Laminar Ulva Species: A Multi-Tool for Humankind?
Abstract
:Featured Application
Abstract
1. Introduction
2. The Biology of Ulva sp.
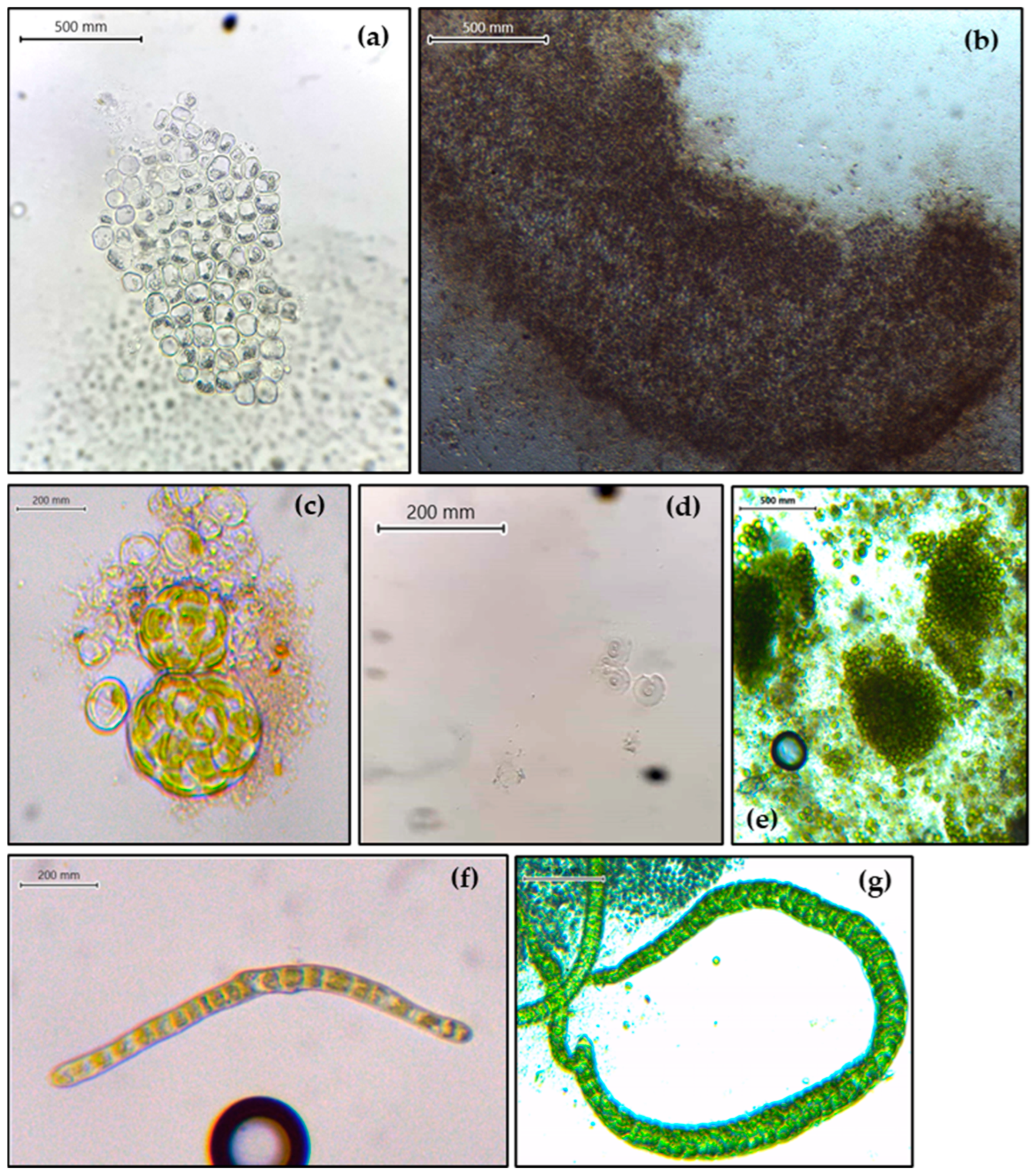
2.1. Reproduction
2.2. Influence of Factors
2.2.1. Biotic Factors
Thallus Age
Phytohormones
Sporulation Inhibitors
Algae Microbial Interaction
Mutualistic Symbiotic Relationships
Metabolomic Profile
2.2.2. Abiotic Factors
Salinity
Light Intensity
Temperature
Nutrients
Thallus Fragmentation
3. Ulva Cultivation Methods: Towards an Innovative Future?
3.1. Vegetative Propagation Method
3.2. Life Cycle Method
- (I)
- Connected, germinating cell
- (II)
- Germinating cell with a discernible germination tube
- (III)
- Initiation of distal cell division
- (IV)
- Onset of lateral cell division
- (V)
- Juvenile thallus displaying evident expansion resulting from distal and lateral cell divisions [105]
3.3. In Vitro
- (1)
- The plant of interest must be chosen; this is normally determined by the purpose of the study, although disease- and insect-free plants are desired; if the plant demands it, pre-treatments can be used.
- (2)
- The start of the in vitro culture necessitates the removal of tiny plant parts (explants) or the use of seeds with their surfaces sterilized.
- (3)
- The explants are then put in appropriate culture conditions and cultured for a short amount of time, with infected explants destroyed. The stages that follow differ based on the intended culture.
- (4)
- Organogenesis is the propagation phase in which explants are cultivated on suitable culture conditions for shoot or root multiplication, while in callogenesis, the callus is multiplied.
- (5)
- Callus and root cultures are grown in bioreactors, while propagated shoots are moved to root-promoting culture medium in the case of micropropagation.
- (6)
- Micropropagated plants are toughened in order to produce individual photosynthesis-capable plants. Hardening is done gradually, allowing the plants to adjust to ex vitro environments [107].
3.4. Cultivation Control or Monitoring Techniques
3.5. Cultivation Security and Safety Measures
4. Ulva Compounds of Interest
4.1. Ulvan
4.2. Pigments
4.2.1. Chlorophylls
4.2.2. Carotenoids
4.3. Phenolic Compounds
4.4. Fatty Accids
4.5. Proteins
5. Commercial Potential
5.1. Human Food
5.2. Agriculture and Livestock Animals
5.3. Pharmaceuticals
- Anticancer—Aronoff 1957 [129] showed that chlorophylls may be employed as precursors of photosensitizers for photodynamic therapy, namely for cancer treatment and microorganism inactivation [130,195]. Another study carried out by Lee et al. (2004) [196] tested the water-soluble component of U. lactuca extract at concentrations up to 140 µg/mL on human leukemia cells (U937). Surprisingly, 50% growth inhibition was reported following therapy. At a dosage of 100 µg/mL, splenocytes showed increased proliferation. Furthermore, the macrophage cell line (RAW 264.7) increased nitric oxide production, which is thought to regulate cytokine action [173].
- Antioxidant—Antioxidants found in chlorophylls, xanthophylls, and carotenoids can function as free radical scavengers, thus helping to safeguard cellular and tissue integrity against oxidative damage and helping to preserve cellular well-being and mitigate susceptibility to disease chronic illnesses such as cancer and improve immune system response [130,180].
- Antihypercholesterolemic—The high yield of soluble dietary fiber is shown in species like Kappaphycopsis cottonii (formerly Eucheuma cottonii) (Rhodophyta), Caulerpa lentillifera (Chlorophyta), Sargassum polycystum (Phaeophyceae), Gymnogongrus durvillei (formerly Ahnfeltiopsis concinna) (Rhodophyta), Gayralia oxysperma (Chlorophyta), Chondrus ocellatus (Rhodophyta), and U. lactuca (as U. fasciata) (Chlorophyta), which can help reduce cholesterol [130,180]. Another study carried out by Hassan et al. (2011) [197] subjected albino rats as an in vivo model to being fed precipitated polysaccharides for 21 days. The control group was given a reference medication named Lapitor (Atorvastatin Ca). Ulvan-treated rats had decreased levels of blood total lipids and triglycerides, total cholesterol, LDL (low-density lipoprotein), and VLDL. Improved levels of atherogenic index, HDL (high-density lipoprotein) (180%), and enzyme activity creatine kinase and lactate dehydrogenase were also seen. Furthermore, hepatic enzyme activity (glutathione peroxidase, superoxide dismutase, and catalase) were enhanced. On the other hand, a decrease of total glutathione and thiol was also detected, indicating that ulvan is a strong drug against induced hypercholesterolemic situations [173].
- Anti-inflammatory—Awad (2000) [198] extracted a physiologically active steroid (3-O-β-D-glucopyranosyl clerosterol) from U. lactuca and tested it on mice ear edema. At dosages of 1000 and 1500 mg/year, edema decreased significantly (p < 0.05) (62.2 and 72.2%, respectively). The green macroalgae, U. lactuca, has been studied in a rat model to prevent edema by lowering inflammation [173].
- Antimicrobial—It was identified in Abbassy et al., 2014 [199] that U. lactuca has insecticidal properties. The acetone extract was considered the best against inhibiting the mosquito population, killing the larvae of Culex pipiens (a hematophagous mosquito belonging to the Culicidae family, a vector of diseases such as Japanese encephalitis, meningitis, and urticaria). Spavieri et al., 2010 [200] reported that Ulva extract indicated only moderate antiprotozoal (specifically trypanocidal) activity (IC50 = 34.9 µg/mL) against Trypanosoma cruzi but good leishmanicidal activity (IC50 = 12 µg/mL) [173].
- Anticoagulants—These are remarkably exploited in medicines to avoid blood clotting. Mao et al., 2006 [202] identified the anticoagulant properties of pure polysaccharides from Ulva conglobata using a hot water and ethanol extraction procedure, followed by ion exchange and HPLC purification. Sulfated polysaccharides were thought to limit thrombin activity both directly and via increasing the potency of heparin cofactor II (HCII) [173].
- Antiobesity and antidiabetic effects—BelHadj et al. (2013) [200] found that extracts from U. lactuca may offer benefits in managing obesity and diabetes. Components of U. lactuca exhibit anti-obesity effects by inhibiting adipogenesis and enhancing lipid metabolism. Additionally, they have been shown to enhance glucose metabolism and insulin sensitivity, suggesting potential antidiabetic properties [128,203].
5.4. Cosmetics
5.5. Biofuel
5.6. Bioplastic
5.7. Feed Aquaculture and Bioremediation
6. Ulva sp. as Environmental Danger
7. Future Roads
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021; FAO: Rome, Italy, 2021; ISBN 9789251343258. [Google Scholar]
- Graham, L.E.; Wilcox, L.W. Algae; Prentice-Hall: Hoboken, NJ, USA, 2000. [Google Scholar]
- Gallardo, T. Marine Algae: General Aspects (Biology, Systematics, Field and Laboratory Techniques). In Marine Algae; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–67. [Google Scholar]
- Mantri, V.A.; Kazi, M.A.; Balar, N.B.; Gupta, V.; Gajaria, T. Concise Review of Green Algal Genus Ulva Linnaeus. J. Appl. Phycol. 2020, 32, 2725–2741. [Google Scholar] [CrossRef]
- Ben-Ari, T.; Neori, A.; Ben-Ezra, D.; Shauli, L.; Odintsov, V.; Shpigel, M. Management of Ulva lactuca as a Biofilter of Mariculture Effluents in IMTA System. Aquaculture 2014, 434, 493–498. [Google Scholar] [CrossRef]
- García-Poza, S.; Morais, T.; Leandro, A.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. A Comparative Study of the Fatty Acids and Monosaccharides of Wild and Cultivated Ulva sp. J. Mar. Sci. Eng. 2022, 10, 233. [Google Scholar] [CrossRef]
- Zhang, J.; Huo, Y.; Wu, H.; Yu, K.; Kim, J.K.; Yarish, C.; Qin, Y.; Liu, C.; Xu, R.; He, P. The Origin of the Ulva Macroalgal Blooms in the Yellow Sea in 2013. Mar. Pollut. Bull. 2014, 89, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, J.; Zhuang, M.; Zhang, J.; Yu, K.; Zhao, S.; Sun, Y.; Tong, Y.; Xia, L.; Qin, Y.; et al. Controlling the Source of Green Tides in the Yellow Sea: NaClO Treatment of Ulva Attached on Pyropia Aquaculture Rafts. Aquaculture 2021, 535, 736378. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, M.; Zhao, L.; Liu, Y.; Wen, Q.; Fu, M.; Yu, K.; Zhang, J.; He, P. Taxonomy and Genetic Diversity of Amphipods Living on Ulva lactuca L. from Gouqi Coast, China. Pac. Sci. 2020, 74, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, J.; Gao, S.; Huo, Y.; Cui, J.; Shen, H.; Liu, G.; He, P. Annual Patterns of Macroalgal Blooms in the Yellow Sea during 2007–2017. PLoS ONE 2019, 14, e0210460. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.F.; Edrees, M. Seasonal Changes in the Constituents of Ulva lactuca. Phytochemistry 1973, 12, 481–485. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Balar, N.B.; Mantri, V.A. Insights into Life Cycle Patterns, Spore Formation, Induction of Reproduction, Biochemical and Molecular Aspects of Sporulation in Green Algal Genus Ulva: Implications for Commercial Cultivation. J. Appl. Phycol. 2020, 32, 473–484. [Google Scholar] [CrossRef]
- Liu, X.; Blomme, J.; Bogaert, K.A.; D’hondt, S.; Wichard, T.; Deforce, D.; Van Nieuwerburgh, F.; De Clerck, O. Transcriptional Dynamics of Gametogenesis in the Green Seaweed Ulva mutabilis Identifies an RWP-RK Transcription Factor Linked to Reproduction. BMC Plant Biol. 2022, 22, 19. [Google Scholar] [CrossRef]
- Stratmann, J.; Paputsoglu, G.; Oertel, W. Differentiation of Ulva mutabilis (Chlorophyta) Gametangia and Gamete Release Are Controlled by Extracellular Inhibitors 1. J. Phycol. 1996, 32, 1009–1021. [Google Scholar] [CrossRef]
- De Clerck, O.; Kao, S.-M.; Bogaert, K.A.; Blomme, J.; Foflonker, F.; Kwantes, M.; Vancaester, E.; Vanderstraeten, L.; Aydogdu, E.; Boesger, J.; et al. Insights into the Evolution of Multicellularity from the Sea Lettuce Genome. Curr. Biol. 2018, 28, 2921–2933.e5. [Google Scholar] [CrossRef]
- Lüning, K.; Kadel, P.; Pang, S. Control Of Reproduction Rhythmicity by Environmental and Endogenous Signals in Ulva pseudocurvata (Chlorophyta). J. Phycol. 2008, 44, 866–873. [Google Scholar] [CrossRef]
- Katsaros, C.; Weiss, A.; Llangos, I.; Theodorou, I.; Wichard, T. Cell Structure and Microtubule Organisation during Gametogenesis of Ulva mutabilis Føyn (Chlorophyta). Bot. Mar. 2017, 60, 123–135. [Google Scholar] [CrossRef]
- Tan, C.-Y.; Dodd, I.C.; Chen, J.E.; Phang, S.-M.; Chin, C.F.; Yow, Y.-Y.; Ratnayeke, S. Regulation of Algal and Cyanobacterial Auxin Production, Physiology, and Application in Agriculture: An Overview. J. Appl. Phycol. 2021, 33, 2995–3023. [Google Scholar] [CrossRef]
- Jones, B.; Gunnerås, S.A.; Petersson, S.V.; Tarkowski, P.; Graham, N.; May, S.; Dolezal, K.; Sandberg, G.; Ljung, K. Cytokinin Regulation of Auxin Synthesis in Arabidopsis Involves a Homeostatic Feedback Loop Regulated via Auxin and Cytokinin Signal Transduction. Plant Cell 2010, 22, 2956–2969. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Žižková, E.; Kubeš, M.; Dobrev, P.I.; Přibyl, P.; Šimura, J.; Zahajská, L.; Záveská Drábková, L.; Novák, O.; Motyka, V. Control of Cytokinin and Auxin Homeostasis in Cyanobacteria and Algae. Ann. Bot. 2017, 119, 151–166. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hirai, N.; Kurimura, Y.; Ohigashi, H.; Tsuji, Y. Abscisic Acid-Dependent Algal Morphogenesis in the Unicellular Green Alga Haematococcus pluvialis; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1997; Volume 22. [Google Scholar]
- Nimura, K.; Mizuta, H. Inducible Effects of Abscisic Acid on Sporophyte Discs from Laminaria japonica Areschoug (Laminariales, Phaeophyceae). J. Appl. Phycol. 2002, 14, 159–163. [Google Scholar] [CrossRef]
- Nagata, T.; Takebe, I. Plating of Isolated Tobacco Mesophyll Protoplasts on Agar Medium. Planta 1971, 99, 12–20. [Google Scholar] [CrossRef]
- Hu, Y.; Bao, F.; Li, J. Promotive Effect of Brassinosteroids on Cell Division Involves a Distinct CycD3-induction Pathway in Arabidopsis. Plant J. 2000, 24, 693–701. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Skoog, F.; Miller, C.O. Chemical Regulation of Growth and Organ Formation in Plant Tissues Cultured in Vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar]
- Chandimali, N.; Park, E.H.; Bak, S.-G.; Lim, H.-J.; Won, Y.-S.; Lee, S.-J. Seaweed Callus Culture: A Comprehensive Review of Current Circumstances and Future Perspectives. Algal Res. 2024, 77, 103376. [Google Scholar] [CrossRef]
- Yokoya, N.S. Apical Callus Formation and Plant Regeneration Controlled by Plant Growth Regulators on Axenic Culture of the Red Alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Phycol. Res. 2000, 48, 133–142. [Google Scholar] [CrossRef]
- Yokoya, N.S.; West, J.A.; Luchi, A.E. Effects of Plant Growth Regulators on Callus Formation, Growth and Regeneration in Axenic Tissue Cultures of Gracilaria tenuistipitata and Gracilaria perplexa (Gracilariales, Rhodophyta). Phycol. Res. 2004, 52, 244–254. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M.P. In Vitro Plant Propagation: A Review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar]
- Yoon, J.T.; Soh, W.Y. Developmental Morphology on the Regeneration of Pelvetia siliquosa. Algae 1998, 13, 261–270. [Google Scholar]
- Sulistiani, E.; Soelistyowati, T.; Yani, A. Callus Induction and Filaments Regeneration from Callus of Cottonii Seaweed (Doty) Collected from Natuna Islands, Riau Islands Province Kappaphycus alvarezii. Biotropia 2012, 19, 103–114. [Google Scholar]
- Uji, T.; Nanaumi, D.; Kawagoe, C.; Saga, N.; Miyashita, K. Factors Influencing the Induction of Adventitious Bud and Callus in the Brown Alga Sargassum horneri (Turner) C. Agardh. J. Appl. Phycol. 2016, 28, 2435–2443. [Google Scholar] [CrossRef]
- Muhamad, S.N.S.; Ling, A.P.-K.; Wong, C.-L. Effect of Plant Growth Regulators on Direct Regeneration and Callus Induction from Sargassum polycystum C. Agardh. J. Appl. Phycol. 2018, 30, 3299–3310. [Google Scholar] [CrossRef]
- Tabuchi, K.; Mizuta, H.; Yasui, H. Promotion of Callus Propagation by 5-Aminolevulinic Acid in a Laminaria japonica Sporophyte. Aquac. Res. 2009, 41, 1–10. [Google Scholar] [CrossRef]
- Wichard, T.; Oertel, W. Gametogenesis and Gamete Release of Ulva mutabilis and Ulva lactuca (Chlorophyta): Regulatory Effects and Chemical Characterization of the “Swarming Inhibitor”. J. Phycol. 2010, 46, 248–259. [Google Scholar] [CrossRef]
- Kessler, R.W.; Weiss, A.; Kuegler, S.; Hermes, C.; Wichard, T. Macroalgal–Bacterial Interactions: Role of Dimethylsulfoniopropionate in Microbial Gardening by Ulva (Chlorophyta). Mol. Ecol. 2018, 27, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Løvlie, A. Genetic Control of Division Rate and Morphogenesis in Ulva mutabilis Foeyn. Comptes-Rendus Trav. Lab. Carlsberg 1964, 34, 77–168. [Google Scholar]
- Vesty, E.F.; Kessler, R.W.; Wichard, T.; Coates, J.C. Regulation of Gametogenesis and Zoosporogenesis in Ulva linza (Chlorophyta): Comparison with Ulva mutabilis and Potential for Laboratory Culture. Front. Plant Sci. 2015, 6, 126163. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The Seaweed Holobiont: Understanding Seaweed–Bacteria Interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef]
- Hardoim, C.C.P.; Esteves, A.I.S.; Pires, F.R.; Gonçalves, J.M.S.; Cox, C.J.; Xavier, J.R.; Costa, R. Phylogenetically and Spatially Close Marine Sponges Harbour Divergent Bacterial Communities. PLoS ONE 2012, 7, e53029. [Google Scholar] [CrossRef]
- Azam, F.; Malfatti, F. Microbial Structuring of Marine Ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Joint, I.; Callow, M.E.; Callow, J.A. Effect of Marine Bacterial Isolates on the Growth and Morphology of Axenic Plantlets of the Green Alga Ulva linza. Microb. Ecol. 2006, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; De Clerck, O.; Coates, J.C. The Green Seaweed Ulva: A Model System to Study Morphogenesis. Front. Plant Sci. 2015, 6, 123849. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.; Steinberg, P.; Rusch, D.; Kjelleberg, S.; Thomas, T. Bacterial Community Assembly Based on Functional Genes Rather than Species. Proc. Natl. Acad. Sci. USA 2011, 108, 14288–14293. [Google Scholar] [CrossRef] [PubMed]
- Alsufyani, T.; Engelen, A.H.; Diekmann, O.E.; Kuegler, S.; Wichard, T. Prevalence and Mechanism of Polyunsaturated Aldehydes Production in the Green Tide Forming Macroalgal Genus Ulva (Ulvales, Chlorophyta). Chem. Phys. Lipids 2014, 183, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Føyn, B. Über Die Sexualität Und Den Generationswechsel von Ulva mutabilis. Arch. Protistenkd. 1958, 102, 473–480. [Google Scholar]
- Califano, G.; Kwantes, M.; Abreu, M.H.; Costa, R.; Wichard, T. Cultivating the Macroalgal Holobiont: Effects of Integrated Multi-Trophic Aquaculture on the Microbiome of Ulva rigida (Chlorophyta). Front. Mar. Sci. 2020, 7, 52. [Google Scholar] [CrossRef]
- van der Loos, L.M.; D’hondt, S.; Willems, A.; De Clerck, O. Characterizing Algal Microbiomes Using Long-Read Nanopore Sequencing. Algal Res. 2021, 59, 102456. [Google Scholar] [CrossRef]
- Ghaderiardakani, F.; Langhans, L.; Kurbel, V.B.; Fenizia, S.; Wichard, T. Metabolite Profiling Reveals Insights into the Species-Dependent Cold Stress Response of the Green Seaweed Holobiont Ulva (Chlorophyta). Environ. Exp. Bot. 2022, 200, 104913. [Google Scholar] [CrossRef]
- Mcgovern, M. Domestication of the Green Seaweed Ulva Ohnoi Aka “sea Lettuce” for Biofloc Effluent Bioremediation. Master’s Thesis, Universidade do Algarve, Faro, Portugal, 2020. [Google Scholar]
- Duan, D.; Xu, L.; Fei, X.; Xu, H. Marine Organisms Attached to Seaweed Surfaces in Jiaozhou Bay, China. World J. Microbiol. Biotechnol. 1995, 11, 351–352. [Google Scholar] [CrossRef]
- Nakanishi, K.; Nishijima, M.; Nomoto, A.M.; Yamazaki, A.; Saga, N. Requisite Morphologic Interaction for Attachment between Ulva pertusa (Chlorophyta) and Symbiotic Bacteria. Mar. Biotechnol. 1999, 1, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Suzuki, M.; Kasai, H.; Shizuri, Y.; Harayama, S. Isolation and Phylogenetic Characterization of Bacteria Capable of Inducing Differentiation in the Green Alga Monostroma oxyspermum. Environ. Microbiol. 2003, 5, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Isolation of Seaweed-Associated Bacteria and Their Morphogenesis-Inducing Capability in Axenic Cultures of the Green Alga Ulva fasciata. Aquat. Biol. 2011, 12, 13–21. [Google Scholar] [CrossRef]
- Provasoli, L.; Pintner, I.J. Bacteria Induced Polymorphism in an Axenic Laboratory Strain of Ulva lactuca (Chlorophyceae). J. Phycol. 1980, 16, 196–201. [Google Scholar] [CrossRef]
- Fjeld, A. Genetic Control of Cellular Differentiation in Ulva mutabilis. Gene Effects in Early Development. Dev. Biol. 1972, 28, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, G.L.; Tait, K.; Taylor, A.; Brownlee, C.; Joint, I. Acyl-homoserine Lactones Modulate the Settlement Rate of Zoospores of the Marine Alga Ulva intestinalis via a Novel Chemokinetic Mechanism. Plant Cell Environ. 2006, 29, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Nishijima, M.; Nishimura, M.; Kuwano, K.; Saga, N. Bacteria That Induce Morphogenesis in Ulva pertusa (Chlorophyta) Grown Under Axenic Conditions. J. Phycol. 1996, 32, 479–482. [Google Scholar] [CrossRef]
- Spoerner, M.; Wichard, T.; Bachhuber, T.; Stratmann, J.; Oertel, W. Growth and Thallus Morphogenesis of Ulva mutabilis (Chlorophyta) Depends on A Combination of Two Bacterial Species Excreting Regulatory Factors. J. Phycol. 2012, 48, 1433–1447. [Google Scholar] [CrossRef] [PubMed]
- Ghaderiardakani, F.; Coates, J.C.; Wichard, T. Bacteria-Induced Morphogenesis of Ulva Intestinalis and Ulva mutabilis (Chlorophyta): A Contribution to the Lottery Theory. FEMS Microbiol. Ecol. 2017, 93, fix094. [Google Scholar] [CrossRef]
- Grueneberg, J.; Engelen, A.H.; Costa, R.; Wichard, T. Macroalgal Morphogenesis Induced by Waterborne Compounds and Bacteria in Coastal Seawater. PLoS ONE 2016, 11, e0146307. [Google Scholar] [CrossRef]
- Joint, I.; Tait, K.; Wheeler, G. Cross-Kingdom Signalling: Exploitation of Bacterial Quorum Sensing Molecules by the Green Seaweed Ulva. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Ingle, K.N.; Polikovsky, M.; Chemodanov, A.; Golberg, A. Marine Integrated Pest Management (MIPM) Approach for Sustainable Seagriculture. Algal Res. 2018, 29, 223–232. [Google Scholar] [CrossRef]
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J. Chemical Interactions between Marine Macroalgae and Bacteria. Mar. Ecol. Prog. Ser. 2010, 409, 267–299. [Google Scholar] [CrossRef]
- Kamermans, P.; Malta, E.J.; Verschuure, J.M.; Schrijvers, L.; Lentz, L.F.; Lien, A.T.A. Effect of Grazing by Isopods and Amphipods on Growth of Ulva spp. (Chlorophyta). Aquat. Ecol. 2002, 36, 425–433. [Google Scholar] [CrossRef]
- Simon, C.; McHale, M.; Sulpice, R. Applications of Ulva Biomass and Strategies to Improve Its Yield and Composition: A Perspective for Ulva Aquaculture. Biology 2022, 11, 1593. [Google Scholar] [CrossRef] [PubMed]
- Jewett, M.C.; Hofmann, G.; Nielsen, J. Fungal Metabolite Analysis in Genomics and Phenomics. Curr. Opin. Biotechnol. 2006, 17, 191–197. [Google Scholar] [CrossRef]
- Roessner, U.; Bowne, J. What Is Metabolomics All About? Biotechniques 2009, 46, 363–365. [Google Scholar] [CrossRef]
- Alsufyani, T.; Weiss, A.; Wichard, T. Time Course Exo-Metabolomic Profiling in the Green Marine Macroalga Ulva (Chlorophyta) for Identification of Growth Phase-Dependent Biomarkers. Mar. Drugs 2017, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Hu, C.; Sun, X.; Li, Y.; Xu, N. Dynamic Metabolic Profiles of the Marine Macroalga Ulva prolifera during Fragmentation-Induced Proliferation. PLoS ONE 2019, 14, e0214491. [Google Scholar] [CrossRef]
- Lee, T.-M.; Liu, C.-H. Correlation of Decreased Calcium Contents with Proline Accumulation in the Marine Green Macroalga Ulva fasciata Exposed to Elevated NaCl Contents in Seawater. J. Exp. Bot. 1999, 50, 1855–1862. [Google Scholar] [CrossRef]
- Dawson, H.M.; Heal, K.R.; Boysen, A.K.; Carlson, L.T.; Ingalls, A.E.; Young, J.N. Potential of Temperature- and Salinity-Driven Shifts in Diatom Compatible Solute Concentrations to Impact Biogeochemical Cycling within Sea Ice. Elem. Sci. Anthr. 2020, 8, 25. [Google Scholar] [CrossRef]
- Gawryluk, R.M.R.; Tikhonenkov, D.V.; Hehenberger, E.; Husnik, F.; Mylnikov, A.P.; Keeling, P.J. Non-Photosynthetic Predators Are Sister to Red Algae. Nature 2019, 572, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.-G. Signaling Role of Glutamate in Plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L.; Puglisi, M.P. DMSP in Marine Macroalgae and Macroinvertebrates: Distribution, Function, and Ecological Impacts. Aquat. Sci. 2007, 69, 394–402. [Google Scholar] [CrossRef]
- Chávez-Sánchez, T.; Piñón-Gimate, A.; Serviere-Zaragoza, E.; López-Bautista, J.M.; Casas-Valdez, M. Ulva Blooms in the Southwestern Gulf of California: Reproduction and Biomass. Estuar. Coast. Shelf Sci. 2018, 200, 202–211. [Google Scholar] [CrossRef]
- Sousa, A.I.; Martins, I.; Lillebø, A.I.; Flindt, M.R.; Pardal, M.A. Influence of Salinity, Nutrients and Light on the Germination and Growth of Enteromorpha sp. Spores. J. Exp. Mar. Biol. Ecol. 2007, 341, 142–150. [Google Scholar] [CrossRef]
- Han, T.; Kang, S.-H.; Park, J.-S.; Lee, H.-K.; Brown, M.T. Physiological Responses of Ulva pertusa and U. armoricana to Copper Exposure. Aquat. Toxicol. 2008, 86, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Carl, C.; de Nys, R.; Lawton, R.J.; Paul, N.A. Methods for the Induction of Reproduction in a Tropical Species of Filamentous Ulva. PLoS ONE 2014, 9, e97396. [Google Scholar] [CrossRef] [PubMed]
- Pettett, P. Preliminary Investigation into the Induction of Reproduction in Ulva spp. in Southeast Queens-land for Mass Cultivation Purposes. Master’s Thesis, University of the Sunshine Coast, Sippy Downs, QLD, Australia, 2009; pp. 2–71. [Google Scholar]
- Kalita, T.L.; Titlyanov, E.A. Influence of Temperature on the Infradian Growth Rhythm in Ulva lactuca (Chlorophyta). Eur. J. Phycol. 2013, 48, 210–220. [Google Scholar] [CrossRef]
- Niesenbaum, R.A. The Ecology of Sporulation by the Macroalga Ulva lactuca L. (Chlorophyceae). Aquat. Bot. 1988, 32, 155–166. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Reproductive Sterility Increases the Capacity to Exploit the Green Seaweed Ulva rigida for Commercial Applications. Algal Res. 2017, 24, 64–71. [Google Scholar] [CrossRef]
- Pedersen, M.F. Transient Ammonium Uptake in the Macroalga Ulva lactuca (Chlorophyta): Nature, Regulation, And The Consequences for Choice of Measuring Technique. J. Phycol. 1994, 30, 980–986. [Google Scholar] [CrossRef]
- Conway, H.L.; Harrison, P.J. Marine Diatoms Grown in Chemostats under Silicate or Ammonium Limitation. IV. Transient Response of Chaetoceros debilis, Skeletonema costatum, and Thalassiosira gravida to a Single Addition of the Limiting Nutrient. Mar. Biol. 1977, 43, 33–43. [Google Scholar] [CrossRef]
- Kalita, T.L.; Titlyanov, E.A. The Effect of Temperature on Infradian Rhythms of Reproduction in Ulva fenestrata Postels et Ruprecht, 1840 (Chlorophyta: Ulvales). Russ. J. Mar. Biol. 2011, 37, 52–61. [Google Scholar] [CrossRef]
- Hiraoka, M.; Enomoto, S. The Induction of Reproductive Cell Formation of Ulva pertusa Kjellman (Ulvales, Ulvophyceae). Phycol. Res. 1998, 46, 199–203. [Google Scholar] [CrossRef]
- Gao, S.; Chen, X.; Yi, Q.; Wang, G.; Pan, G.; Lin, A.; Peng, G. A Strategy for the Proliferation of Ulva prolifera, Main Causative Species of Green Tides, with Formation of Sporangia by Fragmentation. PLoS ONE 2010, 5, e8571. [Google Scholar] [CrossRef]
- Gao, G. Developing Systems for the Commercial Culture of Ulva Species in the UK. Ph.D. Thesis, Newcastle University, Newcastle, UK, 2016. [Google Scholar]
- Zhao, S.; Xia, Z.; Liu, J.; Sun, J.; Zhang, J.; He, P. Morphology, Growth, and Photosynthesis of Ulva prolifera O.F. Müller (Chlorophyta, Ulvophyceae) Gametophytes, the Dominant Green Tide Species in the Southern Yellow Sea. J. Sea Res. 2023, 193, 102375. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, S.; Satish, L.; Dhanya, N.; Malar Vizhi, J.; Nadukkattu Nayagi, N.; Gopala Krishnan, S.; Ganesan, M. Tank Cultivation of Edible Seaweeds: An Overview of the Indian Perspective for Opportunities and Challenges. Biomass Convers. Biorefin 2023. [Google Scholar] [CrossRef]
- Mullikin, R.K.; Rorrer, G.L. A Tubular Recycle Photobioreactor for Macroalgal Suspension Cultures. In BioHydrogen; Springer: Boston, MA, USA, 1998; pp. 403–414. [Google Scholar]
- Liu, Q.; Yu, R.-C.; Yan, T.; Zhang, Q.-C.; Zhou, M.-J. Laboratory Study on the Life History of Bloom-Forming Ulva prolifera in the Yellow Sea. Estuar. Coast. Shelf Sci. 2015, 163, 82–88. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D.; Mao, Y.; Li, Y.; Xue, S.; Zou, J.; Lian, W.; Liang, C.; Zhuang, Z.; Wang, Q.; et al. Settlement of Vegetative Fragments of Ulva prolifera Confirmed as an Important Seed Source for Succession of a Large-scale Green Tide Bloom. Limnol. Oceanogr. 2011, 56, 233–242. [Google Scholar] [CrossRef]
- de Góes, H.G.; Reis, R.P. An Initial Comparison of Tubular Netting versus Tie–Tie Methods of Cultivation for Kappaphycus alvarezii (Rhodophyta, Solieriaceae) on the South Coast of Rio de Janeiro State, Brazil. J. Appl. Phycol. 2011, 23, 607–613. [Google Scholar] [CrossRef]
- Redmond, S.; Green, L.; Yarish, C.; Kim, J.; Neefus, C. New England Seaweed Culture Handbook; University of Connecticut: Groton, CT, USA, 2014. [Google Scholar]
- Zhang, J.; Kim, J.K.; Yarish, C.; He, P. The Expansion of Ulva prolifera O.F. Müller Macroalgal Blooms in the Yellow Sea, PR China, through Asexual Reproduction. Mar. Pollut. Bull. 2016, 104, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Shen, S.; Wang, J.; Yan, B. Reproduction Diversity of Enteromorpha prolifera. J. Integr. Plant Biol. 2008, 50, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Intrinsic and Extrinsic Control of Reproduction in the Green Tide-Forming Alga, Ulva rigida. Environ. Exp. Bot. 2017, 139, 14–22. [Google Scholar] [CrossRef]
- Steinhagen, S.; Larsson, K.; Olsson, J.; Albers, E.; Undeland, I.; Pavia, H.; Toth, G.B. Closed Life-Cycle Aquaculture of Sea Lettuce (Ulva fenestrata): Performance and Biochemical Profile Differ in Early Developmental Stages. Front. Mar. Sci. 2022, 9, 942679. [Google Scholar] [CrossRef]
- Morales-Rubio, M.E.; Espinosa-Leal, C.; Garza-Padrón, R.A. Cultivo de Tejidos Vegetales y Su Aplicación En Productos Naturales. In Investigación en Plantas de Importancia Médica; OmniaScience: Barcelona, Spain, 2016; pp. 351–410. [Google Scholar]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Califano, G.; Wichard, T. Preparation of Axenic Cultures in Ulva (Chlorophyta). In Protocols for Macroalgae Research; CRC: Boca Raton, FL, USA, 2018; pp. 159–171. [Google Scholar]
- Wichard, T. Exploring Bacteria-Induced Growth and Morphogenesis in the Green Macroalga Order Ulvales (Chlorophyta). Front. Plant Sci. 2015, 6, 123850. [Google Scholar] [CrossRef] [PubMed]
- Chemodanov, A.; Robin, A.; Golberg, A. Design of Marine Macroalgae Photobioreactor Integrated into Building to Support Seagriculture for Biorefinery and Bioeconomy. Bioresour. Technol. 2017, 241, 1084–1093. [Google Scholar] [CrossRef]
- Gore, M. (Ed.) Spectrophotometry and Spectrofluorimetry; Oxford University Press: Oxford, UK, 2000; ISBN 9780199638130. [Google Scholar]
- Vo, K. 2.1.5: Spectrophotometry. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/02%3A_Reaction_Rates/2.01%3A_Experimental_Determination_of_Kinetics/2.1.05%3A_Spectrophotometry (accessed on 15 March 2024).
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union Legislation on Macroalgae Products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- FSSC. Food Safety System Certification 22000 Guidance Document: ISO 22000 Interpretation; FSSC: Gorinchem, The Netherlands, 2019. [Google Scholar]
- SGS. Entendendo a Norma de Certificação de Sistemas de Segurança de Alimentos FSSC 22000 Guia Técnico Sobre Os. Desafios, Impactos e Oportunidades Da FSSC 22000; SGS: Geneva, Switzerland, 2014. [Google Scholar]
- ABNT. Sistemas de Gestão Ambiental-Requisitos Com. Orientações Para. Uso Environmental Management Systems-Requirements with Guidance for Use; ABNT: Rio de Janeiro and São Paulo, Brazil, 2015. [Google Scholar]
- Ociepa-Kubicka, A.; Deska, I.; Ociepa, E. Organizations towards the Evaluation of Environmental Management Tools ISO 14001 and EMAS. Energies 2021, 14, 4870. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Algal Polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 151–212. [Google Scholar]
- Li, C.; Tang, T.; Du, Y.; Jiang, L.; Yao, Z.; Ning, L.; Zhu, B. Ulvan and Ulva Oligosaccharides: A Systematic Review of Structure, Preparation, Biological Activities and Applications. Bioresour. Bioprocess. 2023, 10, 66. [Google Scholar] [CrossRef]
- Kraan, S. Mass-Cultivation of Carbohydrate Rich Macroalgae, a Possible Solution for Sustainable Biofuel Production. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Sousa, R.A.; Reis, R.L. A Practical Perspective on Ulvan Extracted from Green Algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef]
- Webster, E.A.; Gadd, G.M. Cadmium Replaces Calcium in the Cell Wall of Ulva lactuca. BioMetals 1996, 9, 241–244. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and Nutritional and Cardiovascular-Health Properties of Seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Schijf, J.; Ebling, A.M. Investigation of the Ionic Strength Dependence of Ulva lactuca Acid Functional Group pKas by Manual Alkalimetric Titrations. Environ. Sci. Technol. 2010, 44, 1644–1649. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Putra, N.R.; Fajriah, S.; Qomariyah, L.; Dewi, A.S.; Rizkiyah, D.N.; Irianto, I.; Rusmin, D.; Melati, M.; Trisnawati, N.W.; Darwati, I.; et al. Exploring the Potential of Ulva lactuca: Emerging Extraction Methods, Bioactive Compounds, and Health Applications—A Perspective Review. S. Afr. J. Chem. Eng. 2024, 47, 233–245. [Google Scholar] [CrossRef]
- Aronoff, S. Photosynthesis. Bot. Rev. 1957, 23, 65–107. [Google Scholar] [CrossRef]
- Martins, M.; Oliveira, R.; Coutinho, J.A.P.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Pinto, D.C.G.A.; Ventura, S.P.M. Recovery of Pigments from Ulva rigida. Sep. Purif. Technol. 2021, 255, 117723. [Google Scholar] [CrossRef]
- Pereira, L.; Kraan, S. Pigments and Minor Compounds in Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 397–461. [Google Scholar]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of Proteins from Two Marine Macroalgae, Ulva sp. and Gracilaria sp., for Food Application, and Evaluating Digestibility, Amino Acid Composition and Antioxidant Properties of the Protein Concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine Bioactives as Functional Food Ingredients: Potential to Reduce the Incidence of Chronic Diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant Activity of Chlorophylls and Their Derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Luo, L.; Lai, X.; Chen, B.; Lin, L.; Fang, L.; Tam, N.F.Y.; Luan, T. Chlorophyll Catalyse the Photo-Transformation of Carcinogenic Benzo[a]Pyrene in Water. Sci. Rep. 2015, 5, 12776. [Google Scholar] [CrossRef]
- Sudakin, D.L. Dietary Aflatoxin Exposure and Chemoprevention of Cancer: A Clinical Review. J. Toxicol. Clin. Toxicol. 2003, 41, 195–204. [Google Scholar] [CrossRef]
- Diogo, P.; Mota, M.; Fernandes, C.; Sequeira, D.; Palma, P.; Caramelo, F.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Gonçalves, T.; Santos, J.M. Is the Chlorophyll Derivative Zn(II)e 6 Me a Good Photosensitizer to Be Used in Root Canal Disinfection? Photodiagn. Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Madhavan, J.; Chandrasekharan, S.; Priya, M.; Godavarthi, A. Modulatory Effect of Carotenoid Supplement Constituting Lutein and Zeaxanthin (10:1) on Anti-Oxidant Enzymes and Macular Pigments Level in Rats. Pharmacogn. Mag. 2018, 14, 268. [Google Scholar] [CrossRef]
- Cotas, J. Marine Phenolics. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 213–245. [Google Scholar]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Bilal Hussain, M.; Hassan, S.; Waheed, M.; Javed, A.; Adil Farooq, M.; Tahir, A. Bioavailability and Metabolic Pathway of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Mukherjee, P.K. Bioactive Phytocomponents and Their Analysis. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–328. ISBN 9780128133743. [Google Scholar]
- Konuk, H.B.; Ergüden, B. Phenolic –OH Group Is Crucial for the Antifungal Activity of Terpenoids via Disruption of Cell Membrane Integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoue, M.; Uzawa, Y.; Kawamura, Y. Anti-tumorigenic Components of a Sea Weed, Eeteromorpha clathrata. BioFactors 2004, 22, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Gheidarloo, R.; Sadati, N.; Shams Ardekani, M.R.; Bagher Nabavi, S.M.; Tavajohi, S.; Ostad, S.N. Cytotoxicity of Fucosterol Containing Fraction of Marine Algae against Breast and Colon Carcinoma Cell Line. Pharmacogn. Mag. 2012, 8, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic Potential of Green Seaweed Enteromorpha prolifera Flavonoids Regulating Insulin Signaling Pathway and Gut Microbiota in Type 2 Diabetic Mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of Green Macroalgae Enteromorpha prolifera Polyphenols in the Modulation of Gene Expression and Intestinal Microflora Profiles in Type 2 Diabetic Mice. Int. J. Mol. Sci. 2018, 20, 25. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Aslan, E.; Aksu, A.; Korkmaz, N.E.; Taskin, O.S.; Caglar, N.B. Monitoring the Antioxidant Activities by Extracting the Polyphenolic Contents of Algae Collected from the Bosphorus. Mar. Pollut. Bull. 2019, 141, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Valério Filho, A.; Santana, L.R.; Motta, N.G.; Passos, L.F.; lnes Wolke, S.; Mansilla, A.; Astorga-España, M.S.; Becker, E.M.; de Pereira, C.M.P.; Carreno, N.L.V. Extraction of Fatty Acids and Cellulose from the Biomass of Algae Durvillaea antarctica and Ulva lactuca: An Alternative for Biorefineries. Algal Res. 2023, 71, 103084. [Google Scholar] [CrossRef]
- Pereira, T.; Horta, A.; Barroso, S.; Mendes, S.; Gil, M.M. Study of the Seasonal Variations of the Fatty Acid Profiles of Selected Macroalgae. Molecules 2021, 26, 5807. [Google Scholar] [CrossRef] [PubMed]
- Fannin, K.F.; Srivastava, V.; Mensinger, J.; Chynoweth, D. Marine Biomass Program: Anaerobic Digestion Systems Development and Stability Study. Final Report; Gas Research Institute: Chicago, IL, USA, 1982. [Google Scholar]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein Content, Amino Acid Composition and Nitrogen-to-Protein Conversion Factors of Ulva rigida and Ulva capensis from Natural Populations and Ulva lactuca from an Aquaculture System, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of Different Extractive Procedures for Proteins from the Edible Seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical Composition and Functional Properties of Ulva lactuca Seaweed Collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- FAO. Global Seaweeds and Microalgae Production, 1950–2019; Food and Agricultural Organization of the United Nations: Rome, Italy, 2021; 172p. [Google Scholar]
- Dan, A.; Hiraoka, M.; Ohno, M.; Critchley, A.T. Observations on the Effect of Salinity and Photon Fluence Rate on the Induction of Sporulation and Rhizoid Formation in the Green Alga Enteromorpha prolifera (Muller) J. Agardh (Chlorophyta, Ulvales). Fish. Sci. 2002, 68, 1182–1188. [Google Scholar] [CrossRef]
- Hiraoka, M.; Oka, N. Tank Cultivation of Ulva Prolifera in Deep Seawater Using a New “Germling Cluster” Method. J. Appl. Phycol. 2008, 20, 97–102. [Google Scholar] [CrossRef]
- Neori, A.; Msuya, F.E.; Shauli, L.; Schuenhoff, A.; Kopel, F.; Shpigel, M. A Novel Three-Stage Seaweed (Ulva lactuca) Biofilter Design for Integrated Mariculture. J. Appl. Phycol. 2003, 15, 543–553. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Buschmann, A.H. Bioremediation Potential, Growth and Biomass Yield of the Green Seaweed, Ulva lactuca in an Integrated Marine Aquaculture System at the Red Sea Coast of Saudi Arabia at Different Stocking Densities and Effluent Flow Rates. Rev. Aquac. 2015, 7, 161–171. [Google Scholar] [CrossRef]
- Robertson-Andersson, D.V.; Potgieter, M.; Hansen, J.; Bolton, J.J.; Troell, M.; Anderson, R.J.; Halling, C.; Probyn, T. Integrated Seaweed Cultivation on an Abalone Farm in South Africa. J. Appl. Phycol. 2008, 20, 579–595. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy Potential of Ulva lactuca: Biomass Yield, Methane Production and Combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Mata, L.; Magnusson, M.; Paul, N.A.; de Nys, R. The Intensive Land-Based Production of the Green Seaweeds Derbesia tenuissima and Ulva ohnoi: Biomass and Bioproducts. J. Appl. Phycol. 2016, 28, 365–375. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; García, V.; Pastén, A.; López, J.; Goñi, G. Effect of Different Drying Methods on Phytochemical Content and Amino Acid and Fatty Acid Profiles of the Green Seaweed, Ulva spp. J. Appl. Phycol. 2019, 31, 1967–1979. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Ripol, A.; Cardoso, C.; Afonso, C.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N.M. Composition, Anti-Inflammatory Activity, and Bioaccessibility of Green Seaweeds from Fish Pond Aquaculture. Nat. Prod. Commun. 2018, 13, 603–608. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2008, 65, 535–543. [Google Scholar] [CrossRef]
- Yu-Qing, T.; Mahmood, K.; Shehzadi, R.; Ashraf, M.F. Ulva lactuca and Its Polysaccharides: Food and Biomedical Aspects. J. Biol. 2016, 6, 140–151. [Google Scholar]
- Hall, A.C.; Fairclough, A.C.; Mahadevan, K.; Paxman, J.R. Ascophyllum Nodosum Enriched Bread Reduces Subsequent Energy Intake with No Effect on Post-Prandial Glucose and Cholesterol in Healthy, Overweight Males. A Pilot Study. Appetite 2012, 58, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Okab, A.B.; Aljumaah, R.S.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A. Nutritional Value of Green Seaweed (Ulva lactuca) for Broiler Chickens. Ital. J. Anim. Sci. 2013, 12, e28. [Google Scholar] [CrossRef]
- Ventura, M.R.; Castañón, J.I.R. The Nutritive Value of Seaweed (Ulva lactuca) for Goats. Small Rumin. Res. 1998, 29, 325–327. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Correia, A.; Rodrigues, A.R.; Cortez, P.P.; Vilanova, M.; Fonseca, A.J.M. Assessing In Vivo Digestibility and Effects on Immune System of Sheep Fed Alfalfa Hay Supplemented with a Fixed Amount of Ulva rigida and Gracilaria vermiculophylla. J. Appl. Phycol. 2017, 29, 1057–1067. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Pereira, N. Seaweed: Promising Plant of the Millennium. Sci. Cult. 2005, 71, 60–66. [Google Scholar]
- Figueira, T.A. Macroalgas e Suas Aplicações Biotecnológicas. In VIII Botânica no Inverno; Instituto de Biociências: São Paulo, Brazil, 2020. [Google Scholar]
- Hashim, R.; Saat, M.A.M. The Utilization of Seaweed Meals as Binding Agents in Pelleted Feeds for Snakehead (Channa striatus) Fry and Their Effects on Growth. Aquaculture 1992, 108, 299–308. [Google Scholar] [CrossRef]
- Mustafa, G.; Wakamatsu, S.; Takeda, T.; Umino, T.; Nakagawa, H. Effects of Algae Meal as Feed Additive on Growth, Feed Efficiency, and Body Composition in Red Sea Bream. Fish. Sci. 1995, 61, 25–28. [Google Scholar] [CrossRef]
- Gabrielsen, B.O.; Austreng, E. Growth, Product Quality and Immune Status of Atlantic Salmon, Salmo salar L., Fed Wet Feed with Alginate. Aquac. Res. 1998, 29, 397–401. [Google Scholar] [CrossRef]
- Naidoo, K.; Maneveldt, G.; Ruck, K.; Bolton, J.J. A Comparison of Various Seaweed-Based Diets and Formulated Feed on Growth Rate of Abalone in a Land-Based Aquaculture System. J. Appl. Phycol. 2006, 18, 437–443. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant Biostimulants: A Review on the Processing of Macroalgae and Use of Extracts for Crop Management to Reduce Abiotic and Biotic Stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Basak, A. Effect of Preharvest Treatment with Seaweed Products, Kelpak® and Goëmar BM 86®, on Fruit Quality in Apple. Int. J. Fruit Sci. 2008, 8, 1–14. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The Effects of a Seaweed Extract in Addition to Nitrogen and Boron Fertilization on Productivity, Fruit Maturation, Leaf Nutritional Status and Oil Quality of the Olive (Olea europaea L.) Cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.R.; Tantaway, A.S.; Hafez, M.M.; Habib, H.A.M. Seaweed Extract Improves Growth, Yield and Quality of Different Watermelon Hybrids. Res. J. Agric. Biol. Sci. 2010, 6, 161–168. [Google Scholar]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a Biostimulant Derived from the Brown Seaweed Ascophyllum nodosum on Ripening Dynamics and Fruit Quality of Grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 462648. [Google Scholar] [CrossRef] [PubMed]
- Hankins, S.D.; Hockey, H.P. The Effect of a Liquid Seaweed Extract from Ascophyllum nodosum (Fucales, Phaeophyta) on the Two-Spotted Red Spider Mite Tetranychus urticae. In Thirteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1990; pp. 555–559. [Google Scholar]
- Subramanian, S.; Sangha, J.S.; Gray, B.A.; Singh, R.P.; Hiltz, D.; Critchley, A.T.; Prithiviraj, B. Extracts of the Marine Brown Macroalga, Ascophyllum nodosum, Induce Jasmonic Acid Dependent Systemic Resistance in Arabidopsis thaliana against Pseudomonas syringae Pv. Tomato DC3000 and Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2011, 131, 237–248. [Google Scholar] [CrossRef]
- Panjehkeh, N.; Abkhoo, J. Influence of Marine Brown Alga Extract (Dalgin) on Damping-off Tolerance of Tomato. J. Mater. Environ. Sci. 2016, 7, 2369–2374. [Google Scholar]
- Eismann, A.I.; Perpetuo Reis, R.; Ferreira da Silva, A.; Negrão Cavalcanti, D. Ulva spp. Carotenoids: Responses to Environmental Conditions. Algal Res. 2020, 48, 101916. [Google Scholar] [CrossRef]
- Lee, D.-G.; Hyun, J.-W.; Kang, K.-A.; Lee, J.-O.; Lee, S.-H.; Ha, B.-J.; Ha, J.-M.; Lee, E.Y.; Lee, J.-H. Ulva lactuca: A Potential Seaweed for Tumor Treatment and Immune Stimulation. Biotechnol. Bioprocess. Eng. 2004, 9, 236–238. [Google Scholar] [CrossRef]
- Hassan, S.; El-Twab, S.A.; Hetta, M.; Mahmoud, B. Improvement of Lipid Profile and Antioxidant of Hypercholesterolemic Albino Rats by Polysaccharides Extracted from the Green Alga Ulva lactuca Linnaeus. Saudi J. Biol. Sci. 2011, 18, 333–340. [Google Scholar] [CrossRef]
- Awad, N.E. Biologically Active Steroid from the Green Alga Ulva lactuca. Phytother. Res. 2000, 14, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Marzouk, M.A.; Rabea, E.I.; Abd-Elnabi, A.D. Insecticidal and Fungicidal Activity of Ulva lactuca Linnaeus (Chlorophyta) Extracts and Their Fractions. Annu. Res. Rev. Biol. 2014, 4, 2252–2262. [Google Scholar] [CrossRef]
- Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Blunden, G.; Tasdemir, D. Antiprotozoal, Antimycobacterial and Cytotoxic Potential of Some British Green Algae. Phytother. Res. 2010, 24, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Sathivel, A.; Raghavendran, H.R.B.; Srinivasan, P.; Devaki, T. Anti-Peroxidative and Anti-Hyperlipidemic Nature of Ulva lactuca Crude Polysaccharide on d-Galactosamine Induced Hepatitis in Rats. Food Chem. Toxicol. 2008, 46, 3262–3267. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated Polysaccharides from Marine Green Algae Ulva conglobata and Their Anticoagulant Activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- BelHadj, S.; Hentati, O.; Elfeki, A.; Hamden, K. Inhibitory Activities of Ulva lactuca Polysaccharides on Digestive Enzymes Related to Diabetes and Obesity. Arch. Physiol. Biochem. 2013, 119, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Biba, E. Protection: The Sunscreen Pill. Nature 2014, 515, S124–S125. [Google Scholar] [CrossRef]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from Algae with Economical Impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Kim, S.-K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the Cosmeceuticals Derived from Marine Organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Leal, M.C.; Rocha, R.J.M.; Rosa, R.; Calado, R. Aquaculture of Marine Non-food Organisms: What, Why and How? Rev. Aquac. 2018, 10, 400–423. [Google Scholar] [CrossRef]
- Sun, Y.; Chavan, M. Cosmetic Compositions Comprising Marine Plants. U.S. Patent No. US9603790B2, 28 March 2017. [Google Scholar]
- Ritchie, H.; Rosado, P. “Fossil Fuels”. 2017. Available online: https://ourworldindata.org/fossil-fuels (accessed on 1 March 2024).
- Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Abomohra, A.E.-F. Screening of Seaweeds for Sustainable Biofuel Recovery through Sequential Biodiesel and Bioethanol Production. Environ. Sci. Pollut. Res. 2020, 27, 32481–32493. [Google Scholar] [CrossRef] [PubMed]
- Korzen, L.; Pulidindi, I.N.; Israel, A.; Abelson, A.; Gedanken, A. Single Step Production of Bioethanol from the Seaweed Ulva rigida Using Sonication. RSC Adv. 2015, 5, 16223–16229. [Google Scholar] [CrossRef]
- Ritchie, H.; Samborska, V.; Roser, M. Plastic Pollution. Our World in Data. 2023. Available online: https://ourworldindata.org/plastic-pollution (accessed on 1 March 2024).
- Lim, C.; Yusoff, S.; Ng, C.G.; Lim, P.E.; Ching, Y.C. Bioplastic Made from Seaweed Polysaccharides with Green Production Methods. J. Environ. Chem. Eng. 2021, 9, 105895. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating Seaweeds into Marine Aquaculture Systems: A Key Toward Sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Chopin, T.; Yarish, C.; Wilkes, R.; Belyea, E.; Lu, S.; Mathieson, A. Developing Porphyra/Salmon Integrated Aquaculture for Bioremediation and Diversification of the Aquaculture Industry. J. Appl. Phycol. 1999, 11, 463–472. [Google Scholar] [CrossRef]
- Troell, M.; Rönnbäck, P.; Halling, C.; Kautsky, N.; Buschmann, A. Ecological Engineering in Aquaculture: Use of Seaweeds for Removing Nutrients from Intensive Mariculture. In Sixteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1999; pp. 603–611. [Google Scholar]
- Hernández, I.; Tovar, A.; Vergara, J.J. Biofiltering Efficiency in Removal of Dissolved Nutrients by Three Species of Estuarine Macroalgae Cultivated with Sea Bass (Dicentrarchus labrax) Waste Waters 2. Ammonium. J. Appl. Phycol. 2002, 14, 375–384. [Google Scholar] [CrossRef]
- Neori, A.; Krom, M.D.; Ellner, S.P.; Boyd, C.E.; Popper, D.; Rabinovitch, R.; Davison, P.J.; Dvir, O.; Zuber, D.; Ucko, M.; et al. Seaweed Biofilters as Regulators of Water Quality in Integrated Fish-Seaweed Culture Units. Aquaculture 1996, 141, 183–199. [Google Scholar] [CrossRef]
- Lüning, K.; Pang, S. Mass Cultivation of Seaweeds: Current Aspects and Approaches. J. Appl. Phycol. 2003, 15, 115–119. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Ulva Rigida in the Future Ocean: Potential for Carbon Capture, Bioremediation and Biomethane Production. GCB Bioenergy 2018, 10, 39–51. [Google Scholar] [CrossRef]
- Kut Güroy, B.; Cirik, Ş.; Güroy, D.; Sanver, F.; Tekinay, A.A. Effects of Ulva rigida and Cystoseira barbata Meals as a Feed Additive on Growth Performance, Feed Utilization, and Body Composition of Nile Tilapia, Oreochromis niloticus. Turk. J. Vet. Anim. Sci. 2007, 31, 91–97. [Google Scholar]
- Gao, G.; Liu, Y.; Li, X.; Feng, Z.; Xu, J. An Ocean Acidification Acclimatised Green Tide Alga Is Robust to Changes of Seawater Carbon Chemistry but Vulnerable to Light Stress. PLoS ONE 2016, 11, e0169040. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.; Neill, K.; D’Archino, R. When Seaweeds Go Bad: An Overview of Outbreaks of Nuisance Quantities of Marine Macroalgae in New Zealand. N. Z. J. Mar. Freshw. Res. 2015, 49, 472–491. [Google Scholar] [CrossRef]
- Thornber, C.S.; Guidone, M.; Deacutis, C.; Green, L.; Ramsay, C.N.; Palmisciano, M. Spatial and Temporal Variability in Macroalgal Blooms in a Eutrophied Coastal Estuary. Harmful Algae 2017, 68, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, J.; Zhang, J.; Shi, J.; Kang, X.; Liu, J.; Wen, Q.; He, P. Reproductive Strategy of the Floating Alga Ulva prolifera in Blooms in the Yellow Sea Based on a Combination of Zoid and Chromosome Analysis. Mar. Pollut. Bull. 2019, 146, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef]
- Yabe, T.; Ishii, Y.; Amano, Y.; Koga, T.; Hayashi, S.; Nohara, S.; Tatsumoto, H. Green Tide Formed by Free-Floating Ulva spp. at Yatsu Tidal Flat, Japan. Limnology 2009, 10, 239–245. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Jiao, N. Tapping Carbon Sequestration Potential of Blooming Macroalgae to Mitigate Climate Change. Ocean-Land-Atmos. Res. 2023, 2, 0033. [Google Scholar] [CrossRef]
- Park, S. Sea Lettuce and Nutrient Monitoring in Tauranga Harbour 1991–2010; Bay of Plenty Regional Council: Whakatane, New Zealand, 2011. [Google Scholar]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of Global Warming Due to Carbon-Cycle Feedbacks in a Coupled Climate Model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justić, D. Global Change and Eutrophication of Coastal Waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Muto, E.Y.; Petti, M.A.V.; Corbisier, T.N.; Soto-Jiménez, M.; et al. Eutrophication and Macroalgal Blooms in Temperate and Tropical Coastal Waters: Nutrient Enrichment Experiments with Ulva spp. Glob. Change Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef]
- Trenberth, K. Changes in Precipitation with Climate Change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef]
- Sydeman, W.J.; García-Reyes, M.; Schoeman, D.S.; Rykaczewski, R.R.; Thompson, S.A.; Black, B.A.; Bograd, S.J. Climate Change and Wind Intensification in Coastal Upwelling Ecosystems. Science 2014, 345, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Green-Gavrielidis, L.A.; Thornber, C.S. Will Climate Change Enhance Algal Blooms? The Individual and Interactive Effects of Temperature and Rain on the Macroalgae Ulva. Estuaries Coasts 2022, 45, 1688–1700. [Google Scholar] [CrossRef]
- Eriksson, B.K.; Ljunggren, L.; Sandström, A.; Johansson, G.; Mattila, J.; Rubach, A.; Råberg, S.; Snickars, M. Declines in Predatory Fish Promote Bloom-forming Macroalgae. Ecol. Appl. 2009, 19, 1975–1988. [Google Scholar] [CrossRef]
- Pereira, F. Agência Ambiental da ONU dá Exemplo em Neutralizar Emissões de Carbono. Available online: https://www.unep.org/pt-br/noticias-e-reportagens/story/agencia-ambiental-da-onu-da-exemplo-em-neutralizar-emissoes-de-carbono (accessed on 1 March 2024).
- Mantri, V.A.; Singh, R.P.; Bijo, A.J.; Kumari, P.; Reddy, C.R.K.; Jha, B. Differential Response of Varying Salinity and Temperature on Zoospore Induction, Regeneration and Daily Growth Rate in Ulva fasciata (Chlorophyta, Ulvales). J. Appl. Phycol. 2011, 23, 243–250. [Google Scholar] [CrossRef]
- MND. Staff ‘Mr. Sargassum’ Has Built 13 Houses with Blocks Made from the Smelly Seaweed. Mexico News Daily, 20 July 2022. [Google Scholar]
- Mkrtichian, E. Sargablock. Available online: https://fortomorrow.org/explore-solutions/sargablock (accessed on 1 March 2024).
- Buckley, S. Seaweed as Insulation? Available online: https://www.architectureanddesign.com.au/news/seaweed-as-insulation (accessed on 1 March 2024).
- Widera, B. Possible Application of Seaweed as Building Material in the Modern Seaweed House on Laesø. In Proceedings of the 30th International PLEA Conference: Sustainable Habitat for Developing Societies—Choosing the Way Forward, CEPT University, Ahmedabad, India, 16–18 December 2014. [Google Scholar]
- Textile Value Chain Fashion from the Sea. Available online: https://textilevaluechain.in/news-insights/fashion-from-the-sea-algae-seaweed/ (accessed on 1 March 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, S.P.; Cotas, J.; Pereira, L. Laminar Ulva Species: A Multi-Tool for Humankind? Appl. Sci. 2024, 14, 3448. https://doi.org/10.3390/app14083448
Costa SP, Cotas J, Pereira L. Laminar Ulva Species: A Multi-Tool for Humankind? Applied Sciences. 2024; 14(8):3448. https://doi.org/10.3390/app14083448
Chicago/Turabian StyleCosta, Sofia Pereira, João Cotas, and Leonel Pereira. 2024. "Laminar Ulva Species: A Multi-Tool for Humankind?" Applied Sciences 14, no. 8: 3448. https://doi.org/10.3390/app14083448







