Application of Response Surface Methodology to Optimize the Extraction Process of Bioactive Compounds Obtained from Coffee Silverskin
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods Used as Reponses in Experimental Design of Extraction Optimization (EO)
2.2.1. Determination of Total Phenolic Compounds (TPC) Content
2.2.2. Determination of Antioxidant Activity by Using ABTS Assay
2.2.3. Determination of Antioxidant Activity by Using FRAP Assay
2.2.4. Determination of Caffeine and Chlorogenic Acids Content by Using HPLC
2.2.5. Determination of Browning Index (BI)
2.3. CS Extracts Prepration Procedure and Statistical Approach of Experimental Design of EO
2.3.1. CS Extracts Preparation Procedure
2.3.2. Statistical Approach of Experimental Design of EO
3. Results and Discussion
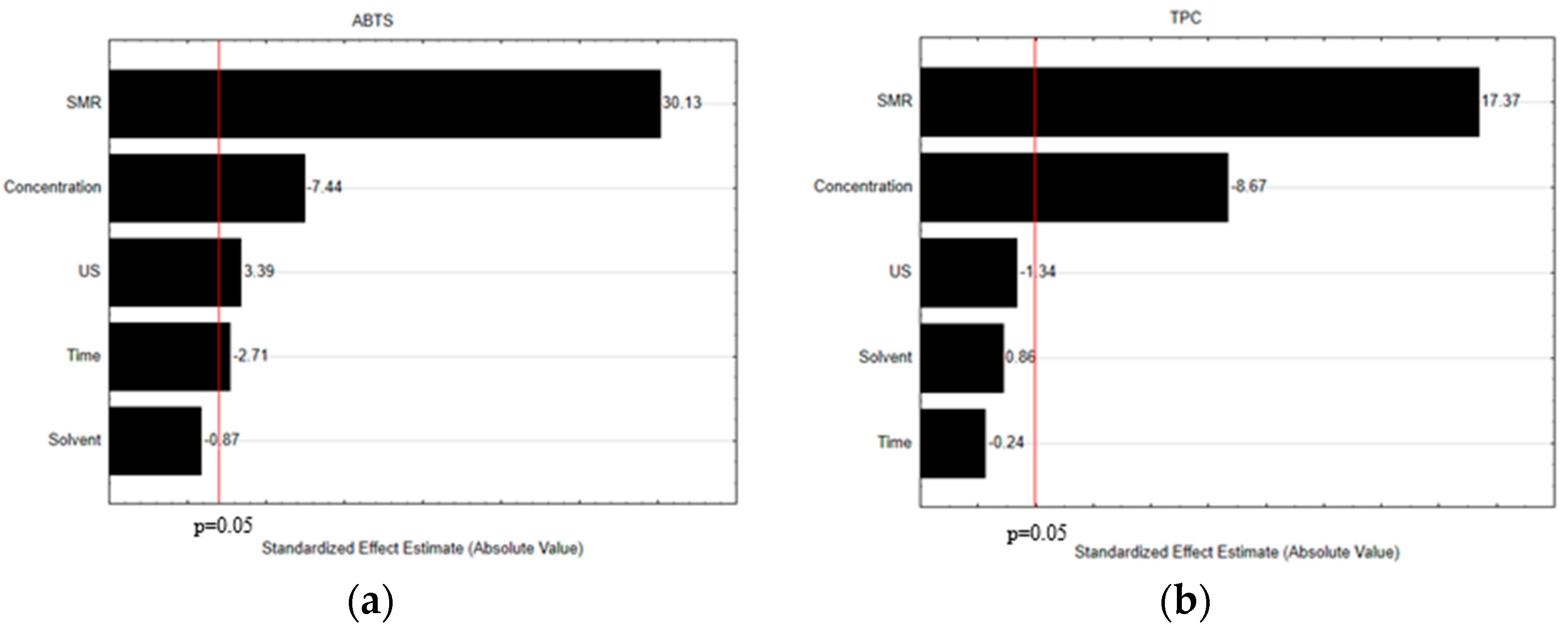
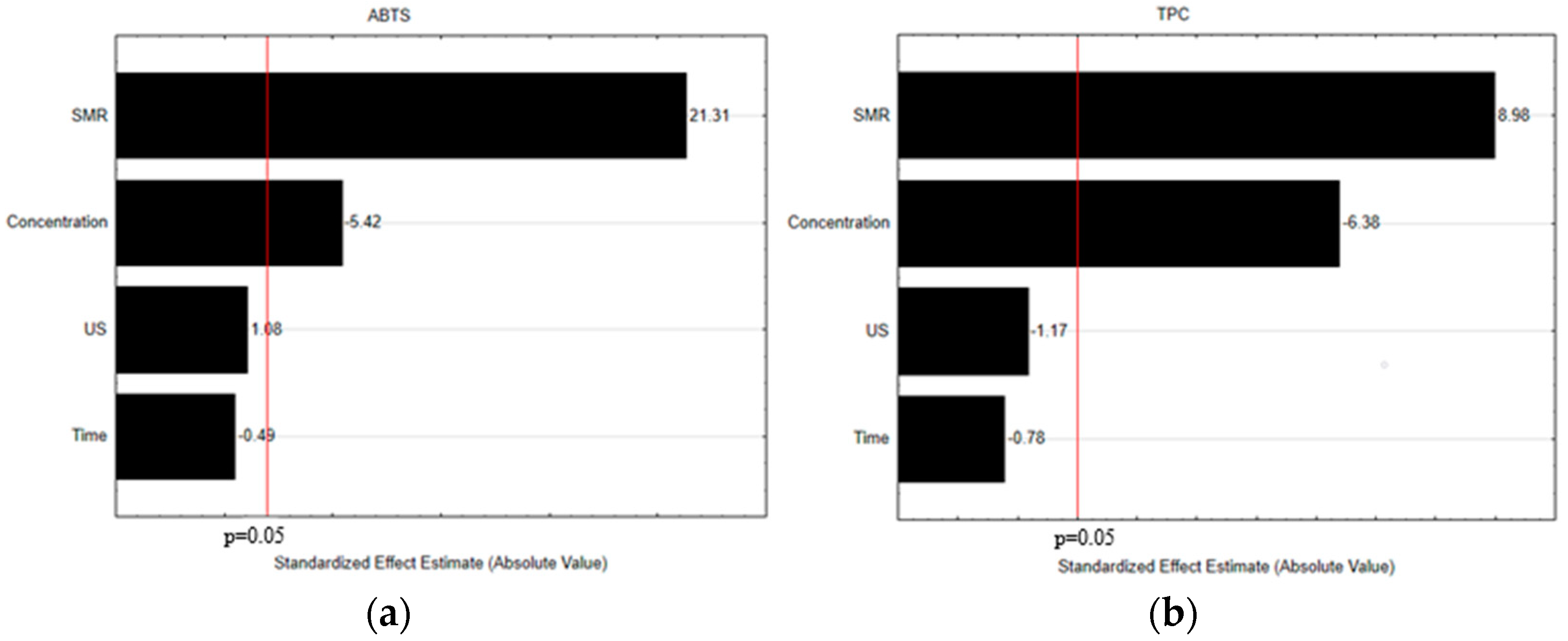
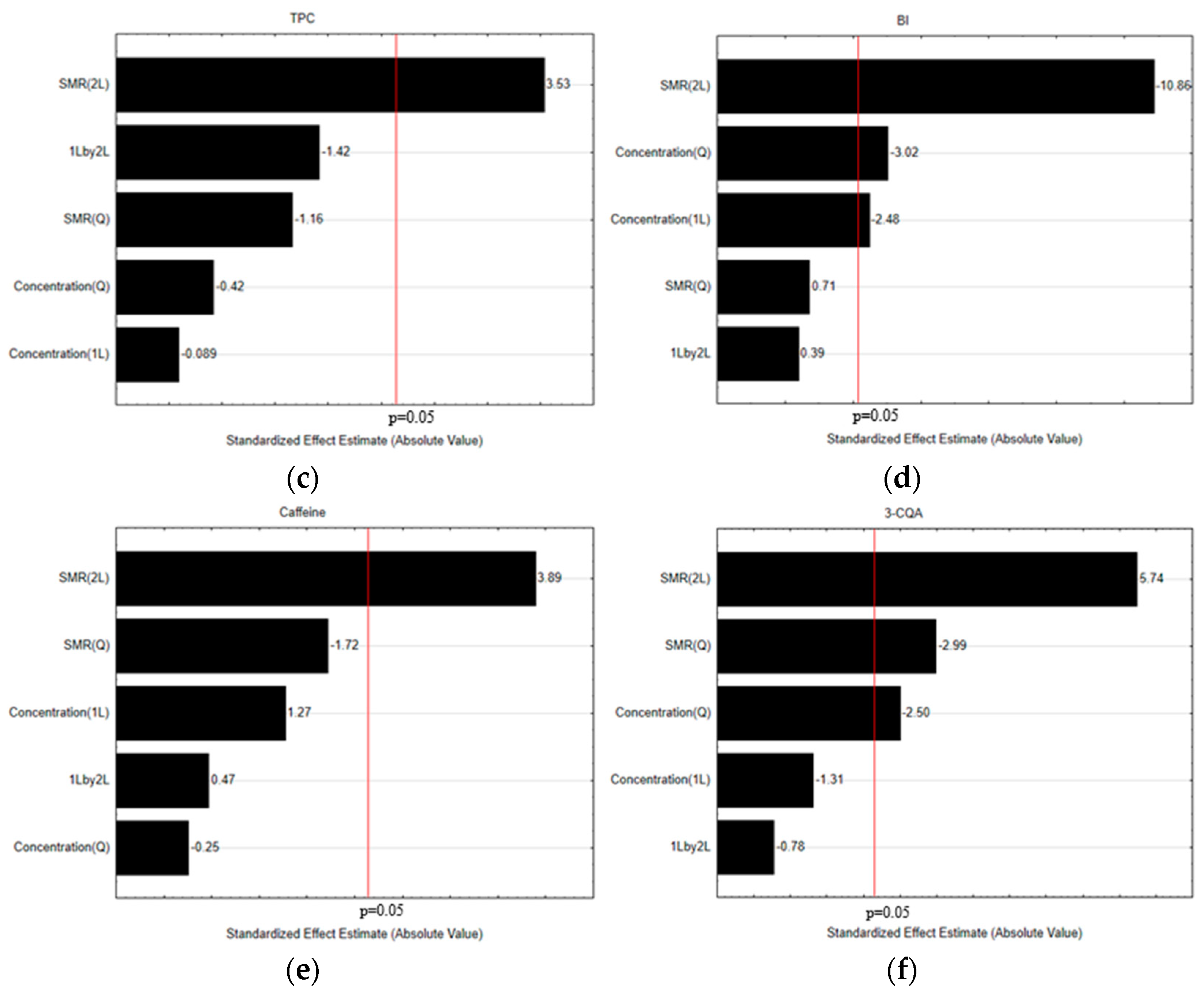
3.1. Selection of the Crucial Operational Extraction Process Variables
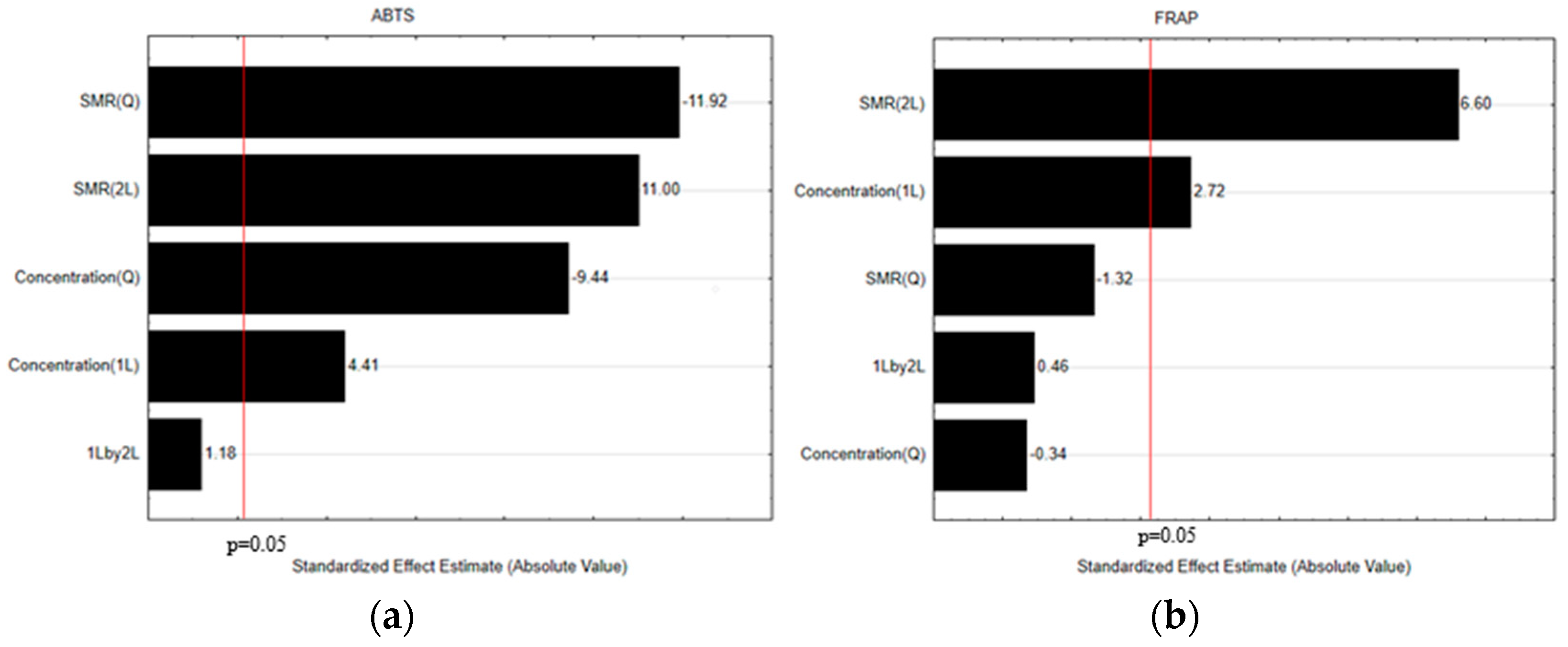
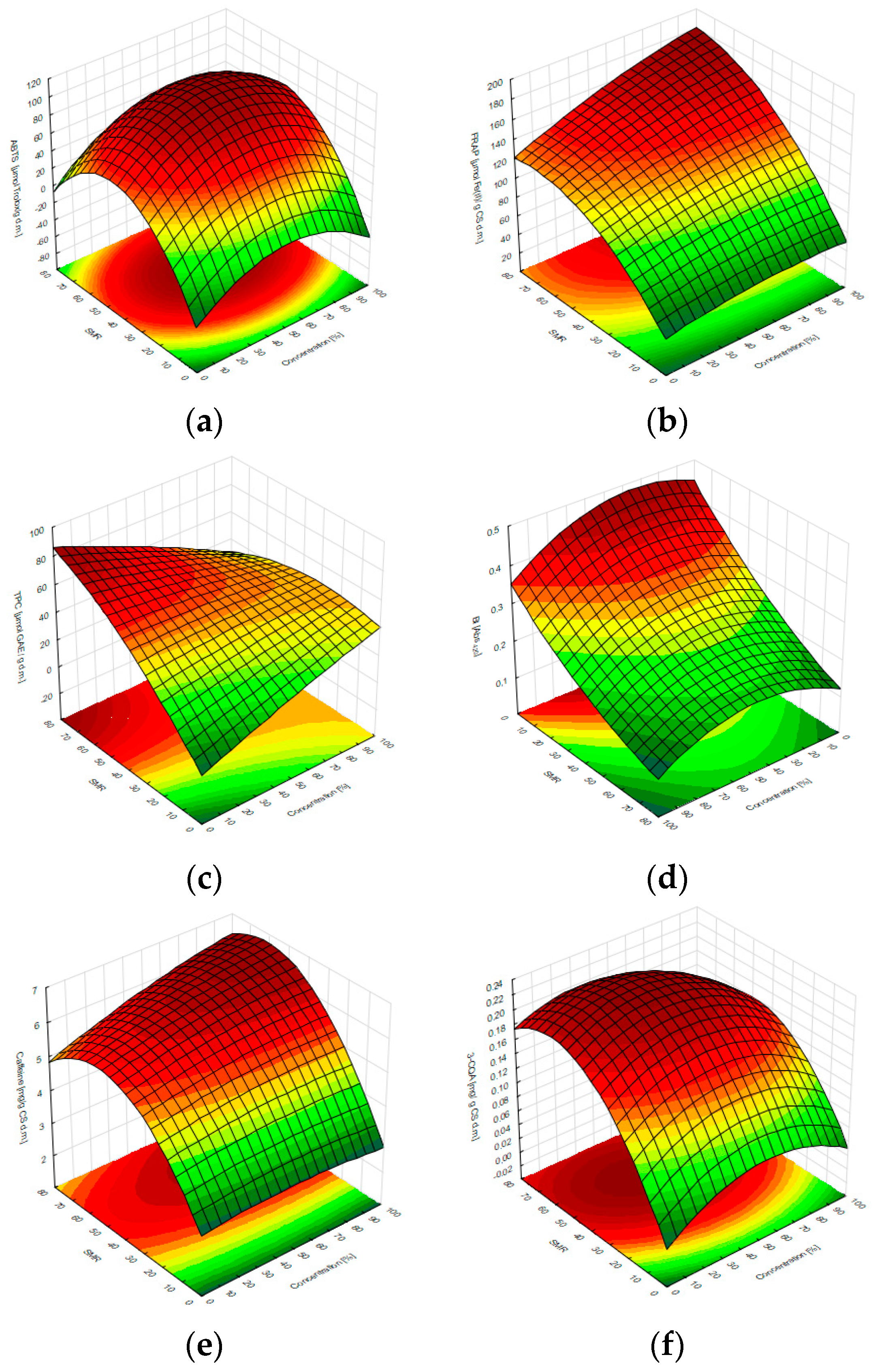
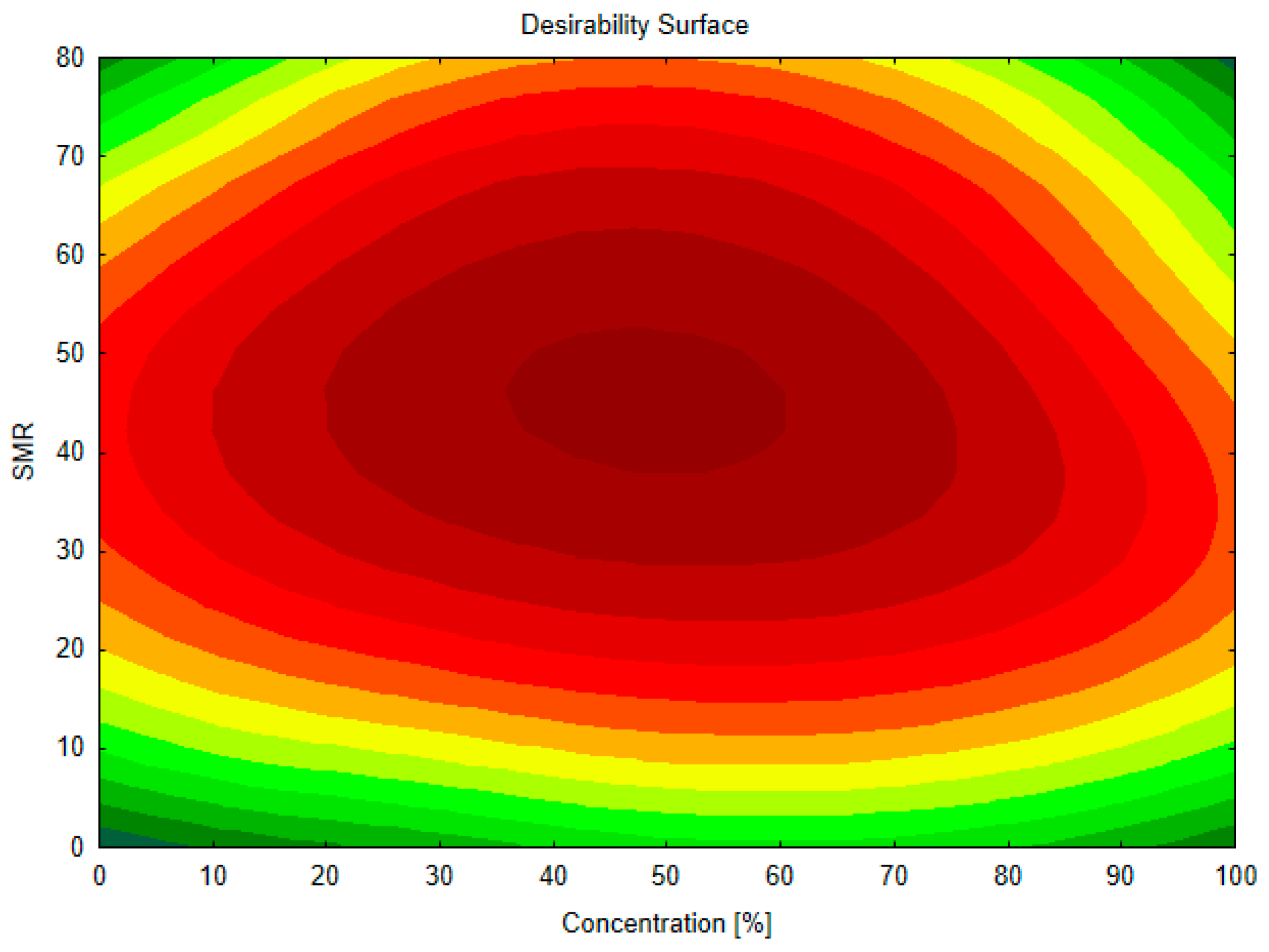
3.2. Optimization of the Extraction Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Farah, A. Coffee Constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y.F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 21–58. [Google Scholar]
- Głowacka, R.; Górska, A.; Wirkowska-Wojdyła, M.; Wołosiak, R.; Majewska, E.; Derewiaka, D. The influence of brewing method on bioactive compounds residues in spent coffee grounds of different roasting degree and geographical origin. Int. J. Food Sci. Technol. 2019, 54, 3008–3014. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Technol. 2021, 59, 429–444. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization (ICO). Coffee Market Report, October 2017. Available online: http://www.ico.org/documents/cy2017-18/cmr-1017-e.pdf (accessed on 9 March 2023).
- International Coffee Organization (ICO). Statistics. Trade Statistics Tables. World Coffee Consumption. Available online: http://www.ico.org/prices/new-consumption-table.pdf (accessed on 9 March 2023).
- International Coffee Organization (ICO). Historical Data on the Global Coffee Trade. Available online: https://www.ico.org/new_historical.asp (accessed on 8 April 2023).
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of Coffee Silver Skin as potential Food-Safe Ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Influence of pretreatment and modifiers on subcritical water liquefaction of spent coffee grounds: A green waste valorization approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Garcia, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Escobar, F.V.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Pearce, D.W.; Turner, R.K. Economics of Natural Resources and the Environment; Johns Hopkins University Press: Baltimore, MD, USA, 1989; pp. 29–42. [Google Scholar]
- Winans, K.; Kendall, A.; Deng, H. The history and current applications of the circular economy concept. Renew. Sust. Energ. Rev. 2017, 68, 825–833. [Google Scholar] [CrossRef]
- Muscio, A.; Sisto, R. Are agri-food systems really switching to a circular economy model? Implications for European research and innovation policy. Sustainability 2020, 12, 5554. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards circular economy in the agri-food sector. A systematic literature review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular economy models in agro-food systems: A review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Brzezińska, R.; Bryś, J.; Giers, O.; Bryś, A.; Górska, A.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M. Quality evaluation of plant oil blends interesterified by using immobilized Rhizomucor miehei lipase. Appl. Sci. 2022, 12, 11148. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Teymourian, T.; Kowsari, E.; Ramakrishna, S.; Ehsani, A. Sustainability and circular economy of food wastes: Waste reduction strategies, higher recycling methods, and improved valorization. Mater. Circ. Econ. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Pashova, S.; Radev, R.; Dimitrov, G.; Ivanov, J. Edible coatings in food industry related to circular economy. Qual. Access Success 2018, 19, 111–117. [Google Scholar]
- Loizia, P.; Neofytou, N.; Zorpas, A.A. The concept of circular economy strategy in food waste management for the optimization of energy production through anaerobic digestion. Environ. Sci. Pollut. Res. 2019, 26, 14766–14773. [Google Scholar] [CrossRef]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Zhang, Q.; Dhir, A.; Kaur, P. Circular economy and the food sector: A systematic literature review. Sustain. Prod. Consum. 2022, 32, 655–668. [Google Scholar] [CrossRef]
- Pannila, N.; Jayalath, M.M.; Thibbotuwawa, A.; Nielsen, I.; Uthpala, T.G.G. Challenges in Applying Circular Economy Concepts to Food Supply Chains. Sustainability 2022, 14, 16536. [Google Scholar] [CrossRef]
- Mayson, S.; Williams, I.D. Applying a circular economy approach to valorize spent coffee grounds. Resour. Conserv. Recycl. 2021, 172, 105659. [Google Scholar] [CrossRef]
- Overturf, E.; Pezzutto, S.; Boschiero, M.; Ravasio, N.; Monegato, A. The CirCo (circular coffee) project: A case study on valorization of coffee silverskin in the context of circular economy in Italy. Sustainability 2021, 13, 9069. [Google Scholar] [CrossRef]
- Del Pozo, C.; Bartrolí, J.; Alier, S.; Puy, N.; Fàbregas, E. Production of antioxidants and other value-added compounds from coffee silverskin via pyrolysis under a biorefinery approach. Waste Manag. 2020, 109, 19–27. [Google Scholar] [CrossRef]
- Bessada, S.M.; Alves, C.R.; Oliveira, M.B.P.P. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Coffee silverskin: A possible valuable cosmetic ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J. Pharmaceut. Biomed. 2010, 51, 456–463. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a new potential functional ingredient: Coffee silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Rehman, Z.U. Evaluation of antioxidant activity of methanolic extract from peanut hulls in fried potato chips. Plant Food Hum. Nutr. 2003, 58, 75–83. [Google Scholar] [CrossRef]
- Anand, S.P.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496–2501. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative methods of bioactive compounds and oils extraction from berry fruit by-products—A review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the solvent and extraction conditions for maximum recovery of antioxidant phenolic compounds from coffee silverskin. Food Bioprocess Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response surface methodology as a tool to optimize the extraction of bioactive compounds from plant sources. J. Sci. Food Agric. 2023, 103, 26–36. [Google Scholar] [CrossRef]
- Box, G.E.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. In Breakthroughs in Statistics; Springer Series in Statistics; Kotz, S., Johnson, N.L., Eds.; Springer: New York, NY, USA, 1992; pp. 270–310. [Google Scholar]
- Candioti, L.V.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef]
- Said, K.A.M.; Amin, M.A.M. Overview on the response surface methodology (RSM) in extraction processes. J. Appl. Sci. Process Eng. 2015, 2, 8–17. [Google Scholar]
- Roshani Neshat, R.; Bimakr, M.; Ganjloo, A. Effects of Zedo gum edible coating enriched with microwave-agitated bed extracted bioactive compounds from lemon verbena leaves on oxidative stability of Oncorhynchus mykiss. J. Food Meas. Charact. 2022, 16, 4388–4401. [Google Scholar] [CrossRef]
- Polish Norm: PN-ISO 3534-2:2010; Statistics—Vocabulary and Symbols—Part 2: Applied Statistics. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, D.H.; Youn, K.S.; Lee, J.B.; Moon, K.D. Optimization of roasting conditions according to antioxidant activity and sensory quality of coffee brews. Food Sci. Biotechnol. 2013, 22, 23–29. [Google Scholar] [CrossRef]
- Glowacka, R.; Górska, A.; Wirkowska-Wojdyla, M. Kawowa łuska srebrzysta-nowa, naturalna alternatywa pozyskiwania wybranych związków bioaktywnych. Zeszyty Problemowe Postępów Nauk Rolniczych 2018, 592, 15–25. [Google Scholar] [CrossRef]
- Wijekoon, M.J.O.; Bhat, R.; Karim, A.A. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J. Food Compos. Anal. 2011, 24, 615–619. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Gokhan, Z.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar]
- Gorinstein, S.; Zachwieja, Z.; Katrich, E.; Pawelzik, E.; Haruenkit, R.; Trakhtenberg, S.; Martin-Belloso, O. Comparison of the contents of the main antioxidant compounds and the antioxidant activity of white grapefruit and his new hybrid. LWT—Food Sci. Technol. 2004, 37, 337–343. [Google Scholar] [CrossRef]
- Sariburun, E.; Sahin, S.; Demir, C.; Turkben, C.; Uylaser, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef]
- Yeong, J.-H.; Jung, H.; Lee, S.-R.; Lee, H.-J.; Hwang, K.T.; Kim, T.-Y. Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem. 2010, 123, 338–344. [Google Scholar]
- Gan, C.Y.; Latiff, A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011, 124, 1277–1283. [Google Scholar] [CrossRef]
- Bhatt, B.D.; Parajuli, G.C. Study on total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activities of Urtica dioica of Nepalese origin. J. Nepal Chem. Soc. 2017, 37, 113–118. [Google Scholar] [CrossRef]
- Yousuf, S.; Shabir, S.; Kauts, S.; Minocha, T.; Obaid, A.A.; Khan, A.A.; Mujalli, A.; Jamous, Y.F.; Almaghrabi, S.; Baothman, B.K.; et al. Appraisal of the antioxidant activity, polyphenolic content, and characterization of selected himalayan herbs: Anti-proliferative potential in HepG2 cells. Molecules 2022, 27, 8629. [Google Scholar] [CrossRef]
- Seal, T.; Chaudhuri, K.; Pillai, B.; Chakrabarti, S.; Mondal, T.; Auddy, B. Evaluation of antioxidant activities, toxicity studies and the DNA damage protective effect of various solvent extracts of Litsea cubeba fruits. Heliyon 2020, 6, e03637. [Google Scholar] [CrossRef]
- Zeroual, A.; Sakar, E.H.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A.; Taleb, M. Effects of extraction technique and solvent on phytochemicals, antioxidant, and antimicrobial activities of cultivated and wild rosemary (Rosmarinus officinalis L.) from taounate region (Northern Morocco). Biointerface Res. Appl. Chem. 2022, 12, 8441–8452. [Google Scholar]
- DiNardo, A.; Brar, H.S.; Subramanian, J.; Singh, A. Optimization of microwave-assisted extraction parameters and characterization of phenolic compounds in Yellow European Plums. Can. J. Chem. Eng. 2018, 97, 256–267. [Google Scholar] [CrossRef]
- Sik, B.; Ajtony, Z.; Lakatos, E.; Szekelyhidi, R. The effects of extraction conditions on the antioxidant activities, total polyphenol and monomer anthocyanin contents of six edible fruits growing wild in Hungary. Heliyon 2022, 8, e12048. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Influence of extraction conditions on the recovery of phenolic antioxidants from spent coffee grounds. Am. J. Appl. Sci. 2013, 10, 478–486. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sanchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput. 2018, 2, 10–14. [Google Scholar]
- Guglielmetti, A.; D’ignoti, V.; Ghirardello, D.; Belviso, S.; Zeppa, G. Optimisation of ultrasound and microwave-assisted extraction of caffeoylquinic acids and caffeine from coffee silverskin using response surface methodology. Ital. J. Food Sci. 2017, 29, 409–423. [Google Scholar]
- Ginting, A.R.; Kit, T.; Mingvanish, W.; Thanasupsin, S.P. Valorization of Coffee Silverskin through Subcritical Water Extraction: An Optimization Based on T-CQA Using Response Surface Methodology. Sustainability 2022, 14, 8435. [Google Scholar] [CrossRef]
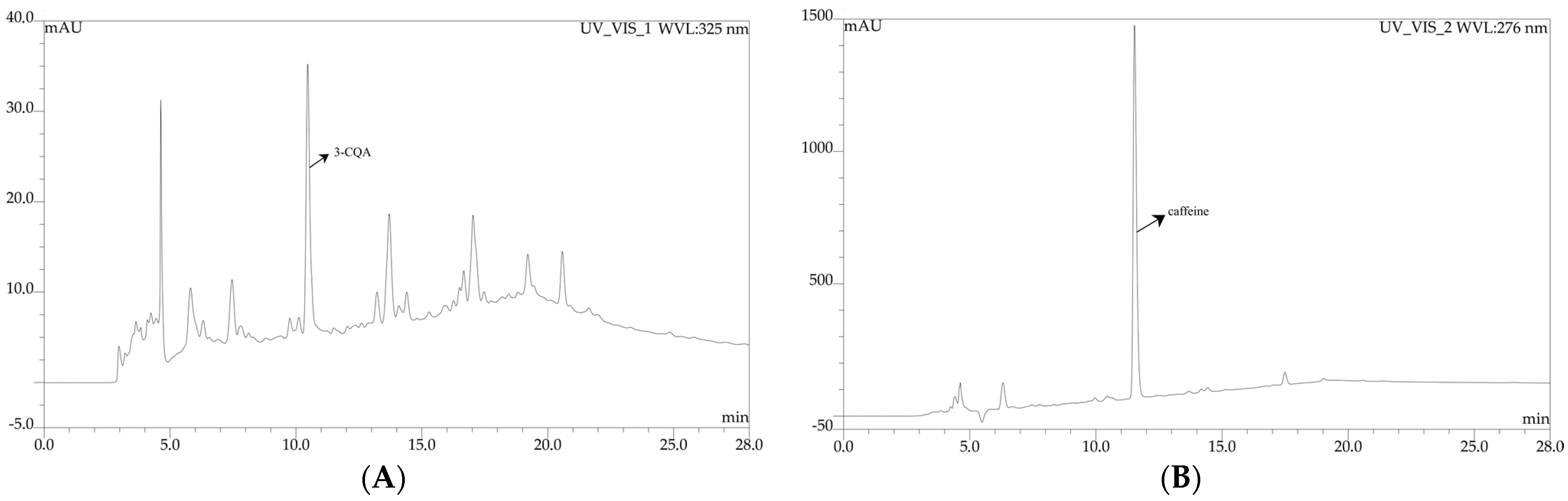
| Type of Sample | Methanol/Ethanol | Water | |||||
|---|---|---|---|---|---|---|---|
| Solvent Concentration [%] | Time [min] | SMR [cm3/g] | US * | Time [min] | SMR [cm3/g] | US * | |
| 1 | 60 (−1) | 30 (−1) | 10 (−1) | w (+1) | 30 (−1) | 10 (−1) | w (+1) |
| 2 | 100 (+1) | 30 (−1) | 10 (−1) | w (+1) | 90 (+1) | 10 (−1) | w (+1) |
| 3 | 60 (−1) | 90 (+1) | 10 (−1) | w (+1) | 30 (−1) | 40 (+1) | w (+1) |
| 4 | 100 (+1) | 90 (+1) | 10 (−1) | w (+1) | 90 (+1) | 40 (+1) | w (+1) |
| 5 | 60 (−1) | 30 (−1) | 40 (+1) | w (+1) | 30 (−1) | 10 (−1) | wo (−1) |
| 6 | 100 (+1) | 30 (−1) | 40 (+1) | w (+1) | 90 (+1) | 10 (−1) | wo (−1) |
| 7 | 60 (−1) | 90 (+1) | 40 (+1) | w (+1) | 30 (−1) | 40 (+1) | wo (−1) |
| 8 | 100 (+1) | 90 (+1) | 40 (+1) | w (+1) | 90 (+1) | 40 (+1) | wo (−1) |
| 9 | 60 (−1) | 30 (−1) | 10 (−1) | wo (−1) | - | - | - |
| 10 | 100 (+1) | 30 (−1) | 10 (−1) | wo (−1) | - | - | - |
| 11 | 60 (−1) | 90 (+1) | 10 (−1) | wo (−1) | - | - | - |
| 12 | 100 (+1) | 90 (+1) | 10 (−1) | wo (−1) | - | - | - |
| 13 | 60 (−1) | 30 (−1) | 40 (+1) | wo (−1) | - | - | - |
| 14 | 100 (+1) | 30 (−1) | 40 (+1) | wo (−1) | - | - | - |
| 15 | 60 (−1) | 90 (+1) | 40 (+1) | wo (−1) | - | - | - |
| 16 | 100 (+1) | 90 (+1) | 40 (+1) | wo (−1) | - | - | - |
| Type of Sample | Solvent Concentration [%] | SMR [cm3/g] |
|---|---|---|
| 1 | 20 (−1) | 20 (−1) |
| 2 | 80 (+1) | 20 (−1) |
| 3 | 20 (−1) | 60 (+1) |
| 4 | 80 (+1) | 60 (+1) |
| 5 | 7.58 (−1.414) | 40 (0) |
| 6 | 92.42 (+1.414) | 40 (0) |
| 7 | 50 (0) | 11.72 (−1.414) |
| 8 | 50 (0) | 68.28 (+1.414) |
| 9 | 50 (0) | 40 (0) |
| 10 | 50 (0) | 40 (0) |
| Type of Sample | Methanol | Ethanol | Water | |||
|---|---|---|---|---|---|---|
| ABTS [μmol Trolox/g d.m.] | TPC [μmol GAE/g d.m.] | ABTS [μmol Trolox/g d.m.] | TPC [μmol GAE/g d.m.] | ABTS [μmol Trolox/g d.m.] | TPC [μmol GAE/g d.m.] | |
| 1 | 33.43 ± 0.62 | 18.91 ± 0.15 | 35.52 ± 0.29 | 27.57 ± 0.27 | 11.66 ± 0.29 | 13.46 ± 0.10 |
| 2 | 24.75 ± 1.05 | 15.47 ± 0.19 | 23.65 ± 1.49 | 22.10 ± 0.17 | 11.65 ± 0.27 | 13.15 ± 0.11 |
| 3 | 26.90 ± 0.84 | 23.08 ± 0.24 | 32.37 ± 0.38 | 27.17 ± 0.14 | 56.65 ± 1.45 | 24.27 ± 0.71 |
| 4 | 22.92 ± 1.14 | 15.96 ± 0.11 | 23.97 ± 1.05 | 24.95 ± 0.24 | 64.16 ± 0.72 | 27.44 ± 0.99 |
| 5 | 71.54 ± 1.01 | 46.39 ± 0.46 | 70.27 ± 0.81 | 48.44 ± 0.64 | 14.94 ± 0.16 | 16.91 ± 0.09 |
| 6 | 73.90 ± 1.33 | 36.30 ± 0.93 | 65.10 ± 1.41 | 25.95 ± 0.88 | 11.16 ± 0.23 | 13.07 ± 0.04 |
| 7 | 52.28 ± 1.43 | 42.46 ± 0.81 | 56.93 ± 1.60 | 37.53 ± 0.49 | 51.46 ± 1.65 | 27.30 ± 0.33 |
| 8 | 62.40 ± 1.17 | 29.60 ± 1.17 | 70.34 ± 0.89 | 34.02 ± 1.36 | 48.00 ± 1.76 | 24.18 ± 1.73 |
| 9 | 41.57 ± 0.45 | 22.98 ± 0.06 | 31.16 ± 0.68 | 21.36 ± 0.11 | - | - |
| 10 | 23.46 ± 0.72 | 16.71 ± 0.05 | 18.62 ± 0.71 | 18.52 ± 0.07 | - | - |
| 11 | 38.64 ± 0.28 | 23.65 ± 0.15 | 25.31 ± 0.55 | 19.94 ± 0.13 | - | - |
| 12 | 26.61 ± 0.20 | 15.69 ± 0.17 | 22.76 ± 0.69 | 17.09 ± 0.20 | - | - |
| 13 | 92.48 ± 1.89 | 37.46 ± 0.56 | 86.61 ± 2.84 | 42.18 ± 0.69 | - | - |
| 14 | 61.43 ± 1.56 | 28.30 ± 0.82 | 60.22 ± 1.06 | 30.06 ± 0.55 | - | - |
| 15 | 80.78 ± 1.02 | 35.61 ± 0.39 | 93.05 ± 1.89 | 44.79 ± 0.65 | - | - |
| 16 | 63.49 ± 1.07 | 42.12 ± 1.21 | 58.22 ± 0.66 | 30.74 ± 1.28 | - | - |
| Type of Sample | ABTS [µmol Trolox/ g CS d.m.] | FRAP [µmol Fe(II)/g CS d.m.] | TPC [µmol GAE/ g CS d.m.] | BI [Abs420] | Caffeine [mg/g CS d.m.] | 3−CQA [mg/g CS d.m.] |
|---|---|---|---|---|---|---|
| 1 | 54.63 ± 0.54 | 90.50 ± 2.88 | 38.73 ± 0.56 | 0.401 ± 0.006 | 5.16 ± 0.26 | 0.324 ± 0.009 |
| 2 | 64.57 ± 1.29 | 124.24 ± 1.05 | 49.29 ± 0.57 | 0.364 ± 0.003 | 6.09 ± 0.02 | 0.428 ± 0.002 |
| 3 | 72.98 ± 1.24 | 139.94 ± 2.48 | 43.88 ± 1.61 | 0.201 ± 0.006 | 6.60 ± 0.01 | 0.356 ± 0.001 |
| 4 | 90.51 ± 1.10 | 183.15 ± 0.74 | 54.37 ± 1.68 | 0.187 ± 0.004 | 6.75 ± 0.06 | 0.415 ± 0.003 |
| 5 | 62.22 ± 0.96 | 131.25 ± 1.37 | 43.46 ± 0.76 | 0.260 ± 0.007 | 6.34 ± 0.06 | 0.401 ± 0.003 |
| 6 | 73.68 ± 0.86 | 138.29 ± 1.37 | 41.07 ± 0.64 | 0.202 ± 0.006 | 6.77 ± 0.27 | 0.383 ± 0.001 |
| 7 | 37.22 ± 0.34 | 65.77 ± 0.44 | 24.25 ± 0.33 | 0.373 ± 0.006 | 3.23 ± 0.06 | 0.292 ± 0.005 |
| 8 | 82.42 ± 2.49 | 161.87 ± 2.30 | 52.67 ± 1.72 | 0.233 ± 0.005 | 6.67 ± 0.10 | 0.200 ± 0.002 |
| 9 | 101.67 ± 0.94 | 130.69 ± 0.71 | 50.31 ± 1.11 | 0.297 ± 0.005 | 5.94 ± 0.06 | 0.447 ± 0.004 |
| 10 | 102.63 ± 0.92 | 132.55 ± 0.71 | 51.04 ± 1.31 | 0.297 ± 0.013 | 6.15 ± 0.06 | 0.430 ± 0.005 |
| Tested RSM Response | Simplified Fitted Function | R2 | Model p-Value | Lack-of-Fit p-Value |
|---|---|---|---|---|
| ABTS assay | −47.02 + 1.74x − 0.02x2 + 4.37y − 0.05y2 + 0.003xy | 0.96 | 0.0051 | 0.3510 |
| FRAP assay | 35.35 + 0.40x − 0.002x2 + 2.53y − 0.02y2 + 0.004xy | 0.79 | 0.0081 | 0.1834 |
| TPC assay | −0.61 + 0.46x − 0.001x2 + 1.57y − 0.01y2 − 0.01xy | 0.53 | 0.0344 | 0.1073 |
| BI (Abs420) | 0.43 + 0.002x − 3 × 10−5x2 − 0.005y + 2 × 10−5y2 + 6 × 10−6xy | 0.91 | 0.0037 | 0.4172 |
| Caffeine (HPLC) | 2.81 + 0.01x − 7 × 10−5x2 + 0.11y − 0.001y2 + 2 × 10−4xy | 0.59 | 0.0473 | 0.0981 |
| 3-CQA (HPLC) | 0.01 + 0.002x – 2 × 10−5x2 + 0.01y – 5 × 10−5y2 − 10−5xy | 0.77 | 0.0105 | 0.2376 |
| Tested RSM Response | Approximated Value | Actual Value | Deviation [%] |
|---|---|---|---|
| ABTS [µmol Trolox/g CS d.m.] | 101.86 | 101.6 | −0.25 |
| FRAP [µmol Fe(II)/g CS d.m.] | 131.96 | 132.3 | 0.25 |
| TPC [µmol GAE/g CS d.m.] | 50.86 | 52.3 | 2.83 |
| BI (Abs420) | 0.30 | 0.29 | −3.33 |
| Caffeine [mg/g CS d.m.] | 6.04 | 5.9 | −2.32 |
| 3-CQA [mg/g CS d.m.] | 0.21 | 0.22 | 4.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, R.; Wirkowska-Wojdyła, M.; Piasecka, I.; Górska, A. Application of Response Surface Methodology to Optimize the Extraction Process of Bioactive Compounds Obtained from Coffee Silverskin. Appl. Sci. 2023, 13, 5388. https://doi.org/10.3390/app13095388
Brzezińska R, Wirkowska-Wojdyła M, Piasecka I, Górska A. Application of Response Surface Methodology to Optimize the Extraction Process of Bioactive Compounds Obtained from Coffee Silverskin. Applied Sciences. 2023; 13(9):5388. https://doi.org/10.3390/app13095388
Chicago/Turabian StyleBrzezińska, Rita, Magdalena Wirkowska-Wojdyła, Iga Piasecka, and Agata Górska. 2023. "Application of Response Surface Methodology to Optimize the Extraction Process of Bioactive Compounds Obtained from Coffee Silverskin" Applied Sciences 13, no. 9: 5388. https://doi.org/10.3390/app13095388





