Preparation and Characterization of Soluble Dextrin Fibre from Potato Starch Obtained on a Semi-Industrial Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
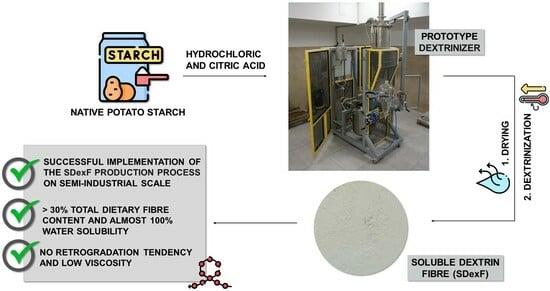
2.2. Preparation of SDexF on a Semi-Industrial Scale
2.3. Total Dietary Fibre Content (TDF)
2.4. Solubility in Water
2.5. Dextrose Equivalent (DE)
2.6. Quantitive Determination of Residual Citric Acid and Ethanol
2.7. The Nutrient Content and Energy Value
2.8. Assessing the Presence of Contaminants
2.9. Fourier Transform Infrared (FT-IR) Spectroscopy
2.10. Scanning Electron Microscopy (SEM)
2.11. Pasting Properties
2.12. Retrogradation Tendency
2.13. Colour Parameters
2.14. Statistical Analysis
3. Results and Discussion
3.1. Preparation of SDexF on a Semi-Industrial Scale
3.2. Total Dietary Fibre Content in SDexF
3.3. Solubility in Water of SDexF
3.4. Dextrose Equivalent (DE) of SDexF
3.5. Quantitive Determination of Citric Acid and Ethanol in SDexF
3.6. The Nutrient Content and Energy Value of SDexF
3.7. Assesing the Presence of Contaminants in SDexF
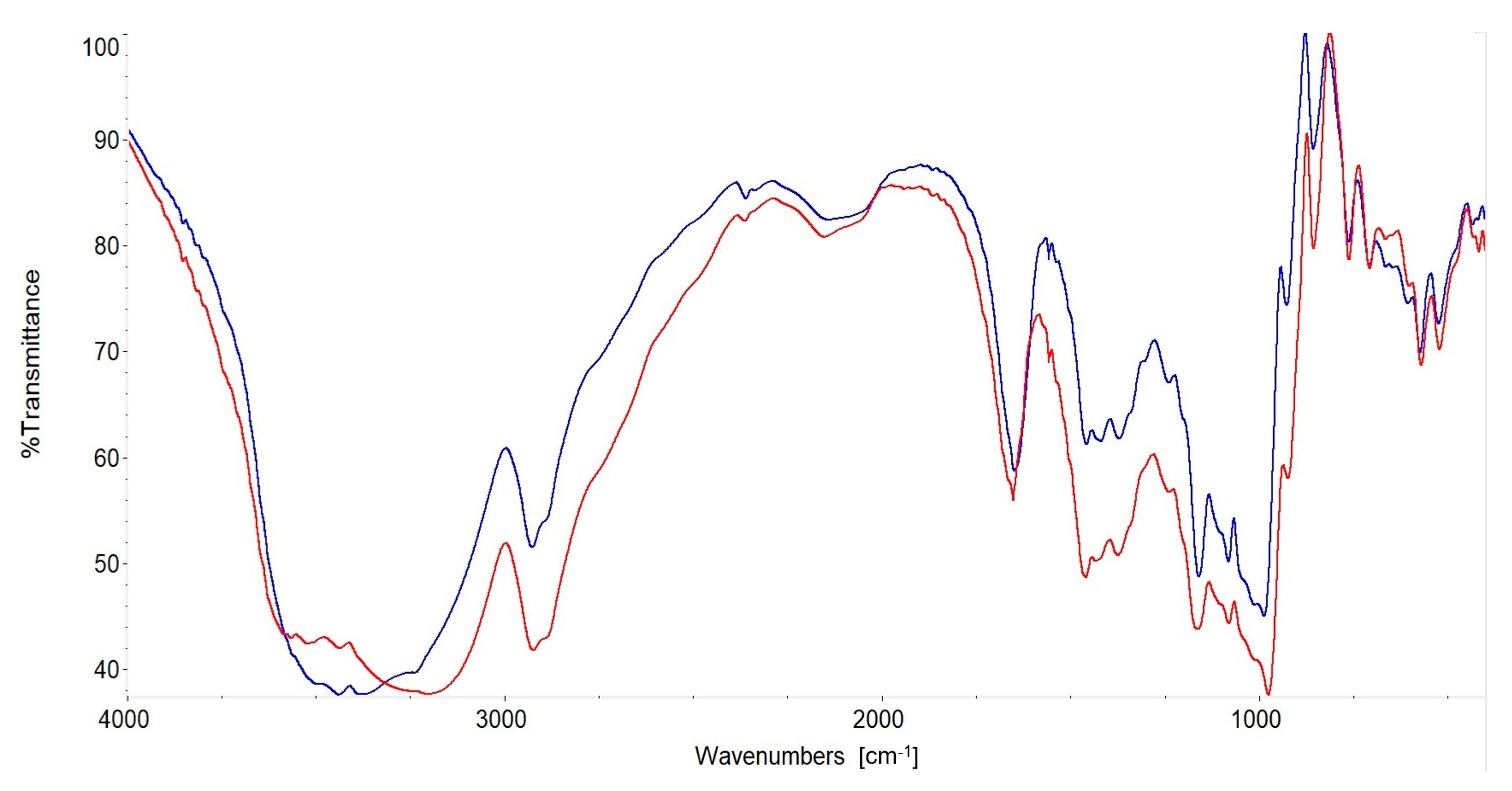
3.8. Fourier Transform Infrared (FT-IR) Spectra of SDexF
3.9. Scanning Electron Microscopy (SEM) of SDexF
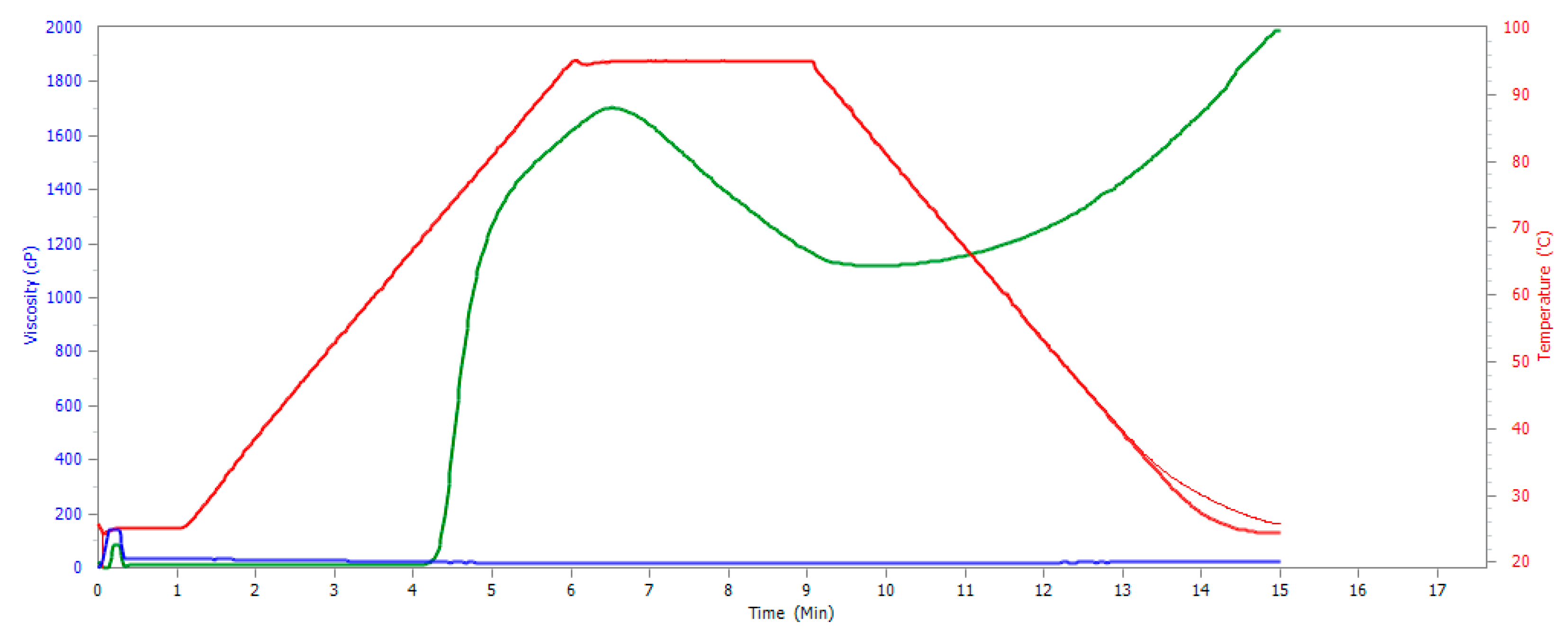
3.10. Pasting Properties of SDexF
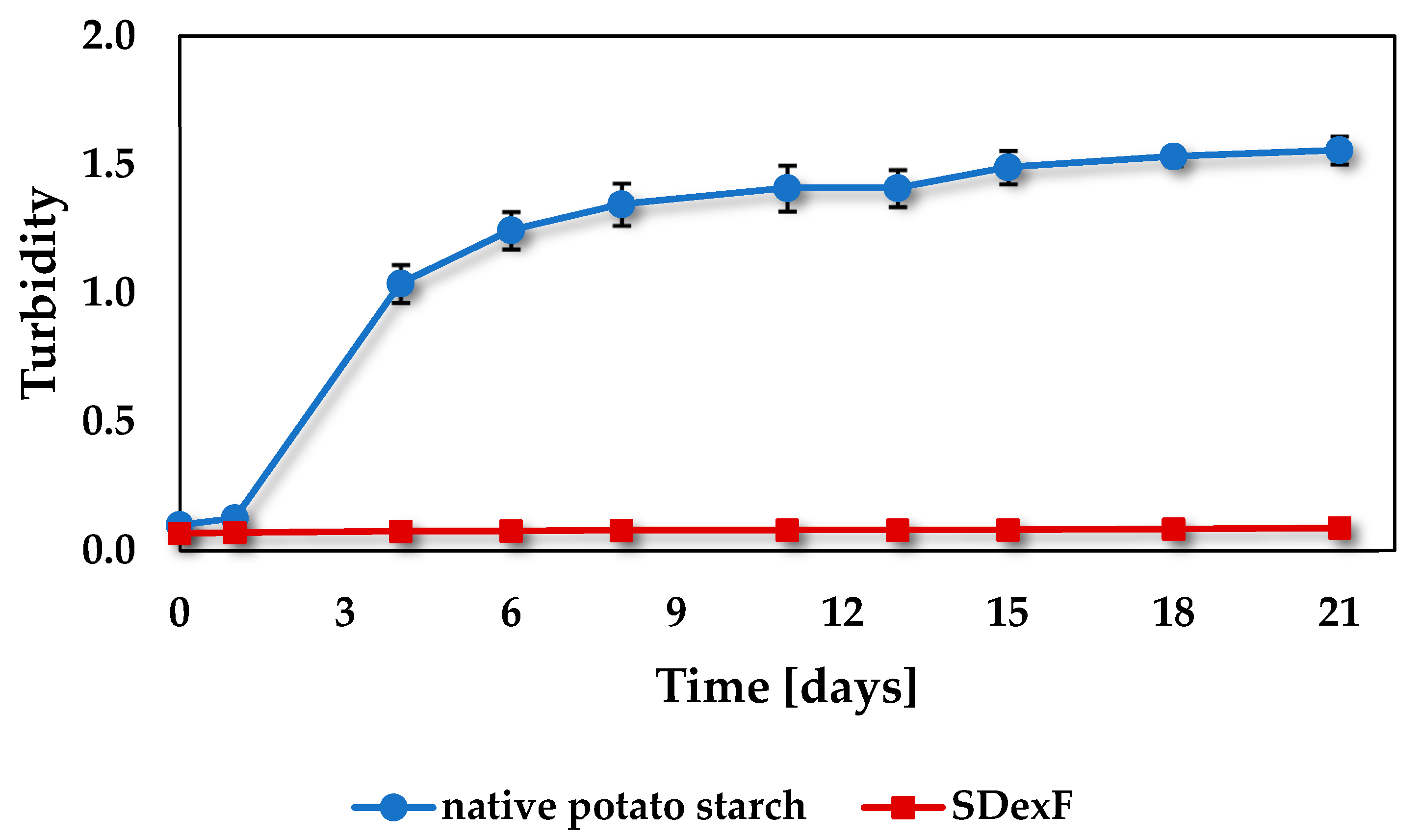
3.11. Retrogradation Tendency of SDexF
3.12. Colour Parameters of SDexF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabete Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Prasadi, N.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.-E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Arumugam, V.; Haugabrooks, E.; Williamson, P.; Hendrich, S. Soluble dietary fiber (Fibersol-2) decreased hunger and increased satiety hormones in humans when ingested with a meal. Nutr. Res. 2015, 35, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, K.S.; Dussort, P.; Erkner, A.; Fiszman, S.; Karnik, K.; Kristensen, M.; Marsaux, C.F.; Miquel-Kergoat, S.; Pentikäinen, S.P.; Putz, P.; et al. A review of the characteristics of dietary fibers relevant to appetite and energy intake outcomes in human intervention trials. Am. J. Clin. Nutr. 2017, 106, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Dayib, M.; Larson, J.; Slavin, J. Dietary fibers reduce obesity-related disorders: Mechanisms of action. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chu, Q.; Ma, S.; Ma, H.; Song, H. Dietary Fiber and Its Potential Role in Obesity: A Focus on Modulating the Gut Microbiota. J. Agric. Food Chem. 2023, 71, 14853–14869. [Google Scholar] [CrossRef] [PubMed]
- Seljak, B.K.; Valenčič, E.; Hristov, H.; Hribar, M.; Lavriša, Ž.; Kušar, A.; Žmitek, K.; Krušič, S.; Gregorič, M.; Blaznik, U.; et al. Inadequate Intake of Dietary Fibre in Adolescents, Adults, and Elderlies: Results of Slovenian Representative SI. Menu Study. Nutrients 2021, 13, 3826. [Google Scholar] [CrossRef]
- Storey, M.; Anderson, P. Income and race/ethnicity influence dietary fiber intake and vegetable consumption. Nutr. Res. 2014, 34, 844–850. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Diep Pham, H.T.; Montez, J.; Mann, J. Dietary fibre intake in childhood or adolescence and subsequent health outcomes: A systematic review of prospective observational studies. Diabetes Obes. Metab. 2020, 22, 2460–2467. [Google Scholar] [CrossRef]
- McKeown, N.M.; Fahey, G.C., Jr.; Slavin, J.; van der Kamp, J.W. Fibre intake for optimal health: How can healthcare professionals support people to reach dietary recommendations? BMJ 2022, 378, e054370. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Jochym, K.; Kapusniak, J.; Barczynska, R.; Sliżewska, K. New starch preparations resistant to enzymatic digestion. J. Sci. Food Agric. 2012, 92, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.; Lambert, J.E.; Parnell, J.A.; Tunnicliffe, J.M.; Nicolucci, A.C.; Han, J.; Sturzenegger, T.; Shearer, J.; Mickiewicz, B.; Vogel, H.J.; et al. Impact of dietary fiber supplementation on modulating microbiota-host-metabolic axes in obesity. J. Nutr. Biochem. 2019, 64, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Kapusniak, J.; Jochym, K.; Barczynska, R.; Slizewska, K.; Libudzisz, Z. Preparation and characteristics of novel enzyme-resistant chemically modified dextrins from potato starch. Zesz. Probl. Postępów Nauk. Rol. 2008, 530, 427–444, (In Polish, with English abstract). [Google Scholar]
- Kapusniak, J.; Kapusniak, K.; Ptak, S.; Zarski, A.; Barczynska, R. Products of thermolysis of potato starch treated with hydrochloric and citric acids as potential prebiotics. Qual. Assur. Saf. 2014, 6, 347–356. [Google Scholar] [CrossRef]
- Kapusniak, J.; Kapusniak, K.; Wojcik, M.; Wrobel, K.; Rosicka-Kaczmarek, J. Assessment of physicochemical and thermal properties of soluble dextrin fiber from potato starch for use in fruit mousses. J. Sci. Food Agric. 2020, 101, 4125–4133. [Google Scholar] [CrossRef] [PubMed]
- Barczynska, R.; Slizewska, K.; Litwin, M.; Szalecki, M.; Zarski, A.; Kapusniak, J. The effect of dietary fibre preparations from potato starch on the growth and activity of bacterial strains belonging to the phyla Firmicutes, Bacteroidetes, and Actinobacteria. J. Funct. Foods. 2015, 19, 661–668. [Google Scholar] [CrossRef]
- Jochym, K.; Kapusniak, J.; Barczynska, R.; Libudzisz, Z.; Slizewska, K. Preparat o właściwościach prebiotycznych (Preparation with prebiotic properties). Polish Patent PL 220965, 16 June 2015. [Google Scholar]
- AOAC 2011.25-2011; Insoluble, Soluble, and Total Dietary Fiber in Foods. Enzymatic-Gravimetric-Liquid Chromatography. AOAC International: Gaithersburg, MD, USA, 2012.
- Richter, M.; Augustat, S.; Schierbaum, F. Ausgewahlte Methoden der Stärkechemie; VEB Fachbuchverlag Leipzig: Leipzig, Germany, 1968. [Google Scholar]
- Kapusniak, K.; Lubas, K.; Wojcik, M.; Rosicka-Kaczmarek, J.; Pavlyuk, V.; Kluziak, K.; Gonçalves, I.; Lopes, J.; Coimbra, M.A.; Kapusniak, J. Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production. Molecules 2021, 26, 5619. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 11085:2015-10; Cereals, Cereals-Based Products and Animal Feeding Stuffs—Determination of Crude Fat and Total Fat Content by the Randall Extraction Method. Sklep PKN: Warsaw, Poland, 2015.
- PN-EN ISO 12966-1:2015-01/AC:2015-06; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. Sklep PKN: Warsaw, Poland, 2015.
- PN-EN ISO 12966-2:2017-05 point 5.2; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl esters of Fatty Acids. ISO: Geneve, Switzerland, 2017.
- PN-A-74108:1996; Pieczywo—Metody Badań, Polski Komitet Normalizacyjny 1996. Sklep PKN: Warsaw, Poland, 2023. (In Polish)
- PN-EN ISO 20483:2014-02; Cereal Grain and Seeds of Legumes—Determination of Nitrogen Content and Conversion to Protein Content—Kjeldahl Method. Sklep PKN: Warsaw, Poland, 2014. (In Polish)
- PN-EN ISO 2171:2010; Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration. Polish Committee for Standardization: Warsaw, Poland, 2010.
- PN-EN ISO 10520:2002; Native Starch—Determination of Starch Content—Ewers Polarimetric Method. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Ewers, E. Die polarimetrisch Analyse des Stärkegehaltes. Ztschr. f. Offentliche Chemie. 1908, 14, 150–157. [Google Scholar]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 (Annex XIV). Available online: http://data.europa.eu/eli/reg/2011/1169/oj (accessed on 18 January 2024).
- PN-EN ISO 6579-1:2017-04; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. GBA POLSKA: Warsaw, Poland, 2017.
- PN-EN ISO 11290-2:2017-7; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp. Part 2: Enumeration method. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN ISO 16649-2:2004; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the enumeration of Beta-Glucuronidase-Positive Escherichia coli. Polish Committee for Standardization: Warsaw, Poland, 2004.
- Zasady Pobierania Próbek Środków Spożywczych w Celu Kontroli Zawartości Ołowiu, Kadmu, Rtęci, Arsenu i 3- MCPD. Przygotowanie próbek i Kryteria Wyboru Metod Analitycznych Stosowanych do Oznaczania Zawartości Metali Szkodliwych dla Zdrowia i 3-MCPD w Środkach Spożywczych; Wydawnictwa Metodyczne Państwowego Zakładu Higieny: Warszawa, Polska, 2003. (In Polish)
- PN-EN 13806:2003; Foodstuffs—Determination of Trace Elements—Determination of Mercury by Cold-Vapour Atomic Absorption Spectrometry (CVAAS) After Pressure Digestion. Sklep PKN: Warsaw, Poland, 2003.
- Jacobson, M.R.; Obanni, M.; BeMiller, J.N. Retrogradation of starches from different botanical sources. Cereal Chem. 1997, 74, 511–518. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Shah, A.; Wani, I.A.; Masoodi, F.A. Preparation, health benefits and applications of resistant starch—A review. Starch-Stärke 2016, 68, 287–301. [Google Scholar] [CrossRef]
- Trithavisup, K.; Kruson, K.; Tananuwong, K. In-depth study of the changes in properties and molecular structure of cassava starch during resistant dextrin preparation. Food Chem. 2019, 297, 124966. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Khaneghah, A.M.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Kwon, S.; Chung, K.M.; Shin, S.I.; Moon, T.W. Contents of Indigestible Fraction, Water Solubility, and Color of Pyrodextrins Made from Waxy Sorghum Starch. Cereal Chem. 2005, 82, 101–104. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, X.; Sun, Y.; Shi, J.; Xu, X.; Shi, Y.-C. Hypoglycemic Effects of Pyrodextrins with Different Molecular Weights and Digestibilities in Mice with Diet-Induced Obesity. J. Agric. Food Chem. 2018, 66, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Weil, W.; Weil, R.C.; Keawsompong, S.; Sriroth, K.; Seib, P.A.; Shi, Y.C. Pyrodextrins from waxy and normal tapioca starches: Molecular structure and in vitro digestibility. Carbohydr. Polym. 2021, 252, 117140. [Google Scholar] [CrossRef] [PubMed]
- Duy Lam, N.; Thi Binh, P.; Cao Tang, P.; Minh Tuan, P. The preparation of enzyme-resistant pyrodextrin from rice starch by pyrolytic reaction with acid catalysts. Vietnam. J. Sci. Technol. 2020, 58, 123–134. [Google Scholar]
- Trithavisup, K.; Shi, Y.C.; Krusong, K.; Tananuwong, K. Molecular structure and properties of cassava-based resistant maltodextrins. Food Chem. 2022, 369, 130876. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Kang, J.; Shi, Y.-C. Changes in molecular size and shape of waxy maize starch during dextrinization. Food Chem. 2021, 348, 128983. [Google Scholar] [CrossRef]
- Han, X.; Kang, J.; Bai, Y.; Xue, M.; Shi, Y.C. Structure of pyrodextrin in relation to its retrogradation properties. Food Chem. 2018, 242, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, T.; Jiang, B.; Chen, J. Purification and Characterization of Resistant Dextrin. Foods 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, J.J.; Chen, Y.; Wei, N.; Hou, Y.; Bai, W.; Hu, S.-Q. Effect of water-soluble dietary fiber resistant dextrin on flour and bread qualities. Food Chem. 2020, 317, 126452. [Google Scholar] [CrossRef]
- Li, H.; Ji, J.; Yang, L.; Lei, N.; Wang, J.; Sun, B. Structural and physicochemical property changes during pyroconversion of native maize starch. Carbohydr. Polym. 2020, 245, 116560. [Google Scholar] [CrossRef]
- Takeiti, C.Y.; Kieckbusch, T.G.; Collares-Queiroz, F.P. Morphological and Physicochemical Characterization of Commercial Maltodextrins with Different Degrees of Dextrose-Equivalent. Int. J. Food Prop. 2010, 13, 411–425. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, R.; Zeng, J.; Li, G.; Li, X. Characterization of dextrins with different Dextrose Equivalents. Molecules 2010, 15, 5162–5173. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Available online: http://data.europa.eu/eli/reg/2008/1333/oj (accessed on 18 January 2024).
- Commission Notice Guidelines for the Implementation of Certain Labelling Provisions of Regulation (EU) 2019/787 of the European Parliament and of the Council of 17 April 2019 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008. Available online: http://data.europa.eu/eli/reg/2019/787/oj (accessed on 18 January 2024).
- Li, L.; He, T.; Ling, Y.; Li, X.; Sui, C.; Cao, R.-A.; Li, C. Preparation, structure characterization and functional properties of pea dregs resistant dextrin. Front. Sustain. Food Syst. 2023, 7, 1182642. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Wang, Z.; Liao, A.; Pan, L.; Zhang, M.; Xue, Y.; Wang, J.; Liu, Y.; Huang, J. A Comparative Study of Resistant Dextrins and Resistant Maltodextrins from Different Tuber Crop Starches. Polymers 2023, 15, 4545. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yang, Y. Molecular characteristics and digestion properties of corn starch esterified by l-malic acid. J. Food Process. Preserv. 2021, 45, e15391. [Google Scholar] [CrossRef]
- Xia, C.; Zhong, L.; Wang, J.; Zhang, L.; Chen, X.; Ji, H.; Ma, S.; Dong, W.; Ye, X.; Huang, Y.; et al. Structural and digestion properties of potato starch modified using an efficient starch branching enzyme AqGBE. Int. J. Biol. Macromol. 2021, 184, 551–557. [Google Scholar] [CrossRef]
- Allan, M.C.; Mauer, L.J. Variable Effects of Twenty Sugars and Sugar Alcohols on the Retrogradation of Wheat Starch Gels. Foods 2022, 11, 3008. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Hu, G.; Guo, K.; Wei, C. Comparison of Physicochemical Properties of Starches from Nine Chinese Chestnut Varieties. Molecules 2018, 23, 3248. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Lin, J.H.; Zeng, H.M.; Wu, Y.H.; Chang, Y.H. Indigestible pyrodextrins prepared from corn starch in the presence of glacial acetic acid. Carbohydr. Polym. 2018, 188, 68–75. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Wang, Z.; Cheng, H.; Zhang, Y.; Lin, B.; Qin, L.; Bai, Y. Effects of reaction condition on glycosidic linkage structure, physical-chemical properties and in vitro digestibility of pyrodextrins prepared from native waxy maize starch. Food Chem. 2020, 320, 126491. [Google Scholar] [CrossRef]



| Sample | IDF [%] | SDFP [%] | SDFS [%] | TDF [%] |
|---|---|---|---|---|
| SDexF1 | 0.75 ± 0.03 b | 10.42 ± 0.06 b | 20.84 ± 1.08 a | 32.01 ± 1.17 ab |
| SDexF2 | 0.67 ± 0.02 a | 7.44 ± 0.16 a | 22.60 ± 0.95 ab | 30.07 ± 0.78 a |
| SDexF3 | 0.71 ± 0.01 b | 8.00 ± 1.22 a | 25.76 ± 0.24 c | 34.47 ± 1.46 b |
| SDexF4 | 0.77 ± 0.05 b | 6.48 ± 1.30 a | 23.54 ± 0.02 b | 30.78 ± 1.27 a |
| Sample | Solubility in Water at 20 °C [%] |
|---|---|
| Native potato starch | 0.40 ± 0.34 a |
| SDexF1 | 95.80 ± 0.85 b |
| SDexF2 | 96.40 ± 0.14 b |
| SDexF3 | 99.15 ± 0.85 c |
| SDexF4 | 100.20 ± 0.49 c |
| Sample | Dextrose Equivalent (DE) |
|---|---|
| Native potato starch | 0.20 ± 0.10 a |
| SDexF1 | 4.48 ± 0.09 b |
| SDexF2 | 4.45 ± 0.05 b |
| SDexF3 | 4.54 ± 0.08 b |
| SDexF4 | 4.57 ± 0.09 b |
| Sample | Citric Acid Content | Ethanol Content |
|---|---|---|
| SDexF1 | 0.006 ± 0.003 a | 4.16 ± 0.02 a |
| SDexF2 | 0.008 ± 0.002 a | 4.22 ± 0.22 a |
| SDexF3 | 0.007 ± 0.002 a | 4.65 ± 0.09 b |
| SDexF4 | 0.008 ± 0.003 a | 4.56 ± 0.06 b |
| Tested Parameter | Result ± Uncertainty | LOQ |
|---|---|---|
| Energy value | 295.66 kcal/100 g 1238.56 kJ/100 g | n.a. (not applicable) |
| Fat, including: | <LOQ | 0.04 g/100 g |
| - SFA | <0.06 g/100 g | n.a. |
| - MUFA | <0.06 g/100 g | n.a. |
| - PUFA | <0.06 g/100 g | n.a. |
| - TFA | <0.06 g/100 g | n.a. |
| - omega-3 | <0.06 g/100 g | n.a. |
| - omega-6 | <0.06 g/100 g | n.a. |
| Digestible carbohydrates, including: | 55.84 g/100 g | From calculations, based on the test results given in this study |
| - total sugars | 4.49 ± 0.24 g/100 g | 0.43 g/100 g |
| Starch | 29.06 ± 2.53 g/100 g | n.a. |
| Dietary fibre | 31.83 ± 1.93/100 g | n.a. |
| Sodium chloride | 0.10 ± 0.01 g/100 g | 0.05 g/100 g |
| Nitrogen | 0.013 ± 0.00 g/100 g | 0.018 g/100 g |
| Protein | 0.08 ± 0.00 g/100 g | n.a. |
| Ash | 0.39 ± 0.01 g/100 g | 0.01 g/100 g |
| Contents of Heavy Metals, Including: | Result | LOQ |
|---|---|---|
| lead | <0.010 mg/kg | 0.010 |
| cadmium | <0.005 mg/kg | 0.005 |
| mercury | <0.005 mg/kg | 0.005 |
| Presence of Bacteria of the Genus | Result |
|---|---|
| Salmonella spp. | not detected in 25 g |
| Listeria monocytogenes | <10 jtk/g |
| β-glucuronidase-positive Escherichia coli | <10 jtk/g |
| Sample | PV | HPV | BD = PV − HPV | FV | SB = FV − HPV |
|---|---|---|---|---|---|
| Native potato starch | 1704 ± 344 | 1117 ± 52 | 587 ± 392 | 1986 ± 114 | 869 ± 62 |
| SDexF | 28 ± 4 | 13 ± 3 | 16 ± 2 | 21 ± 2 | 7 ± 1 |
| Sample | L* | a* | b* | ∆E |
|---|---|---|---|---|
| Native potato starch | 94.90 | −0.19 | 1.00 | - |
| SDexF | 92.11 ± 0.67 | −0.54 ± 0.04 | 10.46 ± 1.73 | 9.88 ± 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcik, M.; Kapusniak, K.; Zarski, A.; Kapusniak, J. Preparation and Characterization of Soluble Dextrin Fibre from Potato Starch Obtained on a Semi-Industrial Scale. Appl. Sci. 2024, 14, 1438. https://doi.org/10.3390/app14041438
Wojcik M, Kapusniak K, Zarski A, Kapusniak J. Preparation and Characterization of Soluble Dextrin Fibre from Potato Starch Obtained on a Semi-Industrial Scale. Applied Sciences. 2024; 14(4):1438. https://doi.org/10.3390/app14041438
Chicago/Turabian StyleWojcik, Malwina, Kamila Kapusniak, Arkadiusz Zarski, and Janusz Kapusniak. 2024. "Preparation and Characterization of Soluble Dextrin Fibre from Potato Starch Obtained on a Semi-Industrial Scale" Applied Sciences 14, no. 4: 1438. https://doi.org/10.3390/app14041438