Photo-Fermentative Bacteria Used for Hydrogen Production
Abstract
:1. Introduction
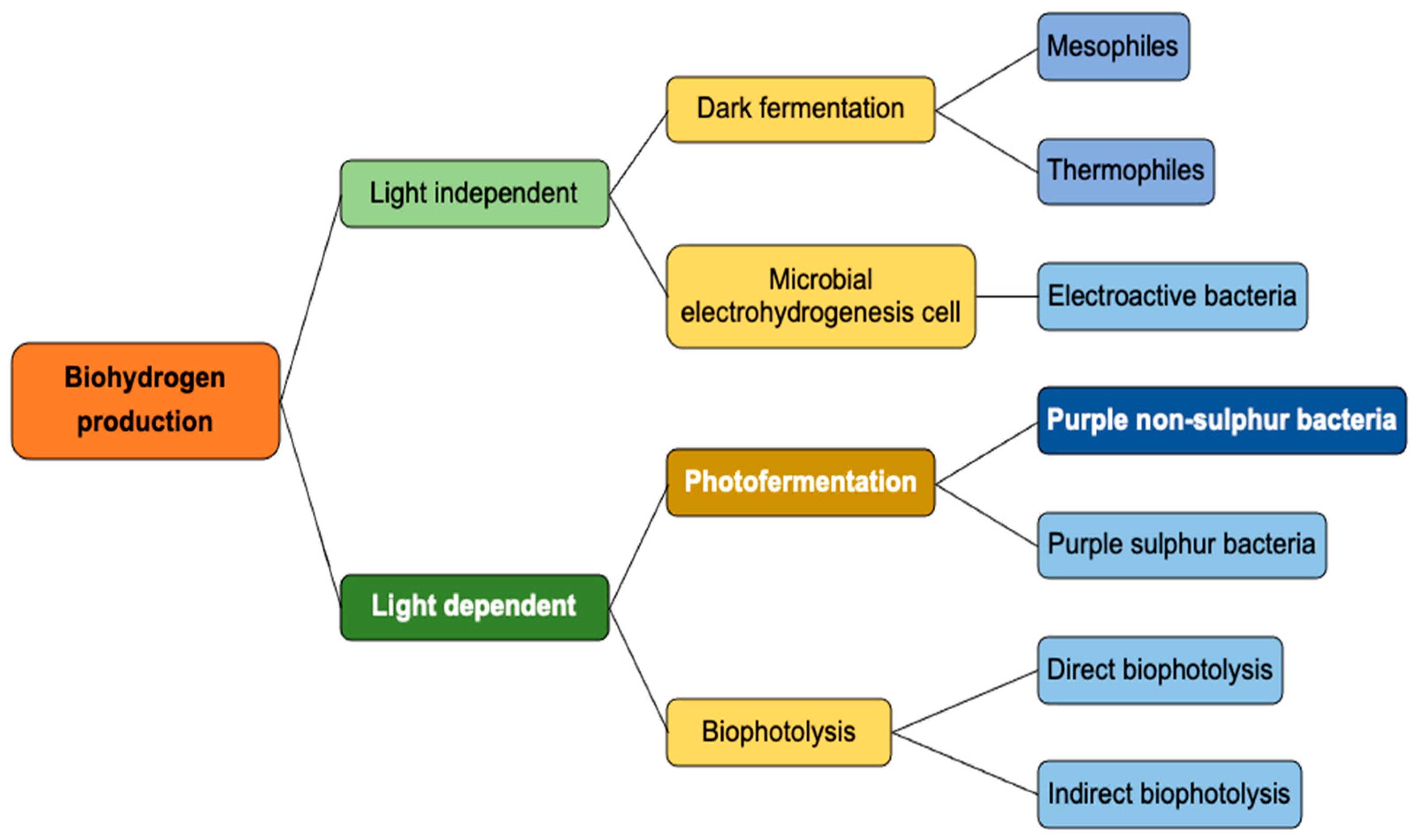
2. Photo-Fermentative Bacteria for Hydrogen Production
2.1. Metabolic Pathway of Photo-Fermentative Bacteria
2.2. Rhodopseudomonas
2.2.1. Rhodopseudomonas palustris
2.2.2. Rhodopseudomonas capsulata
2.3. Rhodobacter
2.3.1. Rhodobacter capsulatus
2.3.2. Rhodobacter sphaeroides
2.4. Rhodospirillum rubrum
2.5. Rhodovulum sulfidophilum
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, W.-C.; Park, J.-Y. Hydrogen gas generation of metal-activated carbon for fuel cells. J. Ind. Eng. Chem. 2007, 13, 578–584. [Google Scholar]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment. Renew. Sust. Energ. Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
- Nortez, K.B.; Movillon, J.L.; Alfafara, C.G.; Sevilla-Nastor, J.B.; Ventura, R.L.G.; Ventura, J.R.S. Optimization of photo-fermentative biohydrogen production in a mixed volatile fatty acid medium by Rhodobacter sp. MAY2: A response surface methodology (RSM) approach. Int. J. Hydrogen Energy 2024, 56, 844–852. [Google Scholar] [CrossRef]
- Jeong, T.Y.; Cha, G.C.; Yeom, S.H.; Choi, S.S. Comparison of hydrogen production by four representative hydrogen-producing bacteria. J. Ind. Eng. Chem. 2008, 14, 333–337. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Ivanenko, A.A.; Laikova, A.A.; Zhuravleva, E.A.; Shekhurdina, S.V.; Vishnyakova, A.V.; Kovalev, A.A.; Kovalev, D.A.; Trchounian, K.A.; Litti, Y.V. Biological production of hydrogen: From basic principles to the latest advances in process improvement. Int. J. Hydrogen Energy 2024, 55, 740–755. [Google Scholar] [CrossRef]
- Vargas, S.R.; Macêdo, W.V.; Trindade, L.F.; Zaiat, M. Influence of organic carbon source on hydrogen production and nutrient removal by microbial consortium in anaerobic photobioreactors. Int. J. Hydrogen Energy 2024, 54, 1160–1168. [Google Scholar] [CrossRef]
- Ren, N.Q.; Liu, B.F.; Ding, J.; Guo, W.Q.; Cao, G.L.; Xie, G.J. The effect of butyrate concentration on photo-hydrogen production from acetate by Rhodopseudomonas faecalis RLD-53. Int. J. Hydrogen Energy 2008, 33, 5981–5985. [Google Scholar] [CrossRef]
- Reungsang, A.; Zhong, N.; Yang, Y.; Sittijunda, S.; Xie, A.; Liao, Q. Hydrogen from Photo Fermentation. In Bioreactors for Microbial Biomass and Energy Conversion; Liao, Q., Chang, J., Herrmann, C., Xia, A., Eds.; Springer Nature: Singapore, 2018; pp. 221–317. [Google Scholar] [CrossRef]
- Xie, G.J.; Liu, B.F.; Ding, J.; Ren, H.Y.; Xing, D.F.; Ren, N.Q. Hydrogen production by photo-fermentative bacteria immobilized on fluidized bio-carrier. Int. J. Hydrogen Energy 2011, 36, 13991–13996. [Google Scholar] [CrossRef]
- Genç, Ş.; Koku, H. A preliminary techno-economic analysis of photo-fermentative hydrogen production. Int. J. Hydrogen Energy 2024, 52, 212–222. [Google Scholar] [CrossRef]
- Tiang, M.F.; Hanipa, M.A.; Abdul, P.M.; Jahim, J.M.; Mahmod, S.S.; Takriff, M.S.; Lay, C.; Resunsang, A.; Wu, S.Y. Recent advanced biotechnological strategies to enhance photo-fermentative biohydrogen production by purple non-sulfur bacteria: An overview. Int. J. Hydrogen Energy 2020, 45, 13211–13230. [Google Scholar] [CrossRef]
- Monir, M.U.; Aziz, A.A.; Ahmed, M.T.; Hasan, M.Y. Hydrogen energy–potential in developing countries. In Renewable Energy and Sustainability; Khan, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 299–325. [Google Scholar] [CrossRef]
- Gest, H.; Kamen, M.D. Photoproduction of molecular hydrogen by Rhodospirillum rubrum. Science 1949, 109, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.S. Degradation of aromatic compounds by purple nonsulfur bacteria. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 577–594. [Google Scholar] [CrossRef]
- Assawamongkholsiri, T.; Reungsang, A. Photo-fermentational hydrogen production of Rhodobacter sp. KKU-PS1 isolated from an UASB reactor. Electron. J. Biotechnol. 2015, 18, 221–230. [Google Scholar] [CrossRef]
- Tao, Y.; He, Y.; Wu, Y.; Liu, F.; Li, X.; Zong, W.; Zhou, Z. Characteristics of a new photosynthetic bacterial strain for hydrogen production and its application in wastewater treatment. Int. J. Hydrogen Energy 2008, 33, 963–973. [Google Scholar] [CrossRef]
- Obeid, J.; Flaus, J.M.; Adrot, O.; Magnin, J.P.; Willison, J.C. State estimation of a batch hydrogen production process using the photosynthetic bacteria Rhodobacter capsulatus. Int. J. Hydrogen Energy 2010, 35, 10719–10724. [Google Scholar] [CrossRef]
- Liu, B.F.; Jin, Y.R.; Cui, Q.F.; Xie, G.J.; Wu, Y.N.; Ren, N.Q. Photo-fermentation hydrogen production by Rhodopseudomonas sp. nov. strain A7 isolated from the sludge in a bioreactor. Int. J. Hydrogen Energy 2015, 40, 8661–8668. [Google Scholar] [CrossRef]
- Bianchi, L.; Mannelli, F.; Viti, C.; Adessi, A.; De, P.R. Hydrogen-producing purple non-sulfur bacteria isolated from the trophic lake Averno (Naples, Italy). Int. J. Hydrogen Energy 2010, 35, 12216–12223. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Aghbashlo, M.; Tabatabaei, M.; Younesi, H.; Najafpour, G. Exergy analysis of biohydrogen production from various carbon sources via anaerobic photosynthetic bacteria (Rhodospirillum rubrum). Energy 2015, 93, 730–739. [Google Scholar] [CrossRef]
- Cai, J.; Wang, G. Hydrogen production by a marine photosynthetic bacterium, Rhodovulum sulfidophilum P5, isolated from a shrimp pond. Int. J. Hydrogen Energy 2012, 37, 15070–15080. [Google Scholar] [CrossRef]
- Vignais, P.M.; Billoud, B.; Meyer, J. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev. 2001, 25, 455–501. [Google Scholar] [CrossRef]
- Larimer, F.W.; Chain, P.; Hauser, L.; Lamerdin, J.; Malfatti, S.; Do, L.; Land, M.L.; Pelletier, D.A.; Beatty, J.T.; Lang, A.S.; et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 2004, 22, 55–61. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Rensing, C.; Ye, J.; Nealson, K.H.; Zhou, S. Syntrophic interspecies electron transfer drives carbon fixation and growth by Rhodopseudomonas palustris under dark, anoxic conditions. Sci. Adv. 2023, 7, 1852. [Google Scholar] [CrossRef]
- Androga, D.D.; Özgür, E.; Eroglu, I.; Gündüz, U.; Yücel, M. Photo-fermentative hydrogen production in outdoor conditions. In Hydrogen Energy—Challenges and Perspectives; IntechOpen Limited: London, UK, 2012; pp. 77–120. [Google Scholar] [CrossRef]
- Imhoff, J.F. Anoxygenic phototrophic bacteria from extreme environments. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Hallenbeck, P.C., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 427–480. [Google Scholar] [CrossRef]
- Surachat, K.; Kantachote, D.; Deachamag, P.; Wonglapsuwan, M. In silico genomic analysis of Rhodopseudomonas palustris strains revealed potential biocontrol agents and crop yield enhancers. Biol. Control 2022, 176, 105085. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liao, Q.; Zhu, X.; Li, J.; Lee, D.J. Effect of culture conditions on the kinetics of hydrogen production by photosynthetic bacteria in batch culture. Int. J. Hydrogen Energy 2011, 36, 14004–14013. [Google Scholar] [CrossRef]
- Wu, X.M.; Zhu, L.Y.; Zhu, L.Y.; Wu, L. Improved ammonium tolerance and hydrogen production in nifA mutant strains of Rhodopseudomonas palustris. Int. J. Hydrogen Energy 2016, 41, 22824–22830. [Google Scholar] [CrossRef]
- Sagir, E.; Alipour, S. Photo-fermentative hydrogen production by immobilized photosynthetic bacteria: Current perspectives and challenges. Renew. Sust. Energ. Rev. 2021, 141, 110796. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Ma, Y.; Huang, H.; Wang, G.; Ma, S.; Li, Z.; Han, M. Improving photo-fermentative hydrogen productivity of photosynthetic bacteria using a formulated Fe and Mo metal supplemented lignocellulosic substrate. Int. J. Hydrogen Energy 2024, 49, 516–531. [Google Scholar] [CrossRef]
- Guo, C.L.; Zhu, X.; Liao, Q.; Wang, Y.Z.; Chen, R.; Lee, D.J. Enhancement of photo-hydrogen production in a biofilm photobioreactor using optical fiber with additional rough surface. Bioresour. Technol. 2011, 102, 8507–8513. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Li, Y.; Zhong, N.; Liao, Q.; Huang, Y.; Xia, A.; Zhu, X.; Hou, Y. A novel biofilm photobioreactor using light guide plate enhances the hydrogen production, Int. J. Hydrogen Energy 2017, 42, 27523–27531. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, N.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X.; Li, Q. A biomaterial doped with LaB6 nanoparticles as photothermal media for enhancing biofilm growth and hydrogen production in photosynthetic bacteria. Int. J. Hydrogen Energy 2017, 42, 5793–5803. [Google Scholar] [CrossRef]
- Liao, Q.; Zhong, N.; Zhu, X.; Huang, Y.; Chen, R. Enhancement of hydrogen production by optimization of biofilm growth in a photobioreactor. Int. J. Hydrogen Energy 2015, 40, 4741–4751. [Google Scholar] [CrossRef]
- Wen, H.; Cao, G.; Xie, G.; Xing, D.; Yin, T.; Ren, N.; Liu, B. Improved photo-fermentative hydrogen production by biofilm reactor with optimizing carriers and acetate concentration. Int. J. Hydrogen Energy 2019, 44, 25151–25159. [Google Scholar] [CrossRef]
- Basak, N.; Jana, A.K.; Das, D. CFD modeling of hydrodynamics and optimization of photo-fermentative hydrogen production by Rhodopseudomonas palustris DSM 123 in annular photobioreactor. Int. J. Hydrogen Energy 2016, 41, 7301–7317. [Google Scholar] [CrossRef]
- Mabutyana, L.; Pott, R.W. Photo-fermentative hydrogen production by Rhodopseudomonas palustris CGA009 in the presence of inhibitory compounds. Int. J. Hydrogen Energy 2021, 46, 29088–29099. [Google Scholar] [CrossRef]
- Ross, B.S.; Pott, R.W.M. Hydrogen production by immobilized Rhodopseudomonas palustris in packed or fluidized bed photobioreactor systems. Int. J. Hydrogen Energy 2021, 46, 1715–1727. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, N.; Mahbubani, K.T.; del Rio-Chanona, E.A.; Slater, N.K.H.; Vassiliadis, V.S. Bioprocess modelling of biohydrogen production by Rhodopseudomonas palustris: Model development and effects of operating conditions on hydrogen yield and glycerol conversion efficiency. Chem. Eng. Sci. 2015, 130, 68–78. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, X.; Liao, Q.; Wang, Y.; Lee, D. Enhanced hydrogen production by Rhodopseudomonas palustris CQK 01 with ultra-sonication pretreatment in batch culture. Bioresour. Technol. 2011, 102, 8696–8699. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xie, X.W.; Zhu, X.; Liao, Q.; Chen, R.; Zhao, X.; Lee, D.J. Hydrogen production by Rhodopseudomonas palustris CQK 01 in a continuous photobioreactor with ultrasonic treatment. Int. J. Hydrogen Energy 2012, 37, 15450–15457. [Google Scholar] [CrossRef]
- Azbar, N.; Cetinkaya, D.F.T. The effect of dilution and l-malic acid addition on bio-hydrogen production with Rhodopseudomonas palustris from effluent of an acidogenic anaerobic reactor. Int. J. Hydrogen Energy 2010, 35, 5028–5033. [Google Scholar] [CrossRef]
- Jamil, Z.; Annuar, M.S.M.; Ibrahim, S.; Vikineswary, S. Optimization of phototrophic hydrogen production by Rhodopseudomonas palustris PBUM001 via statistical experimental design. Int. J. Hydrogen Energy 2009, 34, 7502–7512. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liao, Q.; Zhu, X.; Chen, R.; Guo, C.L.; Zhou, J. Bioconversion characteristics of Rhodopseudomonas palustris CQK 01 entrapped in a photobioreactor for hydrogen production. Bioresour. Technol. 2013, 135, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lee, C.M.; Chang, J.S. Hydrogen production by indigenous photosynthetic bacterium Rhodopseudomonas palustris WP3-5 using optical fiber-illuminating photobioreactors. Biochem. Eng. J. 2006, 32, 33–42. [Google Scholar] [CrossRef]
- Oh, Y.K.; Seol, E.H.; Lee, E.Y.; Park, S. Fermentative hydrogen production by a new chemoheterotrophic bacterium Rhodopseudomonas palustris P4. Int. J. Hydrogen Energy 2002, 27, 1373–1379. [Google Scholar] [CrossRef]
- Eroglu, I.; Sevinç, P.; Gündüz, U.; Grube, T.; Stolten, D.; Yücel, M. The Effect of Temperature and Light Intensity on Hydrogen Production by Rhodobacter capsulatus. In Proceedings of the 18th World Hydrogen Energy Conference 2010: WHEC 2010 Proceedings Forschungszentrum Jülich, Essen, Germany, 16–21 May 2010. [Google Scholar]
- Baccarini-Melandri, A.; Melandri, B.A. Partial resolution of the photophosphorylating system of Rhodopseudomonas capsulata. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1971; pp. 556–561. [Google Scholar] [CrossRef]
- Shi, X.Y.; Li, W.W.; Yu, H.Q. Key parameters governing biological hydrogen production from benzoate by Rhodopseudomonas capsulata. Appl. Energy 2014, 133, 121–126. [Google Scholar] [CrossRef]
- Shi, X.Y.; Yu, H.Q. Continuous production of hydrogen from mixed volatile fatty acids with Rhodopseudomonas capsulata. Int. J. Hydrogen Energy 2006, 31, 1641–1647. [Google Scholar] [CrossRef]
- Shi, X.Y.; Yu, H.Q. Conversion of individual and mixed volatile fatty acids to hydrogen by Rhodopseudomonas capsulata. Int. Biodeterior. Biodegrad. 2006, 58, 82–88. [Google Scholar] [CrossRef]
- Shi, X.Y.; Yu, H.Q. Response surface analysis on the effect of cell concentration and light intensity on hydrogen production by Rhodopseudomonas capsulata. Process Biochem. 2005, 40, 2475–2481. [Google Scholar] [CrossRef]
- Suresh, G.; Sasikala, C.; Ramana, C.V. Reclassification of Gemmobacter changlensis to a new genus as Cereibacter changlensis gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 794–798. [Google Scholar] [CrossRef]
- Suresh, G.; Lodha, T.D.; Indu, B.; Sasikala, C.; Ramana, C.V. Taxogenomics resolves conflict in the genus Rhodobacter: A two and half decades pending thought to reclassify the genus rhodobacter. Front. Microbiol. 2019, 10, 2480. [Google Scholar] [CrossRef]
- Basak, N.; Das, D. The prospect of purple non-sulfur (PNS) photosynthetic bacteria for hydrogen production: The present state of the art. World J. Microbiol. Biotechnol. 2007, 23, 31–42. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Guo, L.; Wu, X.; Yang, H. Enhanced photo-fermentative hydrogen production by Rhodobacter capsulatus with pigment content manipulation. Bioresour. Technol. 2012, 118, 490–495. [Google Scholar] [CrossRef]
- Abo-Hashesh, M.; Desaunay, N.; Hallenbeck, P.C. High yield single stage conversion of glucose to hydrogen by photo-fermentation with continuous cultures of Rhodobacter capsulatus JP91. Bioresour. Technol. 2013, 128, 513–517. [Google Scholar] [CrossRef]
- Ghosh, D.; Sobro, I.F.; Hallenbeck, P.C. Optimization of the hydrogen yield from single-stage photo-fermentation of glucose by Rhodobacter capsulatus JP91 using response surface methodology. Bioresour. Technol. 2012, 123, 199–206. [Google Scholar] [CrossRef]
- Elkahlout, K.; Sagir, E.; Alipour, S.; Koku, H.; Gunduz, U.; Eroglu, I.; Yucel, M. Long-term stable hydrogen production from acetate using immobilized Rhodobacter capsulatus in a panel photobioreactor. Int. J. Hydrogen Energy 2019, 44, 18801–18810. [Google Scholar] [CrossRef]
- Boran, E.; Özgür, E.; Van Der Burg, J.; Yücel, M.; Gündüz, U.; Eroglu, I. Biological hydrogen production by Rhodobacter capsulatus in solar tubular photo bioreactor. J. Clean. Prod. 2010, 18, S29–S35. [Google Scholar] [CrossRef]
- Androga, D.D.; Sevinç, P.; Koku, H.; Yücel, M.; Gündüz, U.; Eroglu, I. Optimization of temperature and light intensity for improved photo-fermentative hydrogen production using Rhodobacter capsulatus DSM 1710. Int. J. Hydrogen Energy 2014, 39, 2472–2480. [Google Scholar] [CrossRef]
- Sagir, E.; Alipour, S.; Elkahlout, K.; Koku, H.; Gunduz, U.; Eroglu, I.; Yucel, M. Scale-up studies for stable, long-term indoor and outdoor production of hydrogen by immobilized Rhodobacter capsulatus. Int. J. Hydrogen Energy 2017, 42, 22743–22755. [Google Scholar] [CrossRef]
- Magnin, J.P.; Deseure, J. Hydrogen generation in a pressurized photobioreactor: Unexpected enhancement of biohydrogen production by the phototrophic bacterium Rhodobacter capsulatus. Appl. Energy 2019, 239, 635–643. [Google Scholar] [CrossRef]
- Boran, E.; Özgür, E.; Yücel, M.; Gündüz, U.; Eroglu, I. Biohydrogen production by Rhodobacter capsulatus in solar tubular photobioreactor on thick juice dark fermenter effluent. J. Clean. Prod. 2012, 31, 150–157. [Google Scholar] [CrossRef]
- Boran, E.; Özgür, E.; Yücel, M.; Gündüz, U.; Eroglu, I. Biohydrogen production by Rhodobacter capsulatus Hup− mutant in pilot solar tubular photobioreactor. Int. J. Hydrogen Energy 2012, 37, 16437–16445. [Google Scholar] [CrossRef]
- Koku, H.; Eroğlu, I.; Gündüz, U.; Yücel, M.; Türker, L. Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int. J. Hydrogen Energy 2002, 27, 1315–1329. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Łaniecki, M. Hydrogen production from starch by co-culture of Clostridium acetobutylicum and Rhodobacter sphaeroides in one step hybrid dark- and photo-fermentation in repeated fed-batch reactor. Bioresour. Technol. 2017, 224, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Pattanamanee, W.; Choorit, W.; Kantachote, D.; Chisti, Y. Repeated-batch production of hydrogen using Rhodobacter sphaeroides S10. Int. J. Hydrogen Energy 2012, 37, 15855–15866. [Google Scholar] [CrossRef]
- Al-Mohammedawi, H.H.; Znad, H. Impact of metal ions and EDTA on photo-fermentative hydrogen production by Rhodobacter sphaeroides using a mixture of pre-treated brewery and restaurant effluents. Biomass Bioenergy 2020, 134, 105482. [Google Scholar] [CrossRef]
- Hu, J.; Yang, H.; Wang, X.; Cao, W.; Guo, L. Strong pH dependence of hydrogen production from glucose by Rhodobacter sphaeroides. Int. J. Hydrogen Energy 2020, 45, 9451–9458. [Google Scholar] [CrossRef]
- Akroum-Amrouche, D.; Abdi, N.; Lounici, H.; Mameri, N. Effect of physico-chemical parameters on biohydrogen production and growth characteristics by batch culture of Rhodobacter sphaeroides CIP 60.6. Appl. Energy 2011, 88, 2130–2135. [Google Scholar] [CrossRef]
- Krujatz, F.; Härtel, P.; Helbig, K.; Haufe, N.; Thierfelder, S.; Bley, T.; Weber, J. Hydrogen production by Rhodobacter sphaeroides DSM 158 under intense irradiation. Bioresour. Technol. 2015, 175, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Basak, N.; Das, D. Photo-fermentative hydrogen production using purple non-sulfur bacteria Rhodobacter sphaeroides O.U.001 in an annular photobioreactor: A case study. Biomass Bioenergy 2009, 33, 911–919. [Google Scholar] [CrossRef]
- Ma, H.; Zheng, X.; Yang, H. Enhancement on hydrogen production performance of Rhodobacter sphaeroides HY01 by overexpressing fdxN. Int. J. Hydrogen Energy 2018, 43, 17082–17090. [Google Scholar] [CrossRef]
- Al-Mohammedawi, H.H.; Znad, H.; Eroglu, E. Synergistic effects and optimization of photo-fermentative hydrogen production of Rhodobacter sphaeroides DSM 158. Int. J. Hydrogen Energy 2018, 43, 15823–15834. [Google Scholar] [CrossRef]
- Seifert, K.; Waligorska, M.; Laniecki, M. Brewery wastewaters in photobiological hydrogen generation in presence of Rhodobacter sphaeroides O.U. 001. Int. J. Hydrogen Energy 2010, 35, 4085–4091. [Google Scholar] [CrossRef]
- Jaime-Pérez, N.; Kaftan, D.; Bína, D.; Bokhari, S.N.H.; Shreedhar, S.; Küpper, H. Mechanisms of sublethal copper toxicity damage to the photosynthetic apparatus of Rhodospirillum rubrum. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 640–650. [Google Scholar] [CrossRef]
- Dadak, A.; Aghbashlo, M.; Tabatabaei, M.; Younesi, H.; Najafpour, G. Exergy-based sustainability assessment of continuous photobiological hydrogen production using anaerobic bacterium Rhodospirillum rubrum. J. Clean. Prod. 2016, 139, 157–166. [Google Scholar] [CrossRef]
- Maeda, I.; Miyasaka, H.; Umeda, F.; Kawase, M.; Yagi, K. Maximization of hydrogen production ability in high-density suspension of Rhodovulum sulfidophilum cells using intracellular poly(3-hydroxybutyrate) as sole substrate. Biotechnol. Bioeng. 2003, 81, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, G. Photo-biological hydrogen production by an acid-tolerant mutant of Rhodovulum sulfidophilum P5 generated by transposon mutagenesis. Bioresour. Technol. 2014, 154, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Guan, Y.; Jia, T.; Yang, J.; Hu, Y.; Li, P.; Duan, Y.; Zhang, L.; Yu, P. Hydrogen production from high slat medium by co-culture of Rhodovulum sulfidophilum and dark fermentative microflora. Int. J. Hydrogen Energy 2018, 43, 10959–10966. [Google Scholar] [CrossRef]
- Keskin, T.; Hallenbeck, P.C. Hydrogen production from sugar industry wastes using single-stage photo-fermentation. Bioresour. Technol. 2012, 112, 131–136. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Chapter 7—Photo-fermentative Biohydrogen Production. In Biohydrogen; Pandey, A., Chang, J.S., Hallenbecka, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 145–159. [Google Scholar] [CrossRef]



| Ref. | Strain | Substrate (Concentration, mM) | Conditions | Bioreactor | Light Source | Irradiance (W/m2) | Hydrogen Production Rate (mM/h) |
|---|---|---|---|---|---|---|---|
| [33] | CQK 01 | Glucose (56) | pH 7.0, 30 °C | PBR made of polymethyl methacrylate (PMMA), continuous | Tungsten lamp | 31.6 | 1.75 |
| [34] | CQK 01 | Glucose (56) | pH 7.0, 25 °C | PBR 8 × 200 × 200 mm3 of PMMA, continuous | Light guided plate | 10.1 | 1.1 |
| SiO2 chitosan-medium-LGP | 7.8 | 1.4 | |||||
| [35] | CQK 01 | Glucose (56) | pH 7.0, 30 °C | Nanobiofilm of LaB6 powder | LED | 145.7 | 0.12 |
| [36] | CQK 01 | Glucose (55) | pH 7.0, 26.5–31.5 °C | Cylindrical PBR of PMMA with chitosan-medium coated optical fiber | Tungsten lamp | 31.6 | 2.72 |
| [37] | A7 | Acetate (68) | pH 7.0, 35 °C | Anaerobic PBR | Tungsten lamp | 1.05 | |
| [38] | DSM 123 | Malate (20) | pH 6.8, 33 °C | Triple-jacketed vertical annular PBR (1 L) | Tungsten lamp | 15 ± 1.1 | 0.31 |
| [39] | CGA009 | Glycerol (10) | pH 7.0, 35 °C | 250 mL glass bottles (200 mL working volume) | Tungsten lamp | 200 ± 5 | 0.25 |
| [40] | NCIMB 11774 | Glycerol (50) | pH 7.0, 28 ± 1 °C | Fluidized bed PBR (1 L) | Tungsten lamp | 100 | 3.23 |
| [41] | NCIB 11774 | Glycerol (10) | 25 ± 2 °C | Piecewise droop model, batch | Tungsten lamp | 174 | 1.54 |
| [42] | CQK 01 | Glucose (50) | pH 7.0, 30 °C | PBR of PMMA 100 × 50 × 100 mm3 | Monochromatic LED | 47.4 | 0.54 |
| [43] | CQK 01 | Glucose (50) | pH 7.0, 30 °C | PBR 120 × 60 × 190 mm3 | Monochromatic LED | 47.4 | 0.82 |
| [44] | DSM 127 | L-malic acid (30), 50% L-malic acid and 50% raw effluent | pH 6.9, 31 °C | 250 mL serum bottles (200 mL working volume), batch | Halogen lamp | 47.4 | 0.35 |
| [45] | PBUM001 | Palm oil mill effluent (POME) | pH 7.0, 30 °C | 121 mL serum bottles (50 mL working volume) | Tungsten lamp | 31.6 | 0.44 |
| [46] | CQK 01 | Glucose (44.7) | pH 7.0, 30 °C | Flat panel PBR 100 × 40 × 200 mm3, continuous | LEDs | 47.4 | 2.5 |
| [47] | WP3–5 | Acetate (14.8) | pH 7.1, 32 °C | Sealed glass vessel (500 mL) | Tungsten/halogen lamp | 95 | 0.76 |
| [48] | P4 | Glucose (27.8) | pH 7.0, 30 °C | 165 mL serum bottle (50 mL working volume), batch | Not described | Not described | 3.2 |
| Ref. | Substrate (Concentration, mM) | Conditions | PBR | Light Source | Irradiance (W/m2) | Hydrogen Production Rate (mM/h) |
|---|---|---|---|---|---|---|
| [51] | Benzoate (5) | pH 7.0, 30 °C | 150 mL glass PBR | Tungsten lamp | 31.6 | 0.36 |
| [52] | Acetate (30) + propionate (3) + butyrate (12) | pH 6.8, 35 °C | 1500 mL, continuous | Tungsten lamp | 39.5 | 0.56 |
| [53] | Individually and mixture: acetate (32) + propionate (5) + butyrate (10) | pH 6.8, 32 °C | Glass vials (300 mL working volume) | Not described | 31.6 | 0.79 (acetate), 0,71 (propionate), 0.26 (butyrate), 0.65 (mixture) |
| [54] | Acetate (30) + propionate (3) + butyrate (11) | pH 7.0, 31 °C | 300 mL glass PBR | Tungsten lamp | 39.5 | 0.31 |
| Ref. | Strain | Substrate (Concentration, mM) | Conditions | PBR | Light Source | Irradiance (W/m2) | Hydrogen Production Rate (mM/h) |
|---|---|---|---|---|---|---|---|
| [59] | JP91 | Glucose (56) | pH 6.8, 30 °C | 350 mL, continuous | Tungsten lamp | Not described | 1.6–2.3 |
| [60] | JP91 | Glucose (35) | pH 6.8, 30 °C | 100 mL serum bottles, batch | Tungsten lamp | 175 | 2.6 |
| [61] | DSM 1710, YO3 | Acetate (60) | pH 6.7, 30–32 °C | 1.4 L PMMA reactor, batch | Tungsten lamp | 200 | 0.75–1.3 |
| [62] | DSM 1710 | Acetic acid (40) | pH < 8, 10–35 °C | 80 L tubular PBR fed-batch | Artificial light source | >90 | 0.52 |
| [63] | DSM 1710 | Lactic acid (7.5) | pH 6.4, 27.5 °C | 55 mL glass bottle | Tungsten lamp | 287 | 0.566 |
| [64] | YO3 | Sucrose (5 indoors, 10 outdoors) | pH 7.5, 30 °C | 3.64 L PBR of two PMMA with PVC frame, batch | Tungsten lamps (indoors), sunlight (outdoors) | 200 | 0.73 (indoors), 0.87 (outdoors) |
| [65] | Wild type B10 | Lactate (35) | pH 6.9, 30 °C | 350 mL cylindrical glass vessel | Na lamps, LEDs | 812, 479 | 0.26–0.46 |
| [66] | DSM 1710 | Acetate (20) | pH < 8, <35 °C | Tubular PBR (90 L), fed-batch | Halogen lamp | 200 | 0.15 |
| [67] | MT1131 | Acetate (20) | pH < 8, <30 °C | Tubular PBR (90 L), fed-batch | Halogen lamp | 200 | 0.20 |
| Ref. | Strain | Substrate (Concentration, mM) | Conditions | PBR | Light Source | Irradiance (W/m2) | Hydrogen Production Rate (mM/h) |
|---|---|---|---|---|---|---|---|
| [69] | O.U.001 | Acetic (4) + butyric acid (6) | pH 7.0, 32 °C | Batch mode and fed-batch | Ultra-Vitalux lamps (Osram, Premstaetten, Austria) | 192 | 0.89 |
| [70] | S10 | Oil palm + mixture of glucose (36.13), xylose (102.56), acetic acid (48.81) | pH 7.0, 35 °C | 500 mL serum cylinder reactors, batch | Tungsten lamps | 79 | 1.4 |
| [71] | DSM 158 | Brewery effluent (30%) | pH 7.2, 30 °C | 120 mL clear glass | Halogen lamp | 126 | 0.74 |
| Restaurant effluent (70%) | pH 7.6, 30 °C | ||||||
| [72] | HY01 | Glucose (40) | pH 6.9, 35 °C | 350 mL | Halogen lamp | 31.6 | 5.6 |
| [73] | CIP 60.6 | Lactate (50) | pH 7.5, 30 °C | Column reactor | Tungsten lamp | 35.6 | 1.8 |
| [74] | DSM 158 | Lactic acid (40) | pH 7.0, 30 °C | Single-walled glass vessel, batch | Halogen lamp | 2250 | 8.7 |
| 20 L feed tank, continuous | 7.4 | ||||||
| [75] | O.U.001 | DL malic acid (15) | pH 6.8, 33 °C, | Triple-jacketed vertical glass PBR (1 L) | Lumine tubular light (Xiamen, China) | 15 | 0.29 |
| [76] | HY01 | Acetate (25) + butyrate (34) | pH 7.0–8.2, 30 °C | 30 mL syringes, batch | Tungsten lamp | 31.6 | 7.0 |
| [77] | DSM 158 | DL malic acid (7.5) | pH 6.5–8.0, 30 °C | 120 mL glass PBR, batch | Halogen lamp | 35–185 | 1.9 |
| [78] | O.U.001 | L-malic acid (15) | pH 7.0–7.2, 28 °C | 25 mL sodium glass vials | Mercury-tungsten lamp | 116 | 1.2 |
| Biebl and Pfenning media + 20% wastewater | 1.1 |
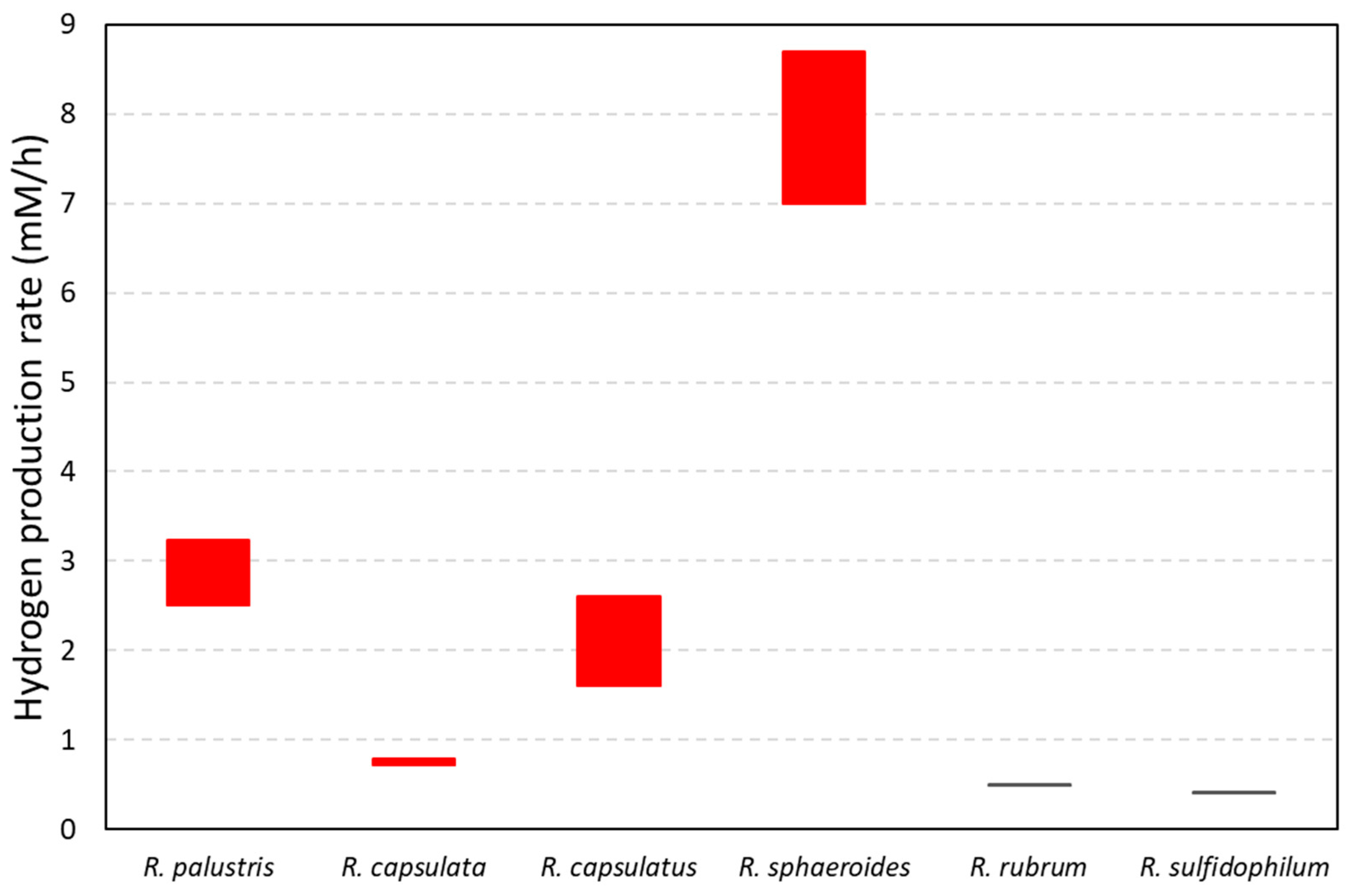
| Ref. | Bacteria | Strain | Substrate (mM) | Maximum Hydrogen Production Rate (mM/h) |
|---|---|---|---|---|
| [36] | R. palustris | CQK01 | Glucose (55) | 2.72 |
| [40] | NCIMB 11774 | Glycerol (50) | 3.23 | |
| [46] | CQK01 | Glucose (44.7) | 2.5 | |
| [48] | P4 | Glucose (27.8) | 3.2 | |
| [53] | R. capsulata | Acetate (32) | 0.79 | |
| Propionate (5) | 0.71 | |||
| [59] | R. capsulatus | JP91 | Glucose (56) | 1.6–2.3 |
| [60] | JP91 | Glucose (35) | 2.6 | |
| [74] | R. sphaeroides | DSM 158 | Lactic acid (40) | 8.7 |
| [76] | HY01 | Acetate (25) + butyrate (34) | 7.0 | |
| [21] | R. rubrum | Acetate | 0.50 | |
| [83] | R. sulfidophilum | TH-102 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. https://doi.org/10.3390/app14031191
Gupta S, Fernandes A, Lopes A, Grasa L, Salafranca J. Photo-Fermentative Bacteria Used for Hydrogen Production. Applied Sciences. 2024; 14(3):1191. https://doi.org/10.3390/app14031191
Chicago/Turabian StyleGupta, Soumya, Annabel Fernandes, Ana Lopes, Laura Grasa, and Jesús Salafranca. 2024. "Photo-Fermentative Bacteria Used for Hydrogen Production" Applied Sciences 14, no. 3: 1191. https://doi.org/10.3390/app14031191





