Review of Flexible Supercapacitors Using Carbon Nanotube-Based Electrodes
Abstract
:1. Introduction
2. Flexible Supercapacitor with Various Types of CNT-Based Electrodes
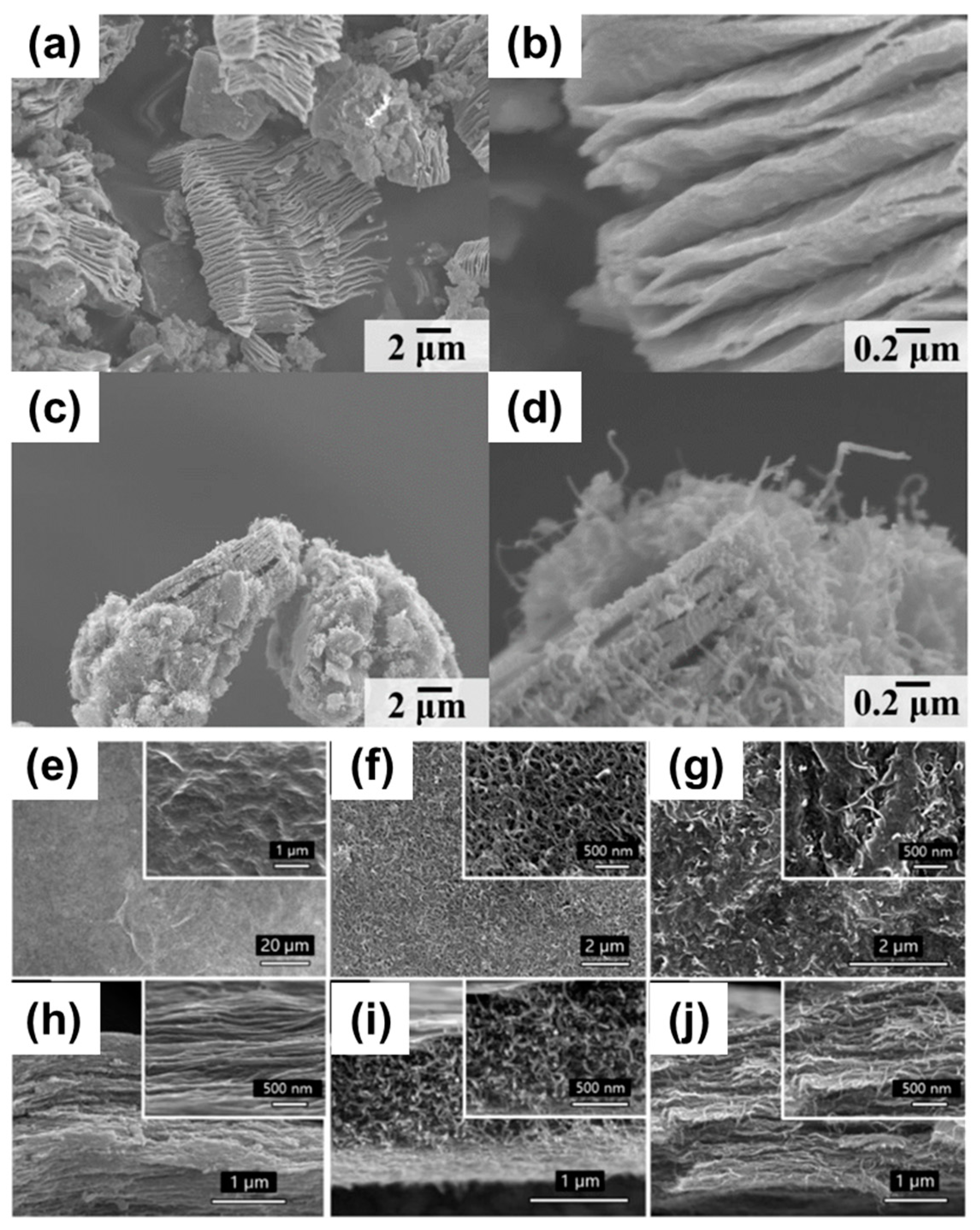
2.1. CNT-FF-Based Electrodes
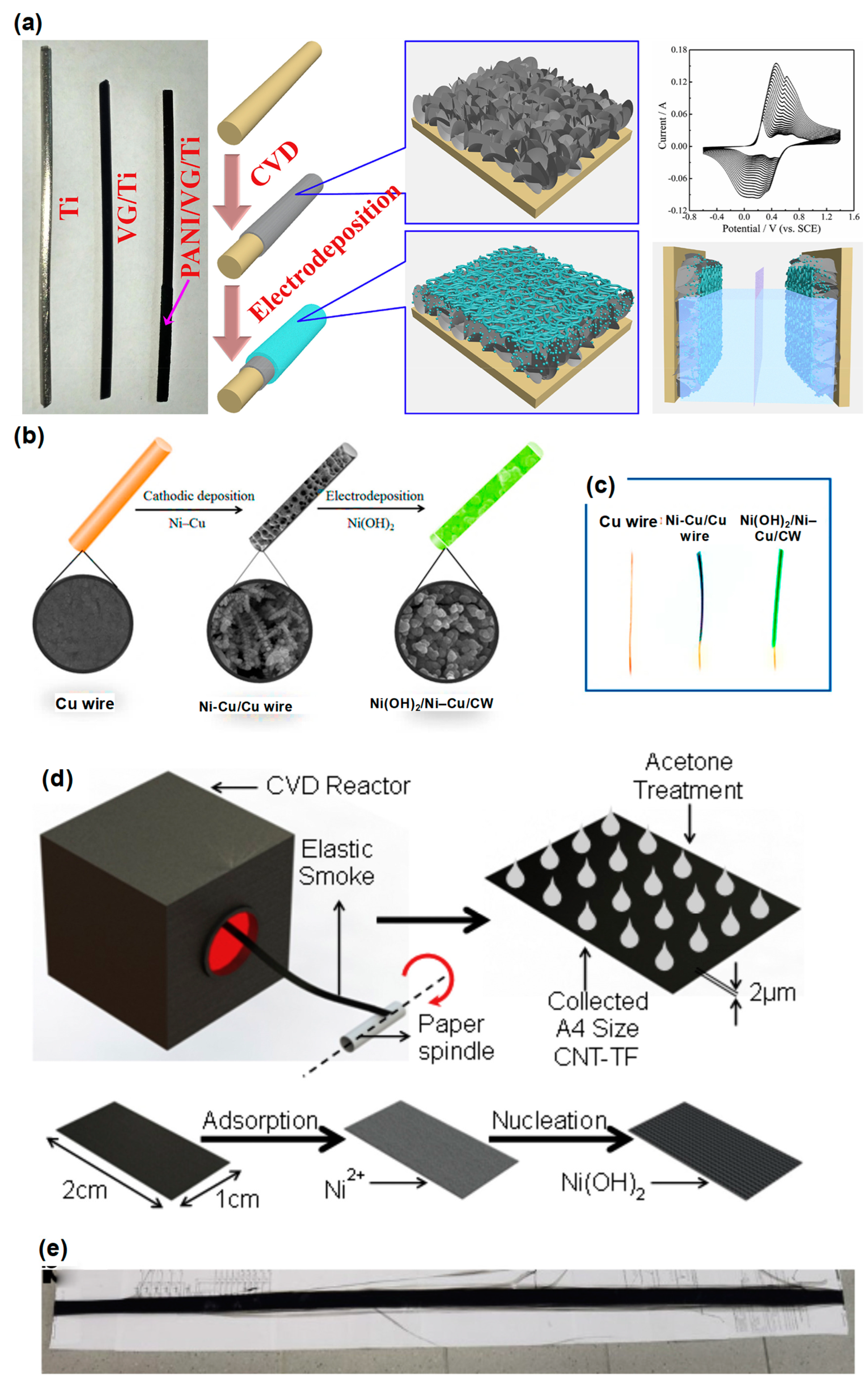
2.2. Carbon Nanotube on Thin Metal Film Electrodes
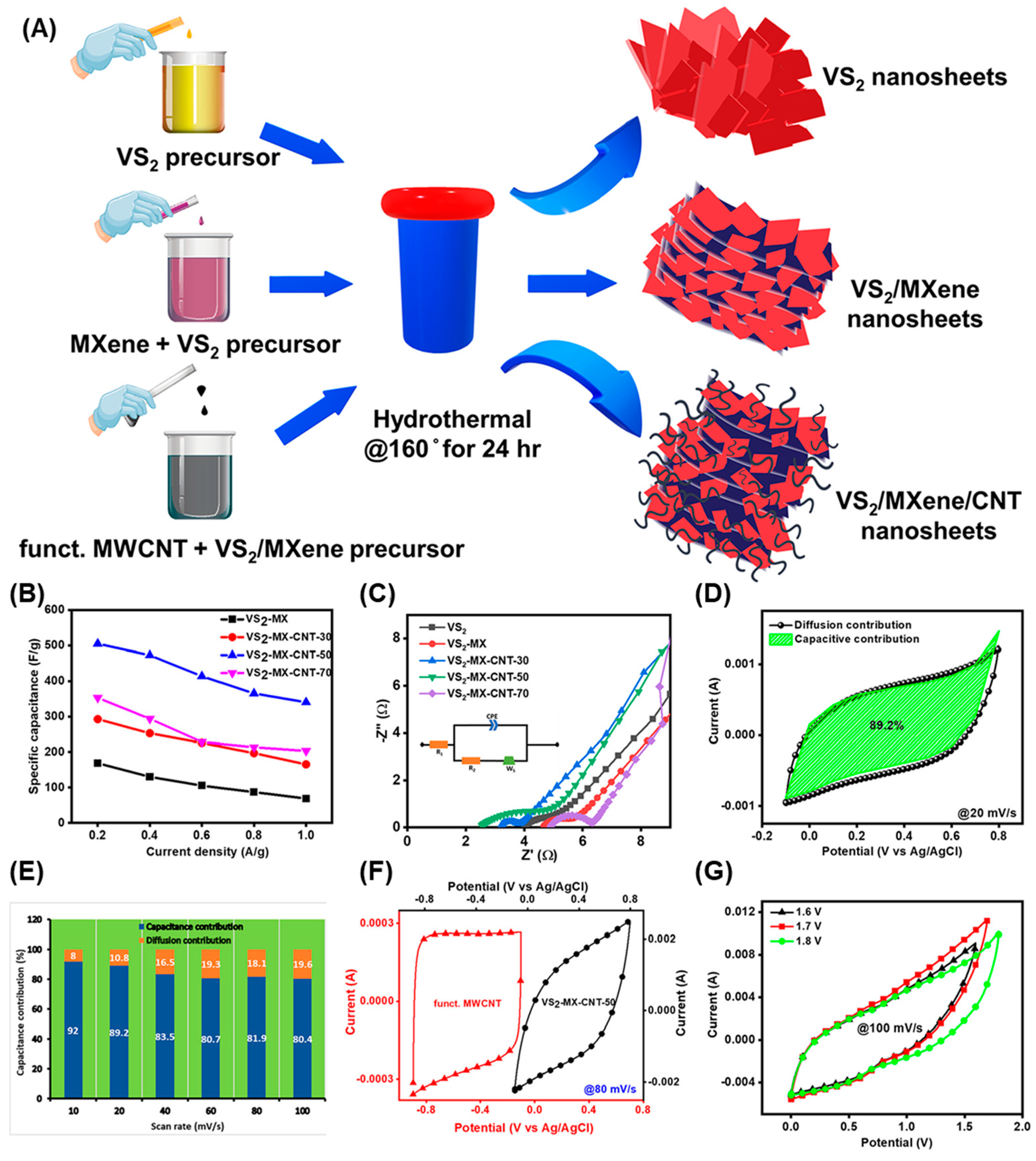
2.3. Hybrid Electrodes with Carbon Nanotube and MXene
2.4. Self-Assembled CNTs with NiCoO2 Electrode
2.5. One-Dimensional Fiber-Based Supercapacitor
3. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arshad, N.; Usman, M.; Adnan, M.; Ahsan, M.T.; Rehman, M.R.; Javed, S.; Ali, Z.; Akram, M.A.; Demopoulos, G.P.; Mahmood, A. Nanoengineering of NiO/MnO2/GO Ternary Composite for Use in High-Energy Storage Asymmetric Supercapacitor and Oxygen Evolution Reaction (OER). Nanomaterials 2023, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Koh, K.L.P.; Liu, P.; Thang, T.Q.; Duong, H.M. Continuous self-assembly of carbon nanotube thin films and their composites for supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 626–632. [Google Scholar] [CrossRef]
- Hu, C.; Miao, L.; Yang, Q.; Yu, X.; Song, L.; Zheng, Y.; Wang, C.; Li, L.; Zhu, L.; Cao, X.; et al. Self-assembly of CNTs on Ni foam for enhanced performance of NiCoO2@CNT@NF supercapacitor electrode. Chem. Eng. J. 2021, 410, 128317. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. MXene–carbon nanotube composite electrodes for high active mass asymmetric supercapacitors. J. Mater. Chem. A 2021, 9, 10335–10344. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. Composite Fe3O4-MXene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method. Materials 2021, 14, 2930. [Google Scholar] [CrossRef]
- He, J.-H.; Elazem, N.Y. The Carbon Nanotube-Embedded Boundary Layer Theory for Energy Harvesting. Facta Univ. Ser. Mech. Eng. 2022, 20, 221–235. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, K.; Huang, T.; Shen, G. Recent advances in fiber supercapacitors: Materials, device configurations, and applications. Adv. Mater. 2020, 32, 1901806. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Qaiser, N.; Hwang, B. Electro-Mechanical Response of Stretchable PDMS Composites with a Hybrid Filler System. Facta Univ. Ser. Mech. Eng. 2023, 2766459-4. [Google Scholar] [CrossRef]
- Wang, B.Q.; Gong, S.H.; Sun, Q.S.; Liu, F.; Wang, X.C.; Cheng, J.P. Carbon nanotubes refined mesoporous NiCoO2 nanoparticles for high−performance supercapacitors. Electrochim. Acta 2022, 402, 139575. [Google Scholar] [CrossRef]
- Wang, C.; Hu, K.; Liu, Y.; Zhang, M.-R.; Wang, Z.; Li, Z. Flexible Supercapacitors Based on Graphene/Boron Nitride Nanosheets Electrodes and PVA/PEI Gel Electrolytes. Materials 2021, 14, 1955. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Tian, G.; Zhang, D. Co@N-CNT/MXenes in situ grown on carbon nanotube film for multifunctional sensors and flexible supercapacitors. Nanoscale 2021, 13, 14460–14468. [Google Scholar] [CrossRef]
- Yang, J.; Mao, Z.; Zheng, R.; Liu, H.; Shi, L. Solution-Blown Aligned Nanofiber Yarn and Its Application in Yarn-Shaped Supercapacitor. Materials 2020, 13, 3778. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, W.; Zhang, P.; Chen, J.; Tian, W.B.; Zhang, Y.M.; Sun, Z.M. MXene/CNTs films prepared by electrophoretic deposition for supercapacitor electrodes. J. Electroanal. Chem. 2018, 830–831, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Wang, J.; Seveno, D.; Fransaer, J.; Locquet, J.-P.; Seo, J.W. Carbon Nanotube Fibers Decorated with MnO2 for Wire-Shaped Supercapacitor. Molecules 2021, 26, 3479. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, L.; Zhang, D.; Chen, S.; Coxon, P.R.; He, X.; Coto, M.; Kim, H.-K.; Xi, K.; Ding, S. A universal synthetic route to carbon nanotube/transition metal oxide nano-composites for lithium ion batteries and electrochemical capacitors. Sci. Rep. 2016, 6, 37752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanoun, O.; Bouhamed, A.; Ramalingame, R.; Bautista-Quijano, J.R.; Rajendran, D.; Al-Hamry, A. Review on conductive polymer/CNTs nanocomposites based flexible and stretchable strain and pressure sensors. Sensors 2021, 21, 341. [Google Scholar] [CrossRef]
- Gnanasekaran, K.; Heijmans, T.; Van Bennekom, S.; Woldhuis, H.; Wijnia, S.; De With, G.; Friedrich, H. 3D printing of CNT-and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl. Mater. Today 2017, 9, 21–28. [Google Scholar] [CrossRef]
- Jing, D.; Hatami, M. Peristaltic Carreau-Yasuda nanofluid flow and mixed heat transfer analysis in an asymmetric vertical and tapered wavy wall channel. Rep. Mech. Eng. 2020, 1, 128–140. [Google Scholar]
- Gregg, A.; De Volder, M.F.; Baumberg, J.J. Light—Actuated Anisotropic Microactuators from CNT/Hydrogel Nanocomposites. Adv. Opt. Mater. 2022, 10, 2200180. [Google Scholar] [CrossRef]
- Du, M.; Xia, W.; Jiao, Z.; Chen, Y.; Demir, M.; Zhang, Y.; Gu, M.; Zhang, X.; Wang, C. Construction of hierarchical sugar gourd-like (Ni,Co)Se2/(Ni,Co)Se2/CC nanostructure with enhanced performance for hybrid supercapacitor. J. Alloy. Compd. 2023, 930, 167459. [Google Scholar] [CrossRef]
- Jiao, Z.; Chen, Y.; Demir, M.; Du, M.; Gu, M.; Wang, C.; Zhang, X.; Deng, Y.; Wang, Z.; Wang, T.; et al. Vanadium-doped Co0.85Se nanowire arrays with high areal capacitance for hybrid supercapacitor electrodes. J. Energy Storage 2022, 52, 104929. [Google Scholar] [CrossRef]
- Jiao, Z.; Chen, Y.; Du, M.; Demir, M.; Yan, F.; Xia, W.; Zhang, Y.; Wang, C.; Gu, M.; Zhang, X.; et al. 3D hollow NiCo LDH nanocages anchored on 3D CoO sea urchin-like microspheres: A novel 3D/3D structure for hybrid supercapacitor electrodes. J. Colloid Interface Sci. 2023, 633, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, H.-X.; Cao, Y.; Yan, X.-H.; Yan, J.; Gao, H.-L.; Luo, H.-W.; Wang, S.-W.; Jia, X.-D.; Kachalova, L.; et al. Recent advances and challenges of electrode materials for flexible supercapacitors. Coord. Chem. Rev. 2021, 438, 213910. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, S.; Chen, M.; Zhang, Y.; Li, Q. Fabrication and functionalization of carbon nanotube films for high-performance flexible supercapacitors. Carbon 2015, 92, 271–296. [Google Scholar] [CrossRef]
- Hillier, N.; Yong, S.; Beeby, S. The good, the bad and the porous: A review of carbonaceous materials for flexible supercapacitor applications. Energy Rep. 2020, 6, 148–156. [Google Scholar] [CrossRef]
- Mikhalchan, A.; Vilatela, J.J. A perspective on high-performance CNT fibres for structural composites. Carbon 2019, 150, 191–215. [Google Scholar] [CrossRef]
- Han, L.; Song, Q.; Sun, J.; Li, K.; Lu, Y. The role of CNT in improving the mechanical strength retention rate of C/C composites during heat treatment. Compos. Part B Eng. 2020, 187, 107856. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Liu, M.; Chen, Q. Mechanical strength of carbon nanotube–nickel nanocomposites. Nanotechnology 2007, 18, 505704. [Google Scholar] [CrossRef]
- Kim, G.; Nam, I.; Yang, B.; Yoon, H.; Lee, H.-K.; Park, S. Carbon nanotube (CNT) incorporated cementitious composites for functional construction materials: The state of the art. Compos. Struct. 2019, 227, 111244. [Google Scholar] [CrossRef]
- Wang, B.; Xin, H.; Li, X.; Cheng, J.; Yang, G.; Nie, F. Mesoporous CNT@ TiO2-C nanocable with extremely durable high rate capability for lithium-ion battery anodes. Sci. Rep. 2014, 4, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadiq, M.; Khan, M.A.; Hasan Raza, M.M.; Aalam, S.M.; Zulfequar, M.; Ali, J. Enhancement of Electrochemical Stability Window and Electrical Properties of CNT-Based PVA–PEG Polymer Blend Composites. ACS Omega 2022, 7, 40116–40131. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z. Curved pi-conjugation, aromaticity, and the related chemistry of small fullerenes (<C60) and single-walled carbon nanotubes. Chem. Rev. 2005, 105, 3643–3696. [Google Scholar]
- Al-Buriahi, M.S.; Hessien, M.; Alresheedi, F.; Al-Baradi, A.M.; Alrowaili, Z.A.; Kebaili, I.; Olarinoye, I.O. ZnO– Bi2O3 nanopowders: Fabrication, structural, optical, and radiation shielding properties. Ceram. Int. 2022, 48, 3464–3472. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Ali, A.M.; Al-Baradi, A.M.; Al-Buriahi, M.S.; Wahab, E.A.A.; Shaaban, K.S. A significant role of MoO3 on the optical, thermal, and radiation shielding characteristics of B2O3–P2O5–Li2O glasses. Opt. Quantum Electron. 2022, 54, 88. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Taha, T.A.; Ibrahim, M.; Saron, K.M.A.; Sriwunkum, C.; Al-Baradi, A.M.; Al-Buriahi, M.S. Synthesis and characterization of B2O3-Ag3PO4-ZnO-Na2O glasses for optical and radiation shielding applications. Optik 2021, 248, 168199. [Google Scholar] [CrossRef]
- Onyibo, E.C.; Safaei, B. Application of finite element analysis to honeycomb sandwich structures: A review. Rep. Mech. Eng. 2022, 3, 192–209. [Google Scholar] [CrossRef]
- Hwang, B.; Han, Y.; Matteini, P. Bending Fatigue Behavior of Ag Nanowire/Cu Thin-Film Hybrid Interconnects for Wearable Electronics. Facta Univ. Ser. Mech. Eng. 2022, 20, 553–560. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, X.; Weng, W.; Hu, Z.; Zhang, Y.; Xu, W.; Bi, Z.; Zhu, M. Activated Carbon Nanotube Fiber Fabric as a High-Performance Flexible Electrode for Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 28433–28441. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, H.; Li, M.; Li, C.; Qian, L.; Su, L.; Yang, B. High-performance aqueous symmetric supercapacitor based on polyaniline/vertical graphene/Ti multilayer electrodes. Electrochim. Acta 2018, 283, 410–418. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Naderi, L.; Mohammadi, R. High-Performance Fiber-Shaped Flexible Asymmetric Microsupercapacitor Based on Ni(OH)2 Nanoparticles-Decorated Porous Dendritic Ni–Cu Film/Cu Wire and Reduced Graphene Oxide/Carbon Fiber Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 14574–14588. [Google Scholar] [CrossRef]
- Sharma, A.; Rout, C.S. 1T metallic vanadium disulfide hybridized with MXene and functionalized-MWCNT as a remarkable electrode for high power density asymmetric supercapacitor applications. Int. J. Energy Res. 2022, 46, 24537–24553. [Google Scholar] [CrossRef]
- Ma, W.; Li, W.; Li, M.; Mao, Q.; Pan, Z.; Hu, J.; Li, X.; Zhu, M.; Zhang, Y. Unzipped Carbon Nanotube/Graphene Hybrid Fiber with Less “Dead Volume” for Ultrahigh Volumetric Energy Density Supercapacitors. Adv. Funct. Mater. 2021, 31, 2100195. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Lee, J.W.; Son, Y.-R.; Lee, J.-H.; Yeo, C.S.; Lam, T.D.; Park, S.Y.; Park, S.-J.; Sinh, L.H.; Shin, M.K. Large-Scale Conductive Yarns Based on Twistable Korean Traditional Paper (Hanji) for Supercapacitor Applications: Toward High-Performance Paper Supercapacitors. Adv. Energy Mater. 2018, 8, 1801854. [Google Scholar] [CrossRef]



| Materials | Electrochemical Performance | [Ref] |
|---|---|---|
| CNTFF | Areal capacitance of up to 353 mF/cm2 and an energy density of up to 247 μW/cm2 | [39] |
| CNT-MnO2 fiber fabrics | A specific capacitance reaching up to 231.3 F/g and an energy density of up to 86 nWh/cm | [14] |
| CNT-graphene fiber fabrics | A specific capacitance of up to 350 mF/cm2 | [10] |
| CNT-NiO fiber fabrics | A specific capacitance of up to 402 F/g and an energy density of up to 28 Wh/kg | [1] |
| Functionalizing CNTs on a Ti thin film with graphene | A specific capacitance of 535.7 F/g and an energy density up to 26.1 Wh/Kg | [40] |
| CNTs on Ni thin films | A specific capacitance of 1200 F/g | [2] |
| MXene (Ti3C2Tx)-MWCNT electrodes | A specific capacitance of 0.94 F cm−2 at scan rate of 2 mV s−1 | [4] |
| A hybrid electrode combining CNTs, Fe3O4, and Ti3C2Tx MXene | A specific capacitance of 5.52 F/cm2 | [5] |
| CNTs and Ti3C2 MXene | A specific capacitance of 134 F/g | [13] |
| VS2-MX-CNT-50//MWCNT | A specific capacitance of 505 F/g and an energy density of 7303 Wh/Kg | [42] |
| NiCoO2/CNT@NF | A specific capacitance of 929 F g−1 | [3] |
| CNTs/NiCoO2 system (3D) | A specific capacitance of 1587 F/g | [9] |
| Self-assembled CNTs/NiCoO2 system | A high specific capacitance of 1360 F/g | [15] |
| SWNTs-coated Korean traditional paper | A specific capacitance of 366 F/g and energy density of 114 Wh/Kg | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Ha, H.; Choi, C.; Yoon, H.; Matteini, P.; Cheong, J.Y.; Hwang, B. Review of Flexible Supercapacitors Using Carbon Nanotube-Based Electrodes. Appl. Sci. 2023, 13, 3290. https://doi.org/10.3390/app13053290
Han Y, Ha H, Choi C, Yoon H, Matteini P, Cheong JY, Hwang B. Review of Flexible Supercapacitors Using Carbon Nanotube-Based Electrodes. Applied Sciences. 2023; 13(5):3290. https://doi.org/10.3390/app13053290
Chicago/Turabian StyleHan, Yurim, Heebo Ha, Chunghyeon Choi, Hyungsub Yoon, Paolo Matteini, Jun Young Cheong, and Byungil Hwang. 2023. "Review of Flexible Supercapacitors Using Carbon Nanotube-Based Electrodes" Applied Sciences 13, no. 5: 3290. https://doi.org/10.3390/app13053290





