Effects of Mechanical Vibration in Equine Osteoarthritis: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
- Lameness assessed as follows: subjective evaluation by two expert equine veterinarians (RG and RR) using the AAEP scale grade from 0 to 5, and an objective gait analysis evaluation using a markerless smartphone computer vision method (Sleip AI);
- Positive result to flexion test;
- Response to intra-articular diagnostic analgesia;
- Radiographic evidence of acute or chronic, localized, or diffuse OA.
- Presence of osteochondritis dissecans (OCD) or articular fracture;
- Lameness due to septic arthritis;
- Joint that undergoes surgical treatment 3 months prior to the study;
- Joint that has received an intra-articular joint injection of anti-arthritic medication 3 months prior to the study;
- horses that received systemic anti-inflammatory drugs 30 days prior the study.
- Two horses met both the inclusion and exclusion criteria.
2.3. Device
2.4. Study Design
2.5. Assessment
2.5.1. Subjective Lameness Evaluation
2.5.2. Objective Lameness Evaluation Using an Artificial Intelligence System
2.5.3. Intra-Articular Diagnostic Analgesia
2.5.4. Radiographic Examination
2.6. Selected Cases
2.7. Treatment Protocol
2.8. Re-Assessment
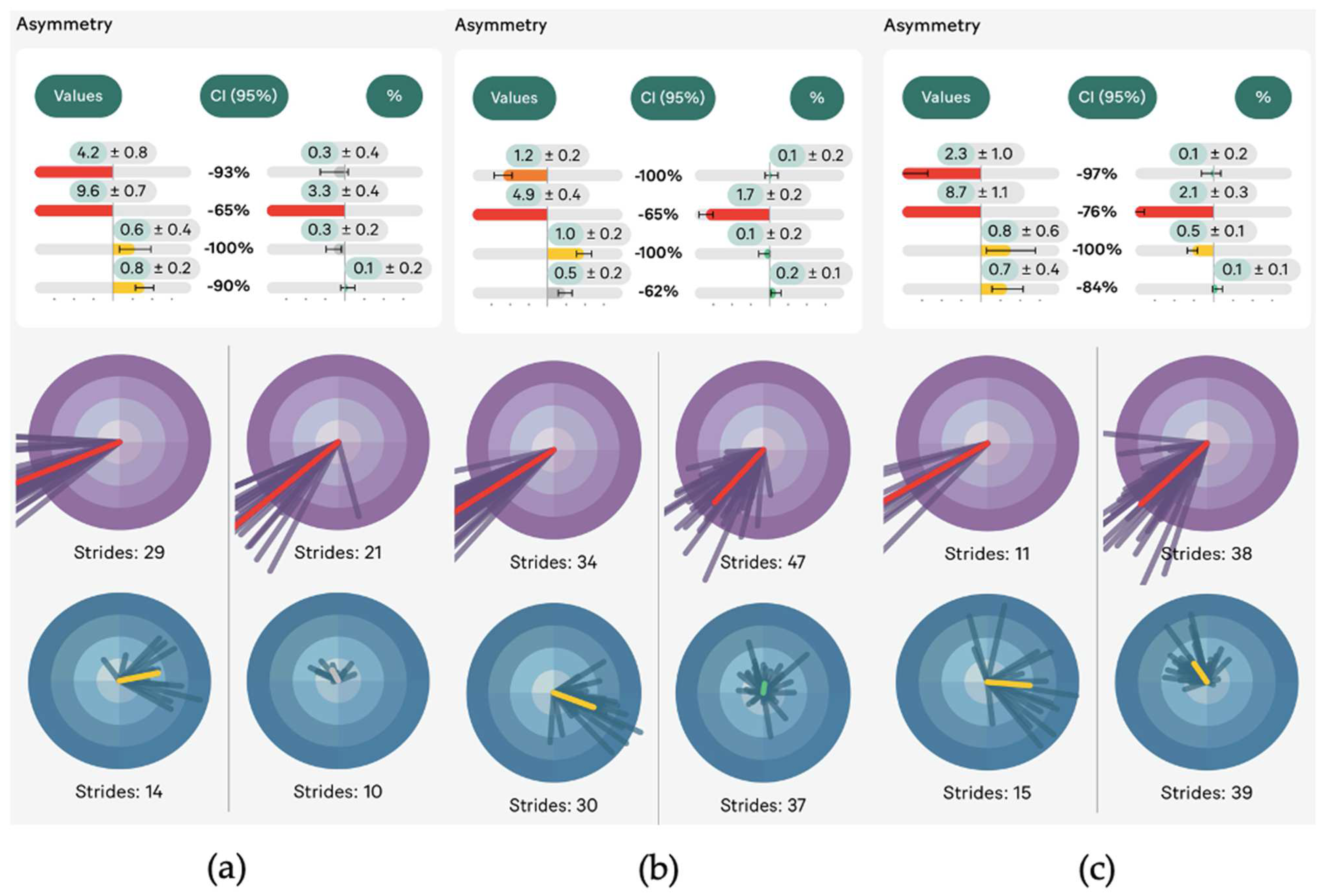
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellomo, R.G.; Carmignano, S.M.; Saggini, R. The Mechanical Vibration: Therapeutic Effects and Applications. In The Applied Mechanical Vibration as Ultrasound Energy; Bentham eBooks: Sharjah, United Arab Emirates, 2017; pp. 89–180. [Google Scholar]
- Nigg, B.M.; Wakeling, J.M. Impact Forces and Muscle Tuning: A New Paradigm. Exerc. Sport Sci. Rev. 2001, 29, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Watson, T. Therapeutic Ultrasound. In Canine Rehabilitation and Physical Therapy; Saunders: Philadelphia, PA, USA, 2022; pp. 328–334. [Google Scholar]
- Boström, A.; Asplund, K.; Bergh, A.; Hyytiäinen, H. Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Therapeutic Ultrasound. Animals 2022, 12, 3144. [Google Scholar] [CrossRef]
- Bergh, A.; Lund, I.; Boström, A.; Hyytiäinen, H.; Asplund, K. A Systematic Review of Complementary and Alternative Veterinary Medicine: “Miscellaneous Therapies”. Animals 2021, 11, 3356. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, B.; Millis, D.L.; Levine, D.; Guevara, J.L. Effect of Therapeutic Ultrasound on Calcaneal Tendon Heating and Extensibility in Dogs. Front. Vet. Sci. 2019, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, U.; Toniato, M.; Harrison, A. Assessment of Noninvasive Low-Frequency Ultrasound as a Means of Treating Injuries to Suspensory Ligaments in Horses: A Research Paper. J. Equine Vet. Sci. 2019, 80, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tezel, A.; Mitragotri, S. Interactions of Inertial Cavitation Bubbles with Stratum Corneum Lipid Bilayers during Low-Frequency Sonophoresis. Biophys. J. 2003, 85, 3502–3512. [Google Scholar] [CrossRef] [PubMed]
- Rinnovati, R.; Forni, G.; Beltrame, A.; Spadari, A. Treatment of Septic Arthritis with Acoustic Cavitation and Lavage: A Case Report. J. Equine Vet. Sci. 2020, 88, 102945. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M. Effects of Physical Agents on Muscle Healing with a Focus on Animal Model Research. Phys. Ther. Res. 2021, 24, 1–8. [Google Scholar] [CrossRef]
- Lindner, A.; Dingerkus, A. Incidence of Training Failure among Thoroughbred Horses at Cologne, Germany. Ger. Prev. Vet. Med. 1993, 16, 85–94. [Google Scholar] [CrossRef]
- Kim, J.; Bae, H.; Han, H.S.; Lee, J. Ultrasonic Enhancement of Chondrogenesis in Mesenchymal Stem Cells by Bolt-Clamped Langevin Transducers. Micromachines 2023, 14, 202. [Google Scholar] [CrossRef]
- Schlachter, C.; Lewis, C. Electrophysical Therapies for the Equine Athlete. Vet. Clin. N. Am.-Equine Pract. 2016, 32, 127–147. [Google Scholar] [CrossRef] [PubMed]
- AAEP. American Association of Equine Practitioners Guide to Veterinary Services for Horse Shows; Manual. American Association of Equine Practitioners: Lexington, KY, USA, 1999. [Google Scholar]
- Sleip. Sleip—Scientifically Validated Equine Gait Analysis App—Tutorial and Guides; Sleip: Stockholm, Sweden, 2023. [Google Scholar]
- Lawin, F.J.; Byström, A.; Roepstorff, C.; Rhodin, M.; Almlöf, M.; Silva, M.; Andersen, P.H.; Kjellström, H.; Hernlund, E. Is Markerless More or Less? Comparing a Smartphone Computer Vision Method for Equine Lameness Assessment to Multi-Camera Motion Capture. Animals 2023, 13, 390. [Google Scholar] [CrossRef]
- Kramer, J.K.G.K. Kinetics of Lameness. In Equine Sports Medicine and Surgery; Saunders Elsevier: Amsterdam, The Netherlands, 2014; pp. 223–238. [Google Scholar]
- Butler, J.A.; Colles, C.; Dyson, S.J.; Kold, S.E.; Poulos, P.W. Metacarpophalangeal and Metatarsophalangeal (Fetlock) Joints. In Clinical Radiology of the Horse; John Wiley & Sons Inc.: Chichester, UK, 2017; pp. 175–213. [Google Scholar]
- Vanderperren, K.; Saunders, J.H. Diagnostic Imaging of the Equine Fetlock Region Using Radiography and Ultrasonography. Part 2: The Bony Disorders. Vet. J. 2009, 181, 123–136. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.D., Jr.; Zachary, J.F. Comparison of Mouse and Rabbit Lung Damage Exposure to 30 KHz Ultrasound. Ultrasound Med. Biol. 1994, 20, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Tezel, A.; Sens, A.; Tuchscherer, J.; Mitragotri, S. Frequency Dependence of Sonophoresis. Pharm. Res. 2001, 18, 700–2694. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Tachibana, K.; Ogawa, K.; Tsujita, N.; Tomita, A. Scanning Electron Microscopic Evaluation of the Skin Surface after Ultrasound Exposure. Anat. Rec. 1997, 247, 455–461. [Google Scholar] [CrossRef]
- Revenaugh, M.S. Extracorporeal Shock Wave Therapy for Treatment of Osteoarthritis in the Horse: Clinical Applications. Vet. Clin. N. Am.-Equine Pract. 2005, 21, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Muste, A.; Muste, M.; Scurtu, L.; Tănase, A.; Ilie, I.; Hodis, L.; Beteg, F. The Improvement of the Joint Inflammatory Status in Dogs through Ultrasound Therapy. Sci. Works. Ser. C Vet. Med. 2015, LXI, 220–223. [Google Scholar]
- Singh, K.I.; Sobti, V.K.; Arora, A.K.; Bhatia, R. Therapeutic Ultrasound [1 Watt/cm2] in Experimental Acute Traumatic Arhtrits in the Equines. Indian Vet. J. Surg. 1996, 17, 81–92. [Google Scholar]
- Singh, K.I.; Sobti, V.K.; Roy, K.S. Gross and Histomorphological Effects of Therapeutic Ultrasound [1 Watt/cm2] in Experimental Acute Traumatic Arthritis in Donkeys. J. Equine Vet. Sci. 1997, 17, 150–155. [Google Scholar] [CrossRef]
- Montgomery, L.; Elliott, S.B.; Adair, H.S. Muscle and Tendon Heating Rates with Therapeutic Ultrasound in Horses. Vet. Surg. 2013, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, W.L. Physical Principles of Ultrasound. In Ultrasound, Its Application in Medicine and Biology; Fry, F.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1978; pp. 1–75. [Google Scholar]
- Khokhlova, V.A.; Bailey, M.R.; Reed, J.A.; Cunitz, B.W.; Kaczkowski, P.J.; Crum, L.A. Effects of Nonlinear Propagation, Cavitation, and Boiling in Lesion Formation by High Intensity Focused Ultrasound in a Gel Phantom. J. Acoust. Soc. Am. 2006, 119, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L. Overview of Experimental Studies of Biological Effects of Medical Ultrasound Caused by Gas Body Activation and Inertial Cavitation. Prog. Biophys. Mol. Biol. 2007, 93, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.P.; Rudenko, O.V.; Nyborg, W.L. Biomedical Applications of Radiation Force of Ultrasound: Historical Roots and Physical Basis. Ultrasound Med. Biol. 2010, 36, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.T.; Forsberg, F.; Tornes, A.; Østensen, J.; Goldberg, B.B. Destruction of Contrast Microbubbles and the Association with the Intertial Cavitation. Ultrasound Med. Biol. 2000, 26, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Carvell, K.J.; Bigelow, T.A. Dependence of Optimal Seed Bubble Size on Pressure Amplitude at Therapeutic Pressure Levels. Ultrasonics 2011, 51, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.N.T. Ultrasonic in Medicine and Biology. Phys. Med. Biol. 1977, 22, 629. [Google Scholar]
- Odegaard, S.; Helge Gilja, O.; Gregersen, H. Basic and New Aspects of Gastrointestinal Ultrasonography; World Scientific: Singapore, 2005. [Google Scholar]
- Holland, C.K.; Apfel, R.E. An Improved Theory for the Prediction of Micro Cavitation Thresholds. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1989, 36, 204–208. [Google Scholar] [CrossRef]
- Robertson VJ, B.K. A Review of Therapeutic Ultrasound: Effectiveness Studies. Phys. Ther. 2001, 81, 1339–1350. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinnovati, R.; Meistro, F.; Ralletti, M.V.; Marzari, F.; Saragoni, G.; Gottarelli, R.; Pasquotto, A.; Spadari, A. Effects of Mechanical Vibration in Equine Osteoarthritis: A Pilot Study. Appl. Sci. 2024, 14, 2762. https://doi.org/10.3390/app14072762
Rinnovati R, Meistro F, Ralletti MV, Marzari F, Saragoni G, Gottarelli R, Pasquotto A, Spadari A. Effects of Mechanical Vibration in Equine Osteoarthritis: A Pilot Study. Applied Sciences. 2024; 14(7):2762. https://doi.org/10.3390/app14072762
Chicago/Turabian StyleRinnovati, Riccardo, Federica Meistro, Maria Virginia Ralletti, Francesca Marzari, Giuditta Saragoni, Roberto Gottarelli, Anna Pasquotto, and Alessandro Spadari. 2024. "Effects of Mechanical Vibration in Equine Osteoarthritis: A Pilot Study" Applied Sciences 14, no. 7: 2762. https://doi.org/10.3390/app14072762




