Corrosion Monitoring Techniques in Subcritical and Supercritical Water Environments
Abstract
:1. Introduction
2. Non-Invasive Monitoring Techniques
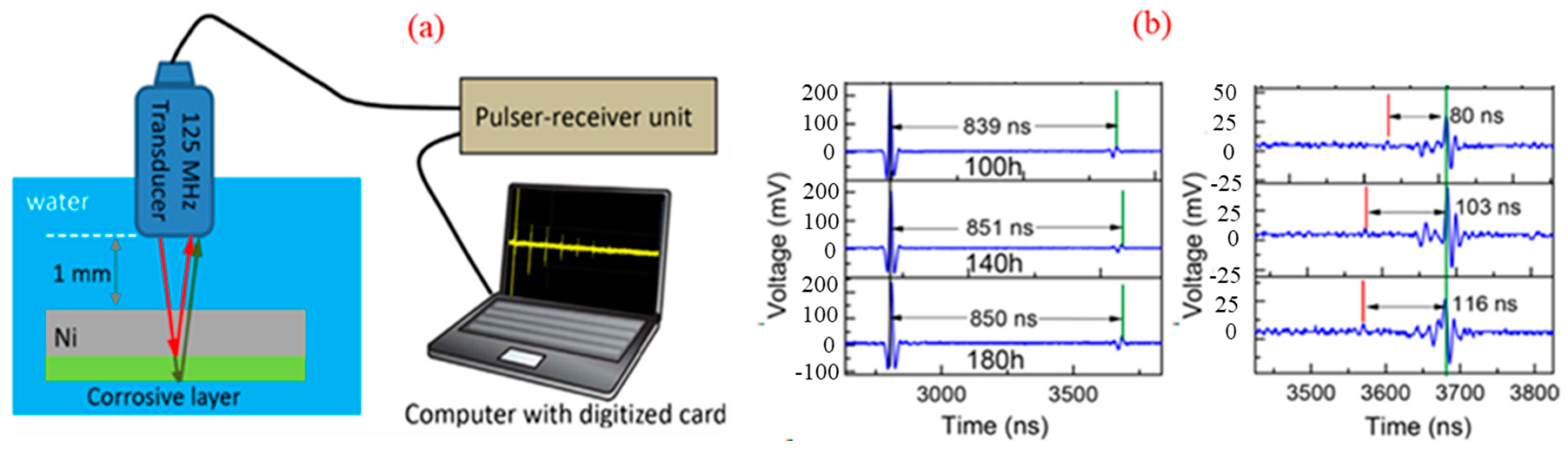
2.1. Ultrasonic Thickness Measurement
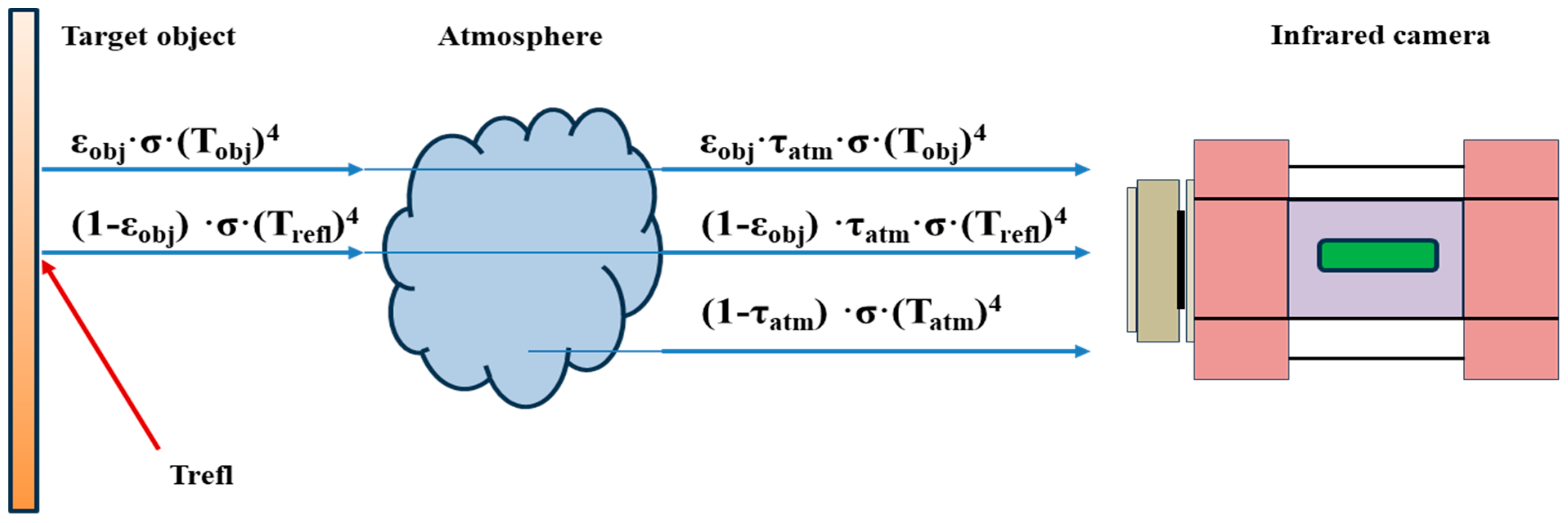
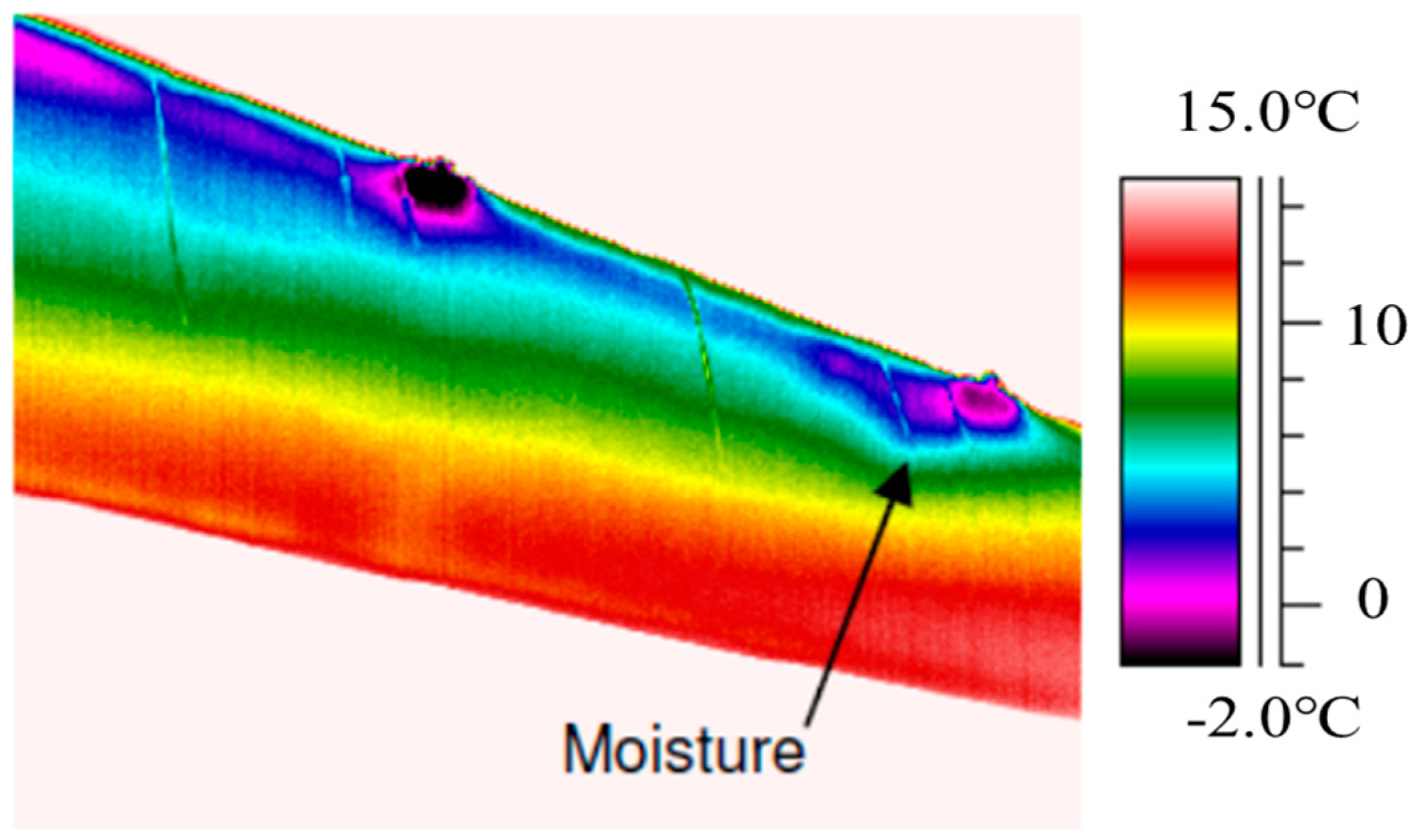
2.2. Infrared Thermography Method
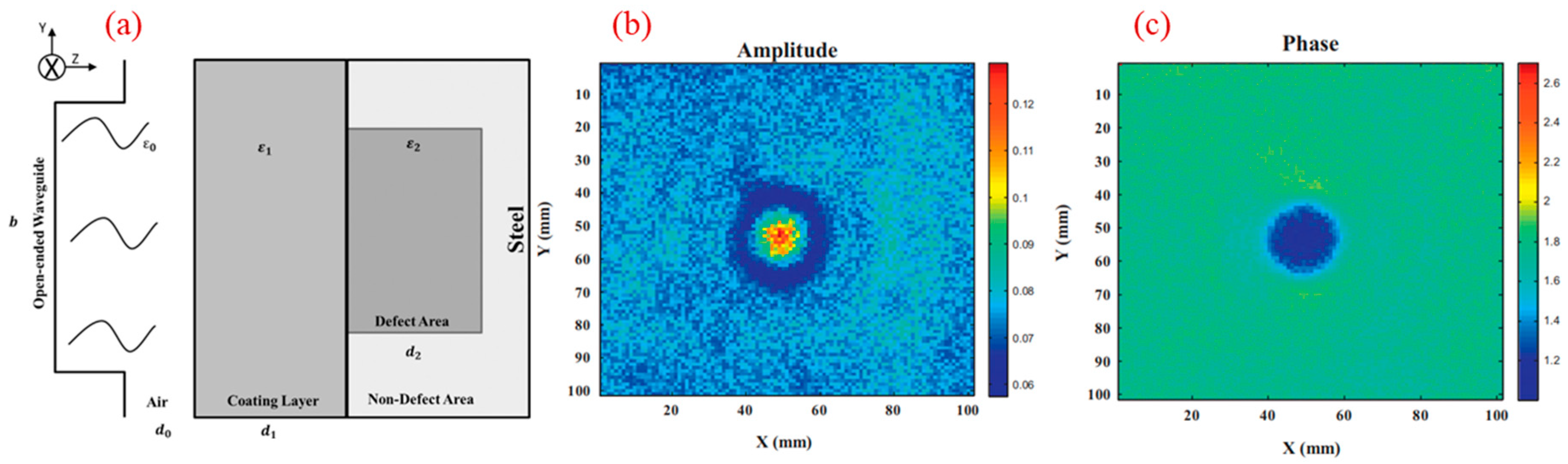
2.3. Microwave Imaging
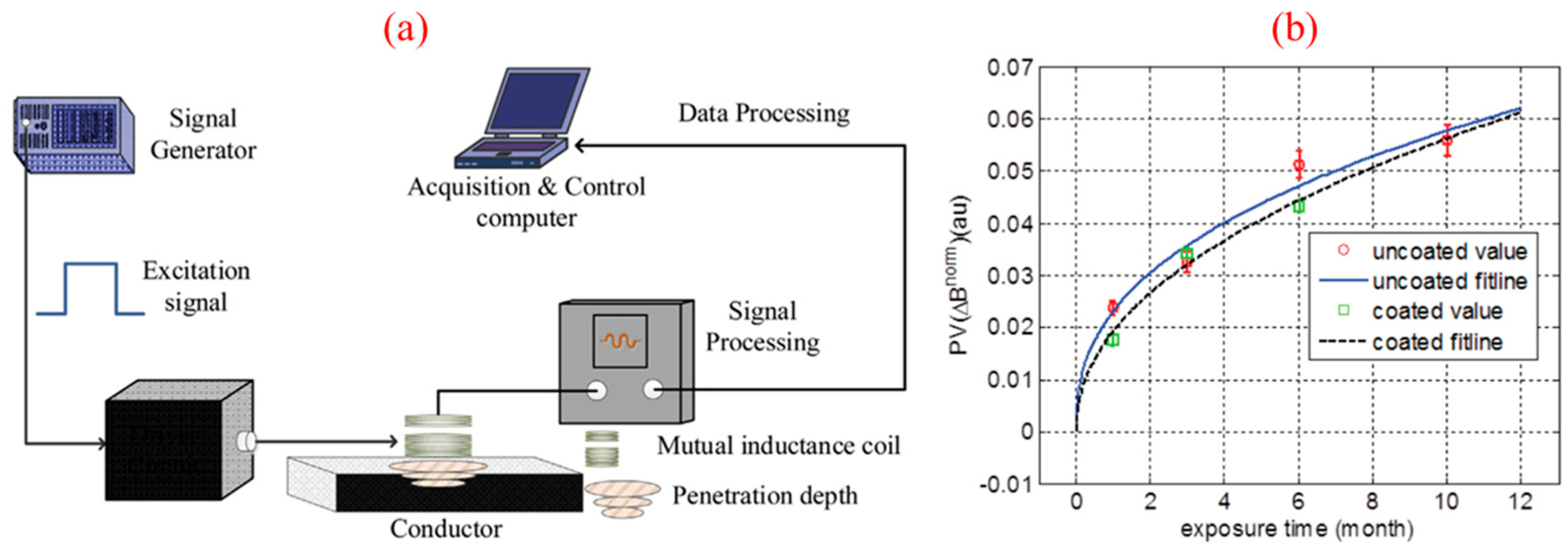
2.4. Eddy Current Detection
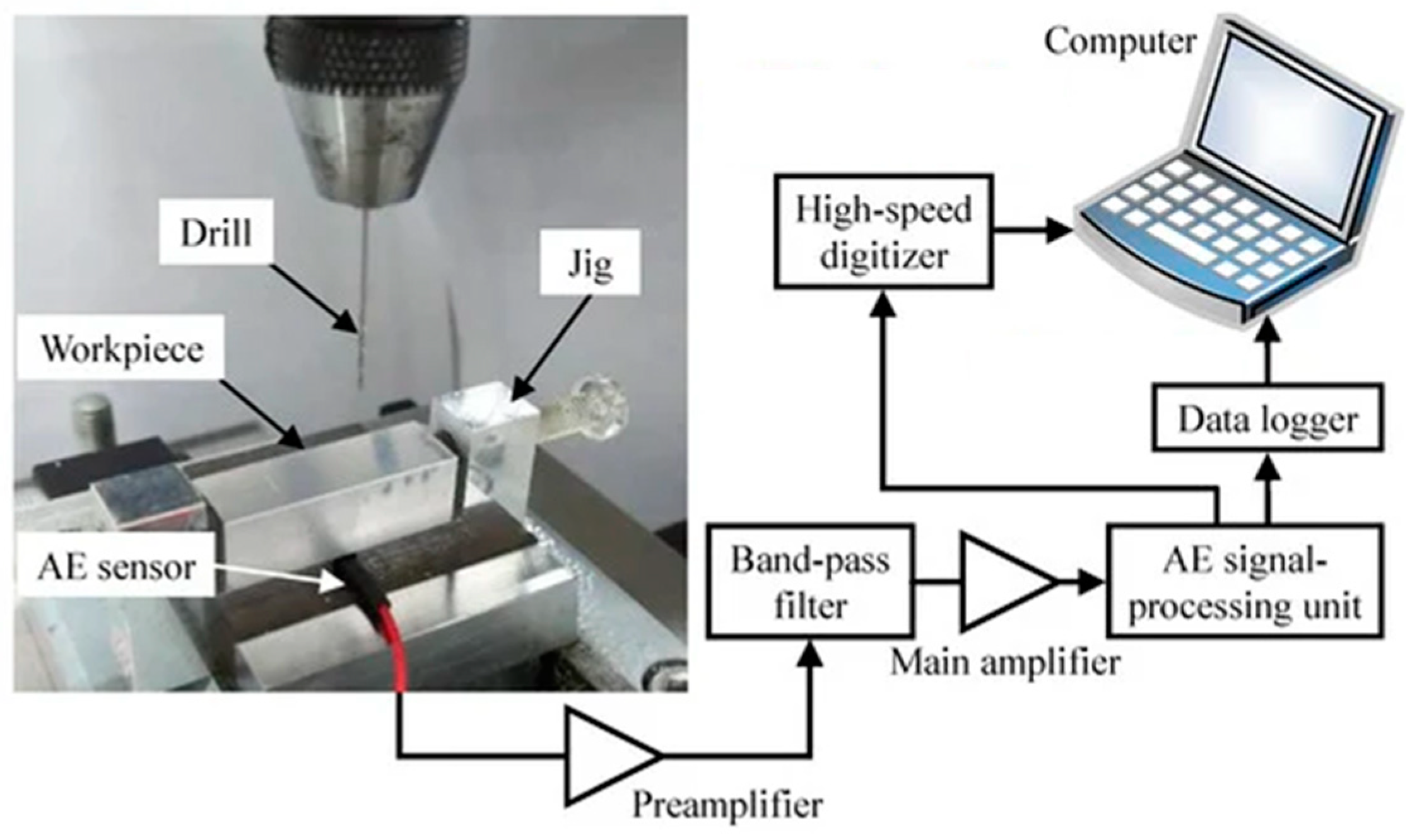
2.5. Acoustic Emission
3. Invasive Corrosion Monitoring Techniques
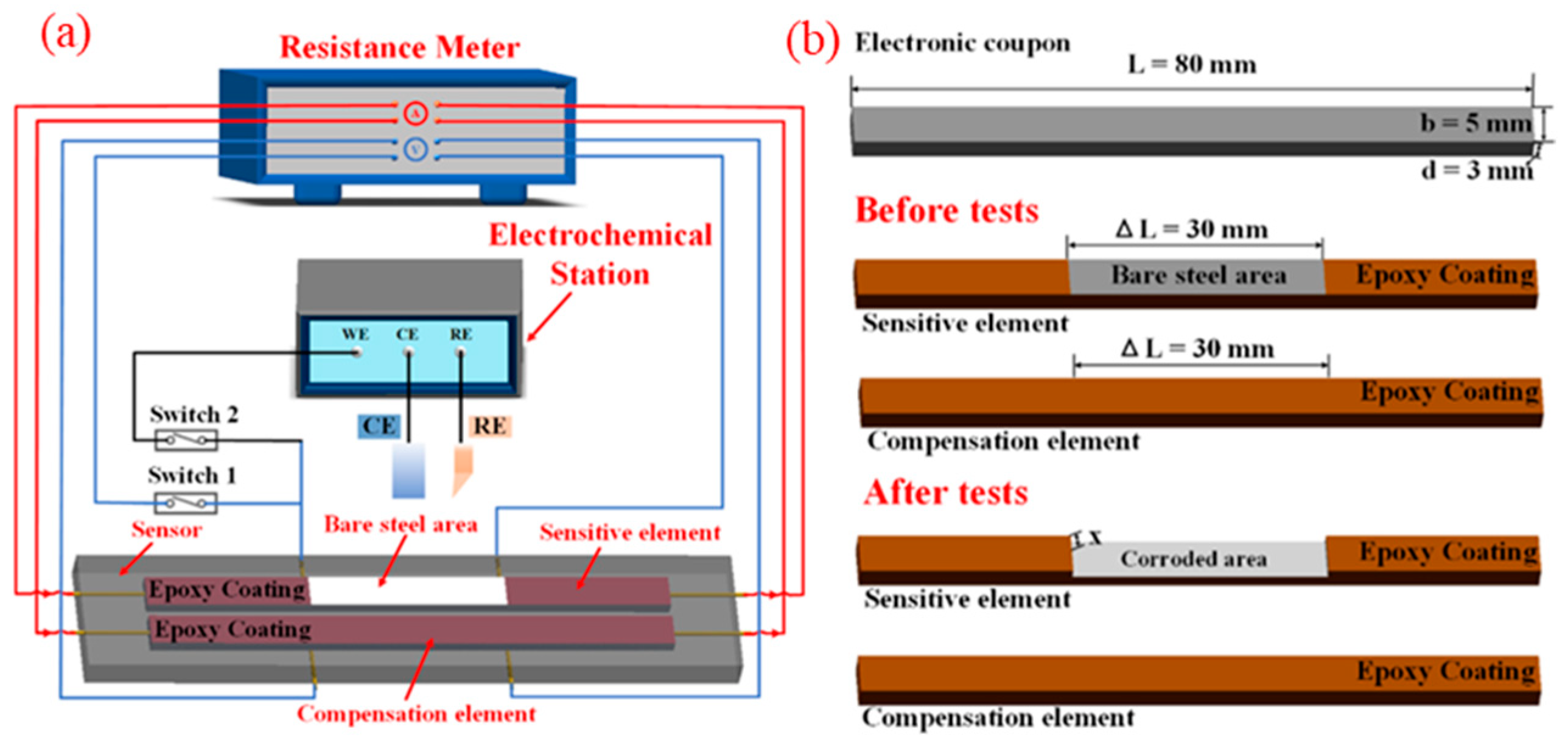
3.1. Electrical Resistance Probe
3.2. Electrochemical Corrosion Potential
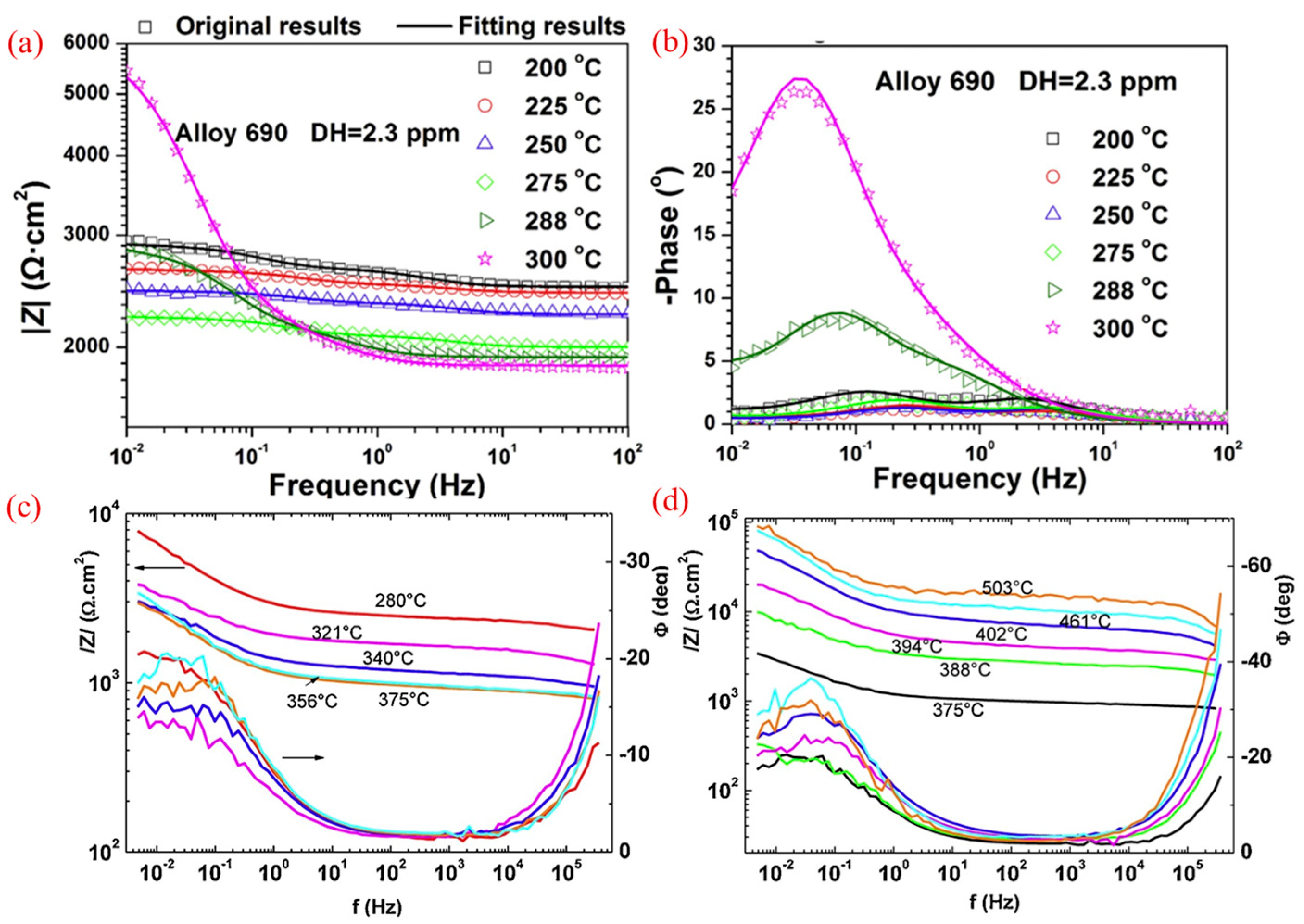
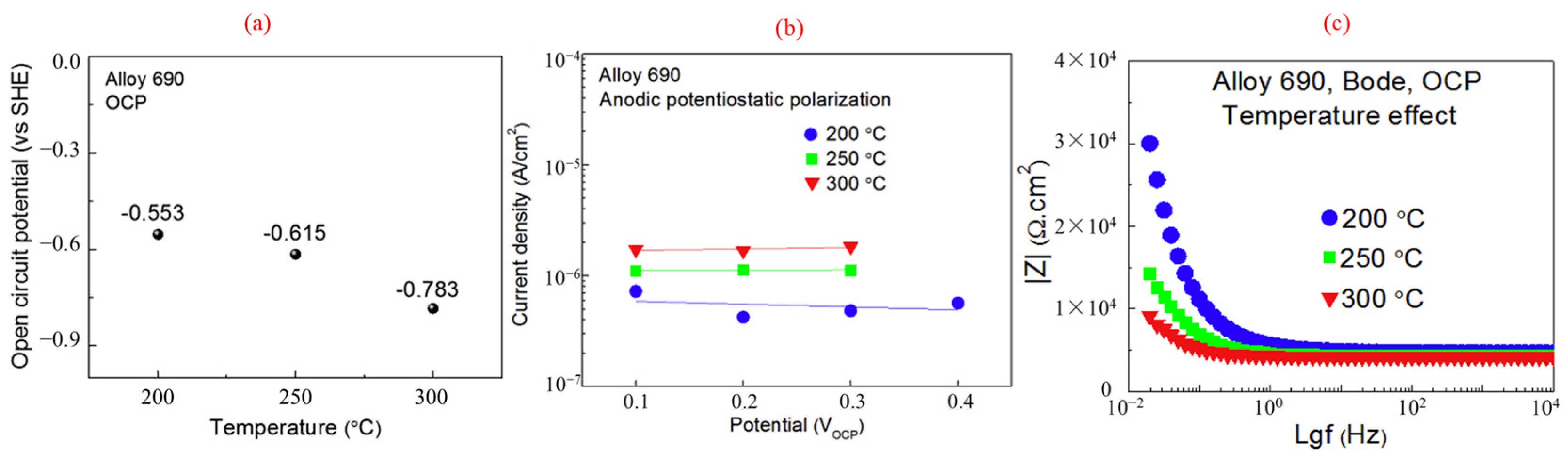
3.3. Electrochemical Impedance Spectroscopy
3.4. Electrochemical Noise
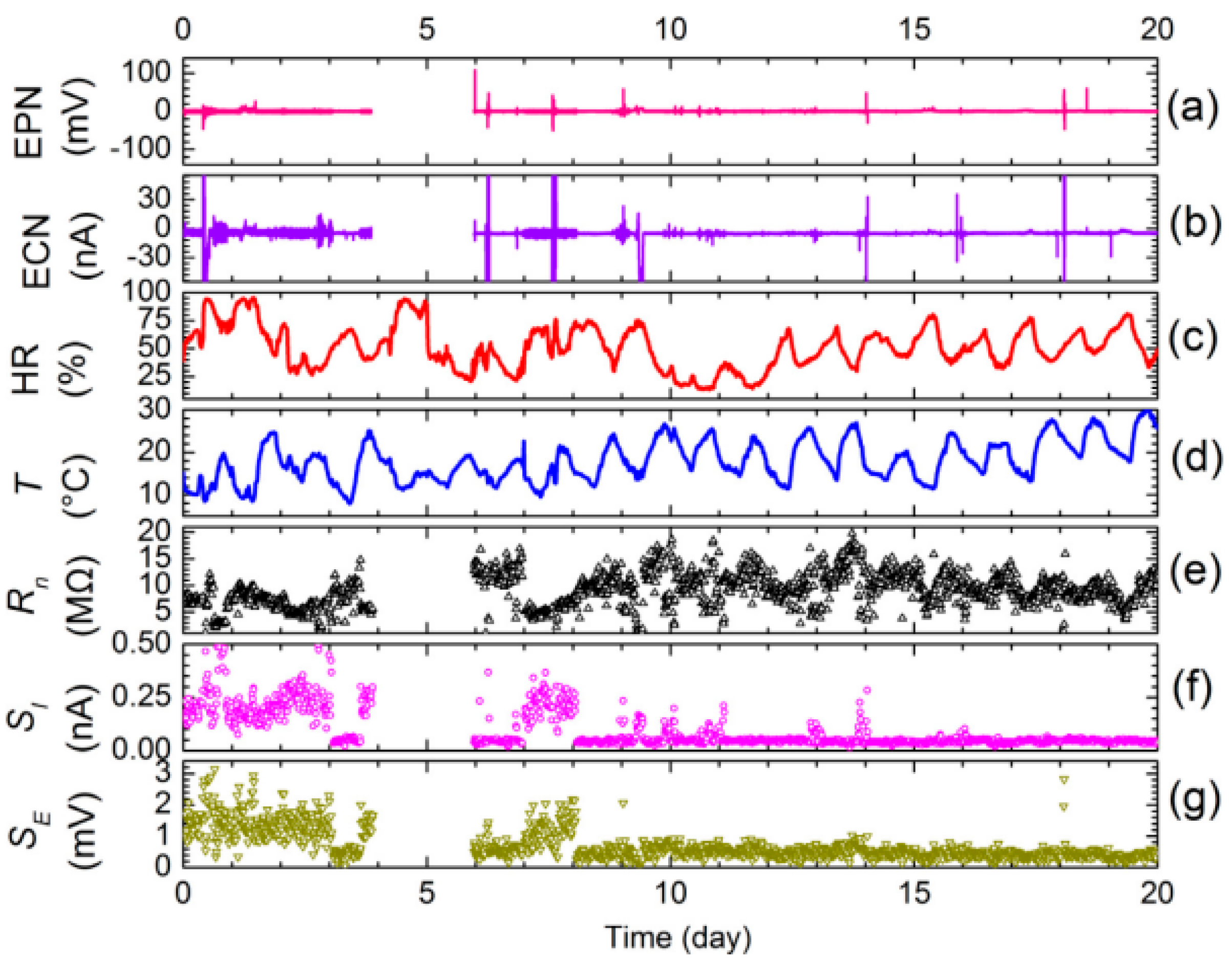
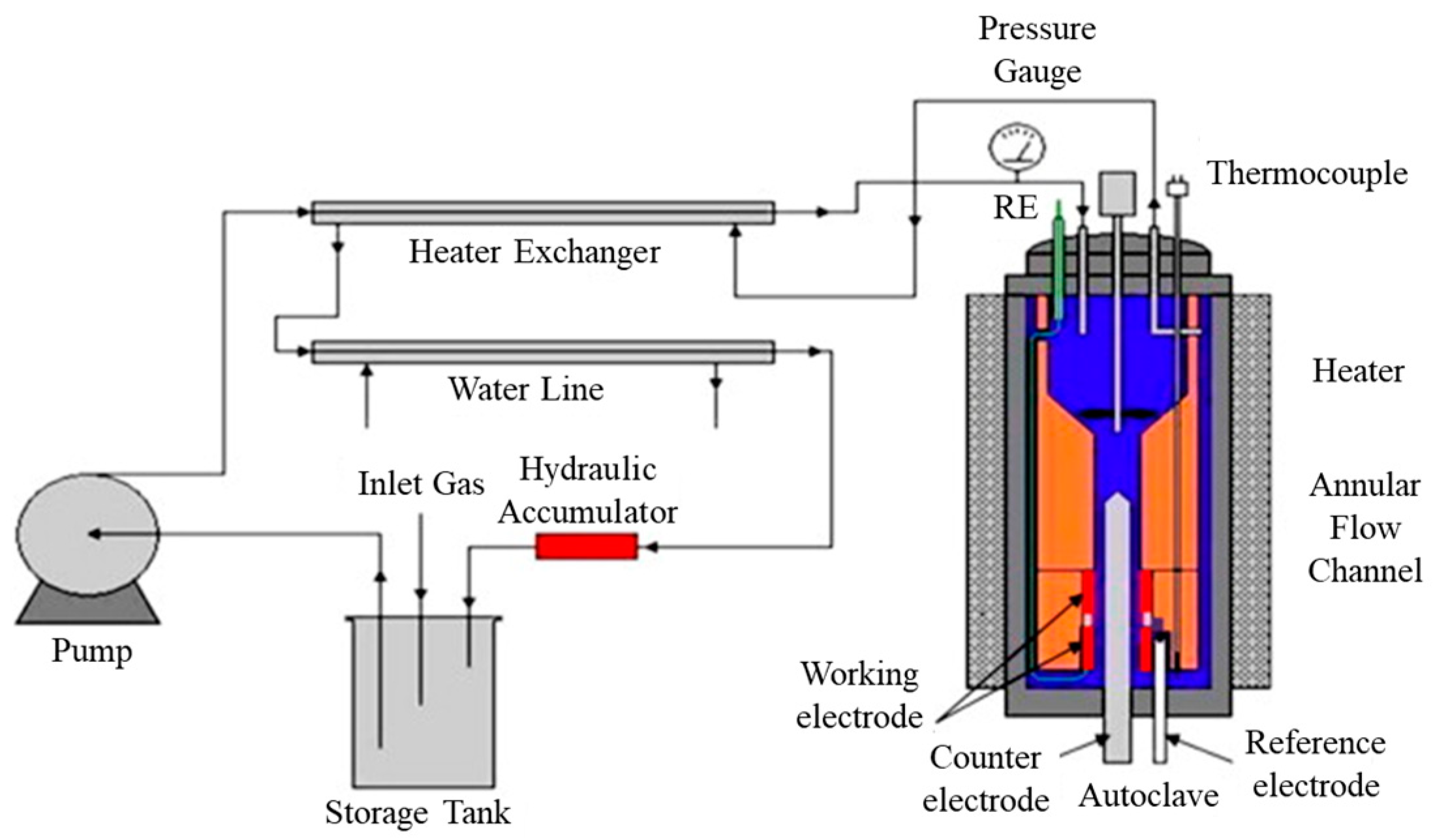
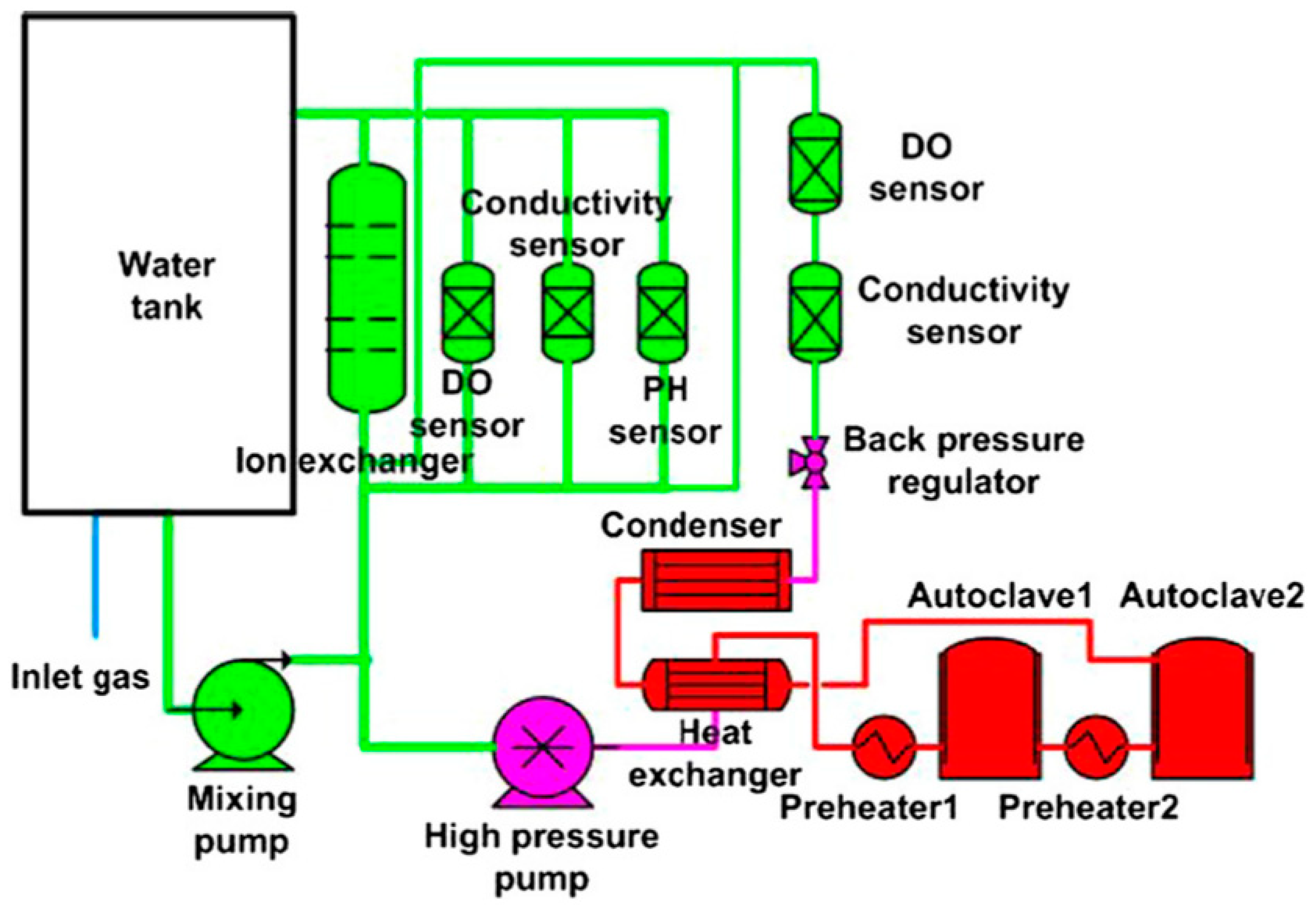
3.5. Research Platform of In Situ Electrochemical Corrosion Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Bai, Z.; Ding, S.; Macdonald, D.D.; Qiu, J.; Wang, K.; Jiang, Z.; Wang, S. Electrochemical techniques and mechanisms for the corrosion of metals and alloys in sub- and supercritical aqueous systems. J. Supercrit. Fluids 2023, 194, 105835. [Google Scholar] [CrossRef]
- Wang, Y.; Oshima, Y.; Akizuki, M. Catalytic Performance of Solid Base Na-ZrO2 in Subcritical Water. J. Chem. Eng. Jpn. 2024, 57, 2293044. [Google Scholar] [CrossRef]
- Ehrlich, K.; Konys, J.; Heikinheimo, L. Materials for high performance light water reactors. J. Nucl. Mater. 2004, 327, 140–147. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.; Kim, S.J.; Lee, Y.K.; Hur, D.H. Fouling behavior of a printed circuit steam generator under simulated operating conditions of a small modular reactor. Ann. Nucl. Energy 2022, 173, 109127. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Urquidi-Macdonald, M. The Electrochemistry of Nuclear Reactor Coolant Circuits. In Encyclopedia of Electrochemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Xiong, X.; Liu, X.; Tan, H.; Deng, S. Investigation on high temperature corrosion of water-cooled wall tubes at a 300 MW boiler. J. Energy Inst. 2020, 93, 377–386. [Google Scholar] [CrossRef]
- Sun, X.; Ning, Y.H.; Yang, J.; Zhao, Y.; Yang, Z.J.; Zhou, X.Z. Study on high temperature corrosion mechanism of water wall tubes of 350 MW supercritical unit. Eng. Fail. Anal. 2021, 121, 105131. [Google Scholar] [CrossRef]
- May, Z.; Alam, M.K.; Nayan, N.A. Recent Advances in Non-destructive Method and Assessment of Corrosion Undercoating in Carbon-Steel Pipelines. Sensors 2022, 22, 6654. [Google Scholar] [CrossRef]
- Solovyeva, V.A.; Almuhammadi, K.H.; Badeghaish, W.O. Current Downhole Corrosion Control Solutions and Trends in the Oil and Gas Industry: A Review. Materials 2023, 16, 1795. [Google Scholar] [CrossRef]
- Jack, T.A.; Szpunar, J. Effect of Nb-induced microstructure on pipeline steel corrosion and stress corrosion cracking performance in acidic environment. Corros. Sci. 2023, 218, 111196. [Google Scholar] [CrossRef]
- Finsgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wang, S.Z.; Xu, T.T.; Li, J.N.; Zhang, Y.S.; Xu, T.T.; Yang, J.Q. Novel designs for the reliability and safety of supercritical water oxidation process for sludge treatment. Process Saf. Environ. Prot. 2021, 149, 385–398. [Google Scholar] [CrossRef]
- Kritzer, P. Corrosion in high-temperature and supercritical water and aqueous solutions: A review. J. Supercrit. Fluids 2004, 29, 1–29. [Google Scholar] [CrossRef]
- De Souza, G.B.M.; Pereira, M.B.; Mourao, L.C.; dos Santos, M.P.; de Oliveira, J.A.; Garde, I.A.A.; Alonso, C.G.; Jegatheesan, V.; Cardozo-Filho, L. Supercritical water technology: An emerging treatment process for contaminated wastewaters and sludge. Rev. Environ. Sci. Bio/Technol. 2022, 21, 75–104. [Google Scholar] [CrossRef]
- Marrone, P.A. Supercritical water oxidation-Current status of full-scale commercial activity for waste destruction. J. Supercrit. Fluids 2013, 79, 283–288. [Google Scholar] [CrossRef]
- Li, Y.; Ding, S.; Bai, Z.; Wang, S.; Zhang, F.; Zhang, J.; Xu, D.; Yang, J. Corrosion characteristics and mechanisms of typical iron/nickel-based alloys in reductive supercritical water environments containing sulfides. J. Supercrit. Fluids 2022, 187, 105599. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Liu, Q.; Xu, L.; Lin, S. Effect of sodium chloride on the electrochemical corrosion of Inconel 625 at high temperature and pressure. J. Alloys Compd. 2017, 703, 523–529. [Google Scholar] [CrossRef]
- Tang, X.; Wang, S.; Qian, L.; Ren, M.; Sun, P.; Li, Y.; Yang, J. Corrosion Properties of Candidate Materials in Supercritical Water Oxidation Process. J. Adv. Oxid. Technol. 2016, 19, 141–157. [Google Scholar] [CrossRef]
- Guo, S.; Xu, D.; Li, Y.; Guo, Y.; Wang, S.; Macdonald, D.D. Corrosion characteristics and mechanisms of typical Ni-based corrosion-resistant alloys in sub- and supercritical water. J. Supercrit. Fluids 2021, 170, 105138. [Google Scholar] [CrossRef]
- Guo, S.; Xu, D.; Liang, Y.; Gong, Y.; Li, Y.; Yang, J. Corrosion characterization of ZrO2 and TiO2 ceramic coatings via air plasma spraying on 316 stainless steel in oxygenated sub- and supercritical water. J. Supercrit. Fluids 2020, 157, 104716. [Google Scholar] [CrossRef]
- Shome, A.; Das, A.; Borbora, A.; Dhar, M.; Manna, U. Role of chemistry in bio-inspired liquid wettability. Chem. Soc. Rev. 2022, 51, 5452–5497. [Google Scholar] [CrossRef]
- Qian, H.; Xu, D.; Du, C.; Zhang, D.; Li, X.; Huang, L.; Deng, L.; Tu, Y.; Mol, J.M.C.; Terryn, H.A. Dual-action smart coatings with a self-healing superhydrophobic surface and anti-corrosion properties. J. Mater. Chem. A 2017, 5, 2355–2364. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Y.; Meng, F.; Zhang, Z.; Li, Y.; Wang, J.; Han, E.-H.; Ming, H. Insights into stress corrosion cracking in scratched area of alloy 690TT steam generator tubes. Acta Mater. 2023, 255, 119083. [Google Scholar] [CrossRef]
- Gholamzadeh, H.; Shaik, A.; Daub, K.; Topping, M.; Daymond, M.R.; Persaud, S.Y. A mechanistic study on dealloying-induced stress corrosion cracking of Alloy 800 in boiling caustic solutions. Corros. Sci. 2023, 220, 111284. [Google Scholar] [CrossRef]
- Wang, X.; Shang, C.; Li, Z.; Bai, Y.; Liu, T.; Lu, Y.; Shoji, T. Study on the SCC behavior induced by creep cavities on scratched surface of Alloy 690TT in high temperature water. Corros. Sci. 2022, 196, 110017. [Google Scholar] [CrossRef]
- Li, J.; Cao, T.; Zhang, C.; Cheng, C.; Zhao, J. Failure analysis of reheater tubes in a 350 MW supercritical circulating fluidized bed boiler. Eng. Failure Anal. 2022, 137, 106285. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, F.; Li, L.; Tan, H. Water Wall Tubes’ High Temperature Corrosion Root Cause Investigation: A 300 MW Level Boiler Case. Energies 2023, 16, 1767. [Google Scholar] [CrossRef]
- Honarvar, F.; Varvani-Farahani, A. A review of ultrasonic testing applications in additive manufacturing: Defect evaluation, material characterization, and process control. Ultrasonics 2020, 108, 106227. [Google Scholar] [CrossRef]
- Marcantonio, V.; Monarca, D.; Colantoni, A.; Cecchini, M. Ultrasonic waves for materials evaluation in fatigue, thermal and corrosion damage: A review. Mech. Syst. Signal Process. 2019, 120, 32–42. [Google Scholar] [CrossRef]
- Liu, W.; Hou, B.P.; Wang, Y.X.; Yao, Y.; Zhou, L. Sparse Structural Principal Component Thermography for Defect Signal Enhancement in Subsurface Defects Detection of Composite Materials. J. Non-Destr. Eval. 2022, 41, 8. [Google Scholar] [CrossRef]
- Doshvarpassand, S.; Wu, C.Z.; Wang, X.Y. An overview of corrosion defect characterization using active infrared thermography. Infrared Phys. Technol. 2019, 96, 366–389. [Google Scholar] [CrossRef]
- Wahab, A.; Aziz, M.M.A.; Sam, A.R.M.; You, K.Y.; Bhatti, A.Q.; Kassim, K.A. Review on microwave non-destructive testing techniques and its applications in concrete technology. Constr. Build. Mater. 2019, 209, 135–146. [Google Scholar] [CrossRef]
- Mazzinghi, A.; Freni, A.; Capineri, L. A microwave non-destructive testing method for controlling polymeric coating of metal layers in industrial products. NDT&E Int. 2019, 102, 207–217. [Google Scholar] [CrossRef]
- Xie, L.; Gao, B.; Tian, G.Y.; Tan, J.D.; Feng, B.; Yin, Y. Coupling pulse eddy current sensor for deeper defects NDT. Sens. Actuator A 2019, 293, 189–199. [Google Scholar] [CrossRef]
- Grosso, M.; Pacheco, C.J.; Arenas, M.P.; Lima, A.H.M.; Margarit-Mattos, I.C.P.; Soares, S.D.; Pereira, G.R. Eddy current and inspection of coatings for storage tanks. J. Mater. Res. Technol. 2018, 7, 356–360. [Google Scholar] [CrossRef]
- Erlinger, T.; Kralovec, C.; Schagerl, M. Monitoring of Atmospheric Corrosion of Aircraft Aluminum Alloy AA2024 by Acoustic Emission Measurements. Appl. Sci. 2023, 13, 370. [Google Scholar] [CrossRef]
- May, Z.; Alam, K.; A’In, N.; Rahman, A.; Nayan, N.A. Denoising of Hydrogen Evolution Acoustic Emission Signal Based on Non-Decimated Stationary Wavelet Transform. Processes 2020, 8, 1460. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Josko, M.; Wieczorek, D.; Sedlak, K.; Nowak, M. The Influence of the Hardness of the Tested Material and the Surface Preparation Method on the Results of Ultrasonic Testing. Appl. Sci. 2023, 13, 9904. [Google Scholar] [CrossRef]
- Ruiz, A.; Ortiz, N.; Medina, A.; Kim, J.Y.; Jacobs, L.J. Application of ultrasonic methods for early detection of thermal damage in 2205 duplex stainless steel. NDT&E Int. 2013, 54, 19–26. [Google Scholar] [CrossRef]
- Li, W.; Cho, Y. Thermal Fatigue Damage Assessment in an Isotropic Pipe Using Nonlinear Ultrasonic Guided Waves. Exp. Mech. 2014, 54, 1309–1318. [Google Scholar] [CrossRef]
- Chillara, V.K.; Lissenden, C.J. Review of nonlinear ultrasonic guided wave non-destructive evaluation: Theory, numerics, and experiments. Opt. Eng. 2016, 55, 9904. [Google Scholar] [CrossRef]
- Fangxin, Z. Data-Enabled Quantitative Corrosion Monitoring using Ultrasound. Data-Enabled Discov. Appl. 2018, 2, 8. [Google Scholar]
- Dace, G.E.; Thompson, R.B.; Brasche, L.J.H.; Rehbein, D.K.; Buck, O. Nonlinear Acoustics, a Technique to Determine Microstructural Changes in Materials; Springer: Boston, MA, USA, 1991. [Google Scholar]
- Hurley, D.C.; Yost, W.T.; Boltz, E.S.; Fortunko, C.M. Experimental Comparison of Ultrasonic Techniques to Determine the Nonlinearity Parameter. In Proceedings of the Ultrasonics Symposium, Lake Buena Vista, FL, USA, 8–11 December 1991. [Google Scholar]
- Tanaka, T.; Izawa, Y. Non-destructive Detection of Small Internal Defects in Carbon Steel by Laser Ultrasonics. Jpn. J. Appl. Phys. 2001, 40, 1477. [Google Scholar] [CrossRef]
- Clorennec, D.; Royer, D.; Walaszek, H. Non-destructive evaluation of cylindrical parts using laser ultrasonics. Ultrasonics 2002, 40, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Kishore Chakrapani, S.; Howard, A.; Barnard, D. Influence of surface roughness on the measurement of acoustic nonlinearity parameter of solids using contact piezoelectric transducers. Ultrasonics 2018, 84, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Ergun, A.S.; Temelkuran, B.; Ozbay, E.; Atalar, A. A new detection method for capacitive micromachined ultrasonic transducers. In Proceedings of the Ultrasonics Symposium, Atlanta, GA, USA, 8–11 October 2002; pp. 1007–1010. [Google Scholar]
- Liu, H.; Zhang, L.; Liu, H.; Chen, S.T.; Wang, S.H.; Wong, Z.Z.; Yao, K. High-frequency ultrasonic methods for determining corrosion layer thickness of hollow metallic components. Ultrasonics 2018, 89, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bagavathiappan, S.; Lahiri, B.B.; Saravanan, T.; Philip, J.; Jayakumar, T. Infrared thermography for condition monitoring—A review. Infrared Phys. Technol. 2013, 60, 35–55. [Google Scholar] [CrossRef]
- Behravan, A.; Tran, T.Q.Q.; Li, Y.; Davis, M.; Shaikh, M.S.; DeJong, M.M.M.; Hernandez, A.; Brand, A.S.S. Field Inspection of High-Density Polyethylene (HDPE) Storage Tanks Using Infrared Thermography and Ultrasonic Methods. Appl. Sci. 2023, 13, 1396. [Google Scholar] [CrossRef]
- Cadelano, G.; Bortolin, A.; Ferrarini, G.; Molinas, B.; Giantin, D.; Zonta, P.; Bison, P. Corrosion Detection in Pipelines Using Infrared Thermography: Experiments and Data Processing Methods. J. Non-Destr. Eval. 2016, 35, 49. [Google Scholar] [CrossRef]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F. Infrared Thermography for Temperature Measurement and Non-Destructive Testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef]
- Ibarra-Castanedo, C.; Tarpani, J.R.; Maldague, X.P.V. Nondestructive testing with thermography. Eur. J. Phys. 2013, 34, S91. [Google Scholar] [CrossRef]
- Cernuschi, F.; Russo, A.; Lorenzoni, L.; Figari, A. In plane thermal diffusivity evaluation by infrared thermography. Rev. Sci. Instrum. 2001, 72, 3988–3995. [Google Scholar] [CrossRef]
- Riegert, G.; Zweschper, T.; Busse, G. Lockin thermography with eddy current excitation. Quant. InfraRed Thermogr. J. 2004, 1, 21–32. [Google Scholar] [CrossRef]
- Favro, L.D.; Han, X.; Ouyang, Z.; Sun, G.; Sui, H.; Thomas, R.L. Infrared imaging of defects heated by a sonic pulse. Rev. Sci. Instrum. 2000, 71, 2418–2421. [Google Scholar] [CrossRef]
- Cuccurullo, G.; Berardi, P.G.; Carfagna, R.; Pierro, V. IR temperature measurements in microwave heating. Infrared Phys. Technol. 2002, 43, 145–150. [Google Scholar] [CrossRef]
- Osiander, R.; Spicer, J.W.M. Time-resolved infrared radiometry with step heating. A review. Rev. Générale Therm. 1998, 37, 680–692. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, B.; Tian, G.Y.; Woo, W.L.; Bai, L.B. Metal defects sizing and detection under thick coating using microwave NDT. NDT&E Int. 2013, 60, 52–61. [Google Scholar] [CrossRef]
- Wang, D.; Che, F.; Wang, Y.; Zhu, L.; Shen, K.; Wang, Y. Application of Microwave Non-destructive Testing of External Anti-Corrosion Coating. Total Corros. Control 2022, 36, 5. [Google Scholar]
- Mazlumi, F.; Sadeghi, S.H.H.; Moini, R. Analysis technique for interaction of rectangular open-ended waveguides with surface cracks of arbitrary shape in metals. NDT&E Int. 2003, 36, 331–338. [Google Scholar] [CrossRef]
- Sayar, M.; Seo, D.; Ogawa, K. Non-destructive microwave detection of layer thickness in degraded thermal barrier coatings using K- and W-band frequency range. NDT&E Int. 2009, 42, 398–403. [Google Scholar] [CrossRef]
- Qaddoumi, N.N.; Saleh, W.M.; Abou-Khousa, M. Innovative near-field microwave nondestructive testing of corroded metallic structures utilizing open-ended rectangular waveguide probes. IEEE Trans. Instrum. Meas. 2007, 56, 1961–1966. [Google Scholar] [CrossRef]
- Zoughi, R.; Kharkovsky, S. Microwave and millimetre wave sensors for crack detection. Fatigue Fract. Eng. Mater. Struct. 2008, 31, 695–713. [Google Scholar] [CrossRef]
- García-Martín, J.; Gómez-Gil, J.; Vázquez-Sánchez, E. Non-Destructive Techniques Based on Eddy Current Testing. Sensors 2011, 11, 2525–2565. [Google Scholar] [CrossRef]
- Newton, M.; Chowdhury, T.; Gravagne, I.; Jack, D. Non-Destructive Evaluation of In-Plane Waviness in Carbon Fiber Laminates Using Eddy Current Testing. Appl. Sci. 2023, 13, 6009. [Google Scholar] [CrossRef]
- Ramos, H.G.; Ribeiro, A.L. Present and Future Impact of Magnetic Sensors in NDE. In Proceedings of the 1st International Conference on Structural Integrity (ICONS), Kalpakkam, India, 4–7 February 2014; pp. 406–419. [Google Scholar]
- Mook, G.; Hesse, O.; Uchanin, V. Deep penetrating eddy currents and probes. Materialprufung 2007, 49, 258–264. [Google Scholar] [CrossRef]
- Papaelias, M.; Cheng, L.; Kogia, M.; Mohimi, A.; Kappatos, V.; Selcuk, C.; Constantinou, L.; Muñoz, C.Q.G.; Marquez, F.P.G.; Gan, T.H. Inspection and Structural Health Monitoring techniques for Concentrated Solar Power plants. Renew. Energy 2016, 85, 1178–1191. [Google Scholar] [CrossRef]
- Rifai, D.; Abdalla, A.N.; Ali, K.; Razali, R. Giant Magnetoresistance Sensors: A Review on Structures and Non-Destructive Eddy Current Testing Applications. Sensors 2016, 16, 298. [Google Scholar] [CrossRef]
- He, Y.Z.; Tian, G.Y.; Zhang, H.; Alamin, M.; Simm, A.; Jackson, P. Steel Corrosion Characterization Using Pulsed Eddy Current Systems. IEEE Sens. J. 2012, 12, 2113–2120. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; He, P.; Wang, G.; Pan, H. Research on Acoustic Emission Characteristics and Crack Evolution during Rock Failure under Tensile and Tensile- and Compressive-Shear Stress States. Appl. Sci. 2024, 14, 545. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Han, E.; Ke, W. Application of acoustic emission technique in study of electrochemical corrosion and stress corrosion cracking progresses of metals. Corros. Sci. Prot. Technol. 2009, 21, 472–476. [Google Scholar] [CrossRef]
- Hase, A. In Situ Measurement of the Machining State in Small-Diameter Drilling by Acoustic Emission Sensing. Coatings 2024, 14, 193. [Google Scholar] [CrossRef]
- Ding, Y.; Reuben, R.L.; Steel, J.A. A new method for waveform analysis for estimating AE wave arrival times using wavelet decomposition. NDT&E Int. 2004, 37, 279–290. [Google Scholar]
- Máthis, K.; Prchal, D.; Novotný, R.; Hähner, P. Acoustic emission monitoring of slow strain rate tensile tests of 304L stainless steel in supercritical water environment. Corros. Sci. 2011, 53, 59–63. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Han, E.H. Acoustic emission response of sensitized 304 stainless steel during intergranular corrosion and stress corrosion cracking. Corros. Sci. 2013, 73, 262–273. [Google Scholar] [CrossRef]
- Yuyama, S.; Kishi, T.; Hisamatsu, Y. Effect of environment, mechanical conditions, and materials characteristics on AE behavior during corrosion fatigue processes of an austenitic stainless steel. Nucl. Eng. Des. 1984, 81, 345–355. [Google Scholar] [CrossRef]
- Alekseev, A.B.; Averin, S.A.; Geferova, M.N.; Kondrat’Ev, V.P.; Shikhalev, V.S. Corrosion resistance of austenitic steels and alloys in high temperature water. J. Nucl. Mater. 1996, 233, 1367–1371. [Google Scholar] [CrossRef]
- Cassagne, T.; Caron, D.; Daret, J.; Proust, A.; Boulanger, D. Initial Results on the Stress Corrosion Cracking Monitoring of Alloy 600 in High Temperature Water Using Acoustic Emission. In Proceedings of the Ninth International Symposium on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, Asheville, NC, USA, 11–15 August 2013. [Google Scholar]
- Xu, J.; Han, E.H.; Wu, X. Acoustic emission response of 304 stainless steel during constant load test in high temperature aqueous environment. Corros. Sci. 2012, 63, 91–99. [Google Scholar] [CrossRef]
- Ritter, S.; Horner, D.A.; Bosch, R.W. corrosion monitoring techniques for detection of crack initiation under simulated light water reactor conditions. Br. Corros. J. 2013, 47, 251–264. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, S.; Lee, T.L.; Fancey, K.S.; Mi, J. Non-destructive testing and evaluation of composite materials/structures: A state-of-the-art review. Adv. Mech. Eng. 2020, 12, 1687814020913761. [Google Scholar] [CrossRef]
- Thompson, N.G.; Yunovich, M.; Dunmire, D. Cost of corrosion and corrosion maintenance strategies. Corros. Rev. 2007, 25, 247–261. [Google Scholar] [CrossRef]
- Kim, M.K.; Chung, D.D.L. Electrical-Connection-Related Stray Inductance Causing Overassessment of the Electrical Resistance Measured by Using the Two-Probe Method. J. Electron. Mater. 2023, 53, 1026–1034. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, W.; Chen, W.; Liu, L.; Xu, Y.; Huang, Y. Probing the Dynamic Progression of Erosion-Corrosion of X65 Pipeline Steel Using the Electrical Resistance Method in Conjunction with Galvanostatic Polarization. Lubricants 2022, 10, 345. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Wang, Z.; Li, G.; Wang, X.; Huang, Y. Probing and separating corrosion and erosion of pipeline steel using electrical resistance method in conjunction with electrochemical measurements. Measurement 2021, 183, 109797. [Google Scholar] [CrossRef]
- Cao, C. Principles of Electrochemistry of Corrosion; Chemical Industry Press: Beijing, China, 2008; pp. 37–38. [Google Scholar]
- Li, X.; Wang, J.; Han, E.H.; Ke, W. Corrosion behavior for Alloy 690 and Alloy 800 tubes in simulated primary water. Corros. Sci. 2013, 67, 169–178. [Google Scholar] [CrossRef]
- Molander, A.; Ullberg, M. New technique for in-plant high mass transfer rate electrochemical potential monitoring. Energy Mat. 2008, 3, 99–103. [Google Scholar] [CrossRef]
- Ramar, A.; Grundler, P.V.; Karastoyanov, V.; Günther-Leopold, I.; Abolhassani-Dadras, S.; Kivel, N.; Ritter, S. Effect of Pt injection rate on corrosion potential and Pt distribution on stainless steel under simulated boiling water reactor conditions. Corros. Eng. Sci. Technol. 2012, 47, 489–497. [Google Scholar] [CrossRef]
- Uchida, S.; Naitoh, M.; Okada, H.; Uehara, Y.; Koshizuka, S.; Lister, D.H. Effects of Water Chemistry on Flow Accelerated Corrosion and Liquid Droplet Impingement. In Proceedings of the Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia, Nagoya, Japan, 28–30 October 2009. [Google Scholar]
- Christensen, H. Remodeling of the oxidant species during radiolysis of high-temperature water in a pressurized water reactor. Nucl. Technol. 1995, 109, 373–382. [Google Scholar] [CrossRef]
- Rosinger, E.L.J.; Dixon, R.S. Mathematical Modelling of Water Radiolysis; A Discussion of Various Methods; Whiteshell Nuclear Research Establishment: Pinawa, MB, Canada, 1977; p. 76. [Google Scholar]
- Burns, W.G.; Moore, P.B. Water radiolysis and its effect upon in-reactor zircaloy corrosion. Radiat. Eff. 1976, 30, 233–242. [Google Scholar] [CrossRef]
- Kim, Y.J.; Niedrach, L.W.; Lin, C.C.K.S. Development of ECP Models for BWR Applications; NACE International: Houston, TX, USA, 1995. [Google Scholar]
- Macdonald, D.D.; Urquidi-Macdonald, M.D. Interpretation of Corrosion Potential Data from Boiling-Water Reactors Under Hydrogen Water Chemistry Conditions. Corrosion 1996, 52, 659–670. [Google Scholar] [CrossRef]
- Macdonald, D.D. Viability of Hydrogen Water Chemistry for Protecting In-Vessel Components of Boiling Water Reactors. Corrosion 1992, 48, 194–205. [Google Scholar] [CrossRef]
- Urquidi-Macdonald, M.; Pitt, J.; Macdonald, D.D. The impact of radiolytic yield on the calculated ECP in PWR primary coolant circuits. J. Nucl. Mater. 2007, 362, 1–13. [Google Scholar] [CrossRef]
- Cheng, X.; Li, C.; Dong, C.; Li, X. Constituent phases of the passive film formed on 2205 stainless steel by dynamic electrochemical impedance spectroscopy. Int. J. Miner. Metall. Mater. 2011, 18, 42–47. [Google Scholar] [CrossRef]
- Lei, X.; Wang, H.; Mao, F.; Zhang, J.; Zhao, M.; Fu, A.; Feng, Y.; Macdonald, D.D. Electrochemical behaviour of martensitic stainless steel after immersion in a H2S-saturated solution. Corros. Sci. 2018, 131, 164–173. [Google Scholar] [CrossRef]
- Euch, S.E.L.; Bricault, D.; Cachet, H.; Sutter, E.M.M.; Tran, M.T.T.; Vivier, V.; Engler, N.; Marion, A.; Skocic, M.; Huerta-Ortega, B. Temperature dependence of the electrochemical behavior of the 690 Ni-base alloy between 25 and 325 °C. Electrochim. Acta 2019, 317, 509–520. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ming, H.; Zhang, Z.; Han, E.-H. Effect of temperature on corrosion behavior of alloy 690 in high temperature hydrogenated water. J. Mater. Sci. Technol. 2018, 34, 1419–1427. [Google Scholar] [CrossRef]
- Macak, J.; Novotny, R.; Sajdl, P.; Bystriansky, V.; Tuma, L.; Novak, M. In-situ electrochemical impedance measurements of corroding stainless steel in high subcritical and supercritical water. Corros. Sci. 2019, 150, 9–16. [Google Scholar] [CrossRef]
- Ai, J.; Chen, Y.; Urquidi-Macdonald, M.; Macdonald, D.D. Electrochemical Impedance Spectroscopic Study of Passive Zirconium: I. High-Temperature, Deaerated Aqueous Solutions. J. Electrochem. Soc. 2007, 154, C43–C51. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.H.; Macdonald, D.D. Effects of temperature and pH on the electrochemical behaviour of alloy 600 in simulated pressurized water reactor primary water. J. Nucl. Mater. 2020, 528, 151850. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Macdonald, D.D.; Wang, S. Study of Steady-state Electrochemical Reactions on Stainless Steels in Elevated Temperature Aqueous Systems. In Proceedings of the 21st International Conference on Water Chemistry in Nuclear Reactor Systems, San Francisco, CA, USA, 9–14 September 2018; p. 12. [Google Scholar]
- Li, Y.; Macdonald, D.D.; Yang, J.; Qiu, J.; Wang, S. Point defect model for the corrosion of steels in supercritical water: Part I, film growth kinetics. Corros. Sci. 2020, 163, 108280. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Wang, J.; Cao, C. Analysis and application of electrochemical noise technology—I. Theory of electrochemical noise analysis. J. Chin. Soc. Corros. Prot. 2001, 21, 11. [Google Scholar]
- Zhang, J.; Zhang, Z.; Wang, J.; Cheng, S.; Cao, C. Analysis and application of electrochemical noise technology II. Application of electrochemical noise. J. Chin. Soc. Corros. Prot. 2002, 22, 241–248. [Google Scholar]
- Guo, Q.; Wu, X.; Xu, S.; Feng, B.; Hu, B.; Lu, J. Research status and development of high temperature and high pressure on-line corrosion monitoring technology. Corros. Sci. Prot. Technol. 2016, 28, 7. [Google Scholar]
- Taylor, D.F.; Hickling, J.; Andresen, P.L. Use of electrochemical noise to detect stress corrosion crack initiation in simulated BWR environments. Mater. Corros. 1999, 49, 651–658. [Google Scholar]
- Ritter, S.; Seifert, H.P. Influence of reference electrode distance and hydrogen content on electrochemical potential noise during SCC in high purity, high temperature water. Br. Corros. J. 2013, 48, 199–206. [Google Scholar] [CrossRef]
- Ma, C.; Song, S.; Gao, Z.; Wang, J.; Xia, D.H. Electrochemical Noise Monitoring of the Atmospheric Corrosion of Steels: Identifying Corrosion Form Using Wavelet Analysis. Corros. Eng. Sci. Technol. 2017, 52, 432–440. [Google Scholar] [CrossRef]
- Eckermann, F.; Suter, T.; Uggowitzer, P.J.; Afseth, A.; Davenport, A.J.; Connolly, B.J.; Larsen, M.H.; Carlo, F.D.; Schmutz, P. In situ monitoring of corrosion processes within the bulk of AlMgSi alloys using X-ray microtomography. Corros. Sci. 2008, 50, 3455–3466. [Google Scholar] [CrossRef]
- Chen, J.F.; Bogaerts, W.F. The physical meaning of noise resistance. Corros. Sci. 1995, 37, 1839–1842. [Google Scholar] [CrossRef]
- Xiao, H.; Mansfeld, F. Evaluation of Coating Degradation with Electrochemical Impedance Spectroscopy and Electrochemical Noise-Analysis. J. Electrochem. Soc. 1994, 141, 2332–2337. [Google Scholar] [CrossRef]
- Iverson, W.P. Transient Voltage Changes Produced in Corroding Metals and Alloys. J. Electrochem. Soc. 1968, 115, 617. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Lvov, S.N.; Wei, X.J.; Benning, L.G.; Macdonald, D.D. Quantitative evaluation of general corrosion of Type 304 stainless steel in subcritical and supercritical aqueous solutions via electrochemical noise analysis. Corros. Sci. 2002, 44, 841–860. [Google Scholar] [CrossRef]
- Macak, J.; Sajdl, P.; Kucera, P.; Novotny, R.; Vosta, J. In situ electrochemical impedance and noise measurements of corroding stainless steel in high temperature water. Electrochim. Acta 2006, 51, 3566–3577. [Google Scholar] [CrossRef]
- Ku?Era, P.; Macák, J.; Sajdl, P.; Novotny, R. Evaluation of 08CH18N10T stainless steel corrosion in subcritical water by electrochemical noise analysis. Mater. Corros. 2008, 59, 719–726. [Google Scholar]
- Song, S.; Wang, J.; Li, J.; Zhao, W.; Gao, Z. Electrochemical noise technology for detecting corrosion damage of nuclear power environmental materials. Prog. Chin. Mater. 2011, 30, 7. [Google Scholar]
- Arganis-Juarez, C.R.; Malo, J.M.; Uruchurtu, J. Electrochemical noise measurements of stainless steel in high temperature water. Nucl. Eng. Des. 2007, 237, 2283–2291. [Google Scholar] [CrossRef]
- Stewart, J.; Wells, D.B.; Scott, P.M.; Williams, D.E. Electrochemical noise measurements of stress corrosion cracking of sensitised austenitic stainless steel in high-purity oxygenated water at 288 °C. Corros. Sci. 1992, 33, 73–88. [Google Scholar] [CrossRef]
- Kim, H.S. A Study for Modeling Electrochemistry in Light Water Reactors. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 2007. [Google Scholar]
- Karaminezhaad-Ranjbar, M.; Mankowski, J.; Macdonald, D.D. Pitting Corrosion of Inconel 600 in High Temperature Chloride Solution under Controlled Hydrodynamic Conditions. Corrosion 1985, 41, 197–204. [Google Scholar] [CrossRef]
- Kim, H.; Macdonald, D.D. Measurement of steady-state hydrogen electrode reactions on Alloys 600 and 690 tubes. Corrosion Science 2010, 52, 1139–1145. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Mankowski, J.; Karaminezhaadranjbar, M.; Hu, Y.H. Apparatus for Controlled Hydrodynamic Electrochemical and Corrosion Studies in High-Temperature Aqueous Systems. Corrosion 1988, 44, 186–192. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.-H.; Rao, J. Effect of alternately changing the dissolved Ni ion concentration on the oxidation of 304 stainless steel in oxygenated high temperature water. Corros. Sci. 2011, 53, 2582–2591. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.-H. The oxidation behaviour of 304 stainless steel in oxygenated high temperature water. Corros. Sci. 2010, 52, 4081–4087. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.H.; Rao, J. The mechanism of oxide film formation on Alloy 690 in oxygenated high temperature water. Corros. Sci. 2011, 53, 3853–3860. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Han, E.-H.; Wu, X. Influence of Zn on oxide films on Alloy 690 in borated and lithiated high temperature water. Corros. Sci. 2011, 53, 3254–3261. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Z.; Ding, S.; Wang, S.; Zhang, F.; Liu, H. An Online Testing System for Electrochemical Corrosion Behavior in Supercritical Water Systems. CN202210499746.6, 29 July 2022. [Google Scholar]
- Yang, J.; Li, Y.; Xu, A.; Fekete, B.; Macdonald, D.D. The electrochemical properties of alloy 690 in simulated pressurized water reactor primary water: Effect of temperature. J. Nucl. Mater. 2019, 518, 305–315. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Z.; Ding, S.; Wang, S.; Li, Z.; Sun, S. A Multifunctional Electrochemical Research Platform Suitable for Sub/Supercritical Water Environments. CN202210499044.8, 5 August 2022. [Google Scholar]
- Lin, S.; Li, H.P.; Xu, L.P.; Zhang, Y.Q.; Cui, C. A novel experimental device for electrochemical measurements in supercritical fluids up to 700 degrees C/1000 bar and its application in the corrosion study of superalloy Inconel 740H. RSC Adv. 2017, 7, 33914–33920. [Google Scholar] [CrossRef]



| Monitoring Techniques | Principle | Data Presentation | Advantages | Disadvantages | Application | Error or Accuracy |
|---|---|---|---|---|---|---|
| Ultrasonic measurement [29,30] | Highly sensitive to structural damage; high permeability to materials | Depth of corrosion pits and thickness of corrosion layer | Real-time, suitable for both internal and external corrosion | Not appropriate for small, thin materials | Large-scale testing of oil pipelines and other facilities | Error less than 15% |
| Infrared thermography [31,32] | Recording electromagnetic waves emitted by objects and establishing a clear relationship between temperature and material corrosion defects | Defect size, depth, and thermal characteristics | Reliable, fast, direct, and wide testing range | Required heating and cooling processes; not suitable for thick materials; costly | Extreme temperatures and environments | Affected by changes in the thermal characteristics of objects and environmental conditions |
| Microwave imaging [33,34] | Based on the interaction between microwaves and dielectric materials | The dielectric performance changes caused by defects or structural abnormalities are converted into readable voltage values and then processed and output as images. | Easily accessible to the coated materials. | Have difficulties penetrating conductive materials; it is limited to surface corrosion detection, whereas the deeper corrosion is undetectable. | Determine concrete properties, etc. | Accuracy better than 1/100 of the period length |
| Eddy current detection [35,36] | Detect discontinuities such as corrosion and material loss by monitoring changes in coil impedance or measuring induced magnetic fields. | Depth of corrosion layer, corrosion rate, conductivity and permeability, etc. | It is fast and most commonly used in conductive materials; it is portable and cheap | It is sensitive to skin effects and is surface-oriented. | Suitable for materials of various shapes | Error less than 5% for the thickness of various corrosion [36] |
| Acoustic emission [37,38] | Materials emit transient sound waves locally due to the rapid release of energy. | Deposition of corrosion products, the rupture of passivation films, and the initiation and propagation of cracks | Passive, non-intrusive, low-cost real-time and remote monitoring | Sensitive to background noise | wavelet analysis, modern spectral analysis, and neural network analysis | The accuracy of signal classification is about 65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Bai, Z.; Xing, L.; Zhang, Q.; Ding, S.; Zhang, Y.; Gao, P.; Yu, Z.; Xu, D. Corrosion Monitoring Techniques in Subcritical and Supercritical Water Environments. Appl. Sci. 2024, 14, 2350. https://doi.org/10.3390/app14062350
Li Y, Bai Z, Xing L, Zhang Q, Ding S, Zhang Y, Gao P, Yu Z, Xu D. Corrosion Monitoring Techniques in Subcritical and Supercritical Water Environments. Applied Sciences. 2024; 14(6):2350. https://doi.org/10.3390/app14062350
Chicago/Turabian StyleLi, Yanhui, Zhouyang Bai, Limei Xing, Qian Zhang, Shaoming Ding, Yinan Zhang, Pengfei Gao, Zhihong Yu, and Donghai Xu. 2024. "Corrosion Monitoring Techniques in Subcritical and Supercritical Water Environments" Applied Sciences 14, no. 6: 2350. https://doi.org/10.3390/app14062350





