, X.; Koleva, D.A.; Ye, G.; van Breugel, K. Insights into the mechanisms of nucleation and growth of C–S–H on fillers. Mater. Struct. Constr. 2017, 50, 213. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.H.; Shi, C.J.; Farzadnia, N.; Shi, Z.G.; Jia, H.F.; Ou, Z.H. A review on use of limestone powder in cement-based materials: Mechanism, hydration and microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Jia, H.; Zeng, R.; Wu, Y.; Lao, L. A quantitative study on physical and chemical effects of limestone powder on properties of cement pastes. Constr. Build. Mater. 2019, 204, 58–69. [Google Scholar] [CrossRef]
- Jiang, D.; Li, X.; Lv, Y.; Zhou, M.; He, C.; Jiang, W.; Liu, Z.; Li, C. Utilization of limestone powder and fly ash in blended cement: Rheology, strength and hydration characteristics. Constr. Build. Mater. 2020, 232, 117228. [Google Scholar] [CrossRef]
- Kechagia, P.; Koutroumpi, D.; Bartzas, G.; Peppas, A.; Samouhos, M.; Deligiannis, S.; Tsakiridis, P.E. Waste marble dust and recycled glass valorization in the production of ternary blended cements. Sci. Total Environ. 2021, 761, 143224. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.; Aponte, D.; Pelà, L.; Roca, P. Influence of recycled limestone filler additions on the mechanical behaviour of commercial premixed hydraulic lime based mortars. Constr. Build. Mater. 2020, 238, 117722. [Google Scholar] [CrossRef]
- Puppala, A.J.; Talluri, N.; Congress, S.S.C.; Gaily, A. Ettringite induced heaving in stabilized high sulfate soils. Innov. Infrastruct. Solutions 2018, 3, 72. [Google Scholar] [CrossRef]
- Celik, E.; Nalbantoglu, Z. Effects of ground granulated blastfurnace slag (GGBS) on the swelling properties of lime-stabilized sulfate-bearing soils. Eng. Geol. 2013, 163, 20–25. [Google Scholar] [CrossRef]
- Caselles, L.D.; Hot, J.; Roosz, C.; Cyr, M. Stabilization of soils containing sulfates by using alternative hydraulic binders. Appl. Geochem. 2020, 113, 104494. [Google Scholar] [CrossRef]
- ASTM International ASTM D2487-11; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2011.
- AENOR UNE EN 197-1:2011; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements 2011. European Union: Brussels, Belgium, 2011; p. 30.
- Pastor, J.L.; Tomás, R.; Cano, M.; Riquelme, A.; Martín, R. Unconfined compressive strength assessment of soils stabilized with cement or lime and carbonate residue additive of ornamental rock (in Spanish). In Proceedings of the XI Simposio nacional Ingeniería Geotécnica, Mieres, Spain, 24–27 May 2022. [Google Scholar]
- Comisión Permanente del Hormigón. Code on Structural Concrete EHE-08; Ministerio de Fomento: Madrid, Spain, 2008.
- Ministerio de Fomento de España. Documento Básico Seguridad Escrutural Cimientos CTE; Ministerio de Fomento de España: Madrid, Spain, 2007.
- AENOR UNE 103400; Simple compression rupture test in soil test specimens (in Spanish). AENOR: Madrid, Spain, 1993.
- Juárez Sanz, M.; Sánchez Sánchez, A.; Jordá Guijarro, J.D.; Sánchez Andreu, J.J. Diagnóstico del Potencial Nutritivo del Suelo (in Spanish); Publicaciones de la Universidad de Alicante: Alicante, Spain, 2004. [Google Scholar]
- Cabeza, M.; Merino, P.; Miranda, A.; Nóvoa, X.R.; Sanchez, I. Impedance spectroscopy study of hardened Portland cement paste. Cem. Concr. Res. 2002, 32, 881–891. [Google Scholar] [CrossRef]
- Monteagudo, S.M.; Moragues, A.; Gálvez, J.C.; Casati, M.J.; Reyes, E. The degree of hydration assessment of blended cement pastes by differential thermal and thermogravimetric analysis. Morphological evolution of the solid phases. Thermochim. Acta 2014, 592, 37–51. [Google Scholar] [CrossRef]
- Mustafa, Y.M.H.; Al-Amoudi, O.S.B.; Ahmad, S.; Maslehuddin, M.; Al-Malack, M.H. Utilization of Portland cement with limestone powder and cement kiln dust for stabilization/solidification of oil-contaminated marl soil. Environ. Sci. Pollut. Res. 2021, 28, 3196–3216. [Google Scholar] [CrossRef]
- Bazarbekova, A.; Shon, C.-S.; Kissambinova, A.; Kim, J.R.; Zhang, D.; Moon, S.-W. Potential of limestone powder to improve the stabilization of sulfate-contained saline soil. IOP Conf. Series Mater. Sci. Eng. 2021, 1040, 12016. [Google Scholar] [CrossRef]
- Ibrahim, H.H.; Alshkane, Y.M.; Mawlood, Y.I.; Noori, K.M.G.; Hasan, A.M. Improving the geotechnical properties of high expansive clay using limestone powder. Innov. Infrastruct. Solutions 2020, 5, 112. [Google Scholar] [CrossRef]
- Al-Mukhtar, M.; Khattab, S.; Alcover, J.-F. Microstructure and geotechnical properties of lime-treated expansive clayey soil. Eng. Geol. 2012, 139, 17–27. [Google Scholar] [CrossRef]
- Harris, P.; von Holdt, J.; Sebesta, S.; Scullion, T. Recommendations for Stabilization of High-Sulfate Soils in Texas. Transp. Res. Rec. J. Transp. Res. Board 2006, 1952, 71–79. [Google Scholar] [CrossRef]
- Behnood, A. Soil and clay stabilization with calcium- and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Du, Y.-J.; Liu, K. Durability of lightweight alkali-activated ground granulated blast furnace slag (GGBS) stabilized clayey soils subjected to sulfate attack. Appl. Clay Sci. 2018, 161, 70–75. [Google Scholar] [CrossRef]



| Property | % |
|---|---|
| Illite | 28.7 |
| Calcite | 22.5 |
| Quartz | 19.4 |
| Gypsum | 7.6 |
| Montmorillonite | 5.9 |
| Kaolinite | 3.9 |
| Sample | Binder | Industrial Waste | Sulfate Solution |
|---|---|---|---|
| LM9LP0 | Lime 9% | 0% | No |
| LM9LP25 | Lime 9% | 25% | No |
| LM9LP0 Sulf | Lime 9% | 0% | Na2SO4 |
| LM9LP25 Sulf | Lime 9% | 25% | Na2SO4 |
| CM9LP0 | Cement 9% | 0% | No |
| CM9LP25 | Cement 9% | 25% | No |
| CM9LP0 Sulf | Cement 9% | 0% | Na2SO4 |
| CM9LP25 Sulf | Cement 9% | 25% | Na2SO4 |
| Binder | Industrial Waste | Sulfate Solution | Curing Age | Weight Loss (%) | ||
|---|---|---|---|---|---|---|
| dh | dx | dc | ||||
| Lime | No | No | 7 days | 7.398 | 1.553 | 9.832 |
| 3 months | 7.346 | 2.016 | 10.944 | |||
| 6 months | 7.479 | 1.974 | 10.729 | |||
| Yes | No | 7 days | 6.074 | 1.275 | 16.364 | |
| 3 months | 6.457 | 1.797 | 17.549 | |||
| 6 months | 6.748 | 1.903 | 17.287 | |||
| No | Yes | 7 days | 7.398 | 1.553 | 9.832 | |
| 3 months | 6.727 | 2.124 | 12.131 | |||
| 6 months | 7.530 | 1.739 | 11.978 | |||
| Yes | Yes | 7 days | 6.074 | 1.275 | 16.364 | |
| 3 months | 5.634 | 1.903 | 17.936 | |||
| 6 months | 6.810 | 1.896 | 17.052 | |||
| Binder | Industrial Waste | Sulfate Solution | Curing Age | Weight Loss (%) | ||
|---|---|---|---|---|---|---|
| dh | dx | dc | ||||
| Cement | No | No | 7 days | 6.984 | 2.089 | 8.674 |
| 3 months | 7.126 | 2.044 | 9.594 | |||
| 6 months | 7.030 | 1.934 | 10.400 | |||
| Yes | No | 7 days | 5.847 | 2.187 | 15.484 | |
| 3 months | 6.254 | 2.077 | 16.262 | |||
| 6 months | 6.481 | 2.041 | 15.276 | |||
| No | Yes | 7 days | 6.984 | 2.089 | 8.674 | |
| 3 months | 7.127 | 2.009 | 9.485 | |||
| 6 months | 7.673 | 2.114 | 9.737 | |||
| Yes | Yes | 7 days | 5.847 | 2.187 | 15.484 | |
| 3 months | 5.897 | 2.074 | 17.763 | |||
| 6 months | 5.714 | 1.610 | 17.447 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, J.L.; Chai, J.; Sánchez, I. Strength and Microstructure of a Clayey Soil Stabilized with Natural Stone Industry Waste and Lime or Cement. Appl. Sci. 2023, 13, 2583. https://doi.org/10.3390/app13042583
Pastor JL, Chai J, Sánchez I. Strength and Microstructure of a Clayey Soil Stabilized with Natural Stone Industry Waste and Lime or Cement. Applied Sciences. 2023; 13(4):2583. https://doi.org/10.3390/app13042583
Chicago/Turabian StylePastor, José Luis, Jinchun Chai, and Isidro Sánchez. 2023. "Strength and Microstructure of a Clayey Soil Stabilized with Natural Stone Industry Waste and Lime or Cement" Applied Sciences 13, no. 4: 2583. https://doi.org/10.3390/app13042583





