The Implementation of AFM-Based Nanoscale Diagnostic Methods in the Investigation of the Degradation Process of Bacteriostatic Acrylic Film with Silver Nanoparticles
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. The Exposure Parameters
- -
- -
- UV radiation typically utilized for the air and room sterilization—500 h (details are shown in Table 2);
- -
- Increased humidity (RH 98%) and temperature (40 °C)—72 h;
- -
- Increased temperature (70 °C)—48 h;
- -
- Chemical disinfection substances typically utilized in medical units, 24 h exposure for each substance:
- ○
- Incidin Liquid Spray;
- ○
- Lysoformin Plus Schaum;
- ○
- Aldewir (10% solution).
- -
- -
- -
- Wettability tests provided complementary information to the roughness measurement concerning the practical aspects of the utilization of the film in particular conditions [25].
- -
- Colorimetric analysis gave information concerning the loss of aesthetical aspects of the film as well as phenomena responsible for the aging process [26].
2.2. AFM Measurements
2.3. Wettability Determination
2.4. Colorimetric Analysis
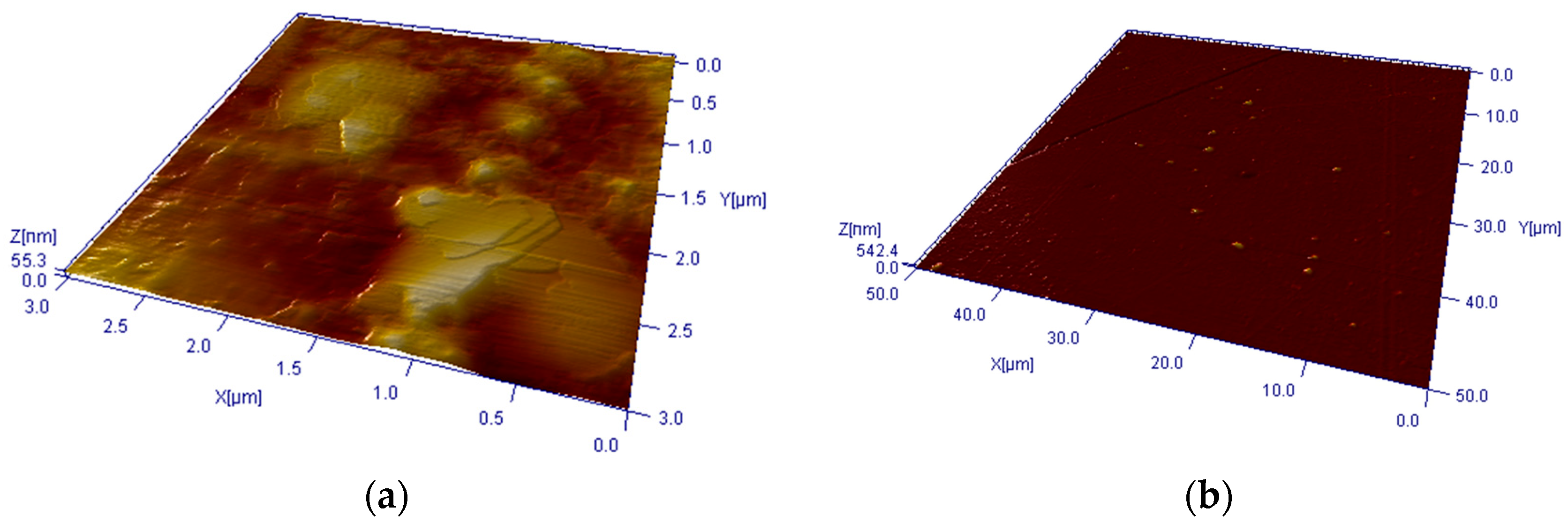
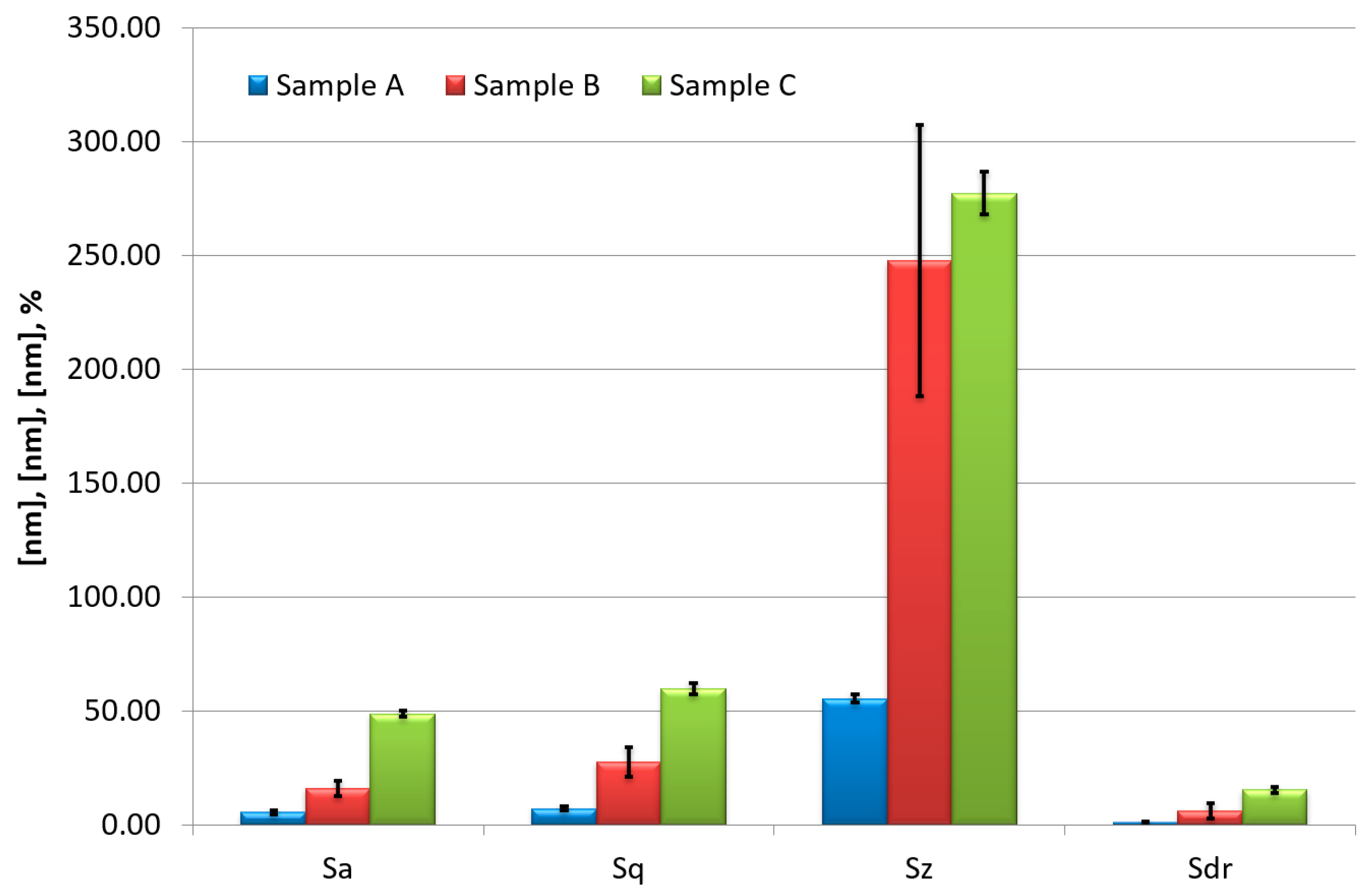
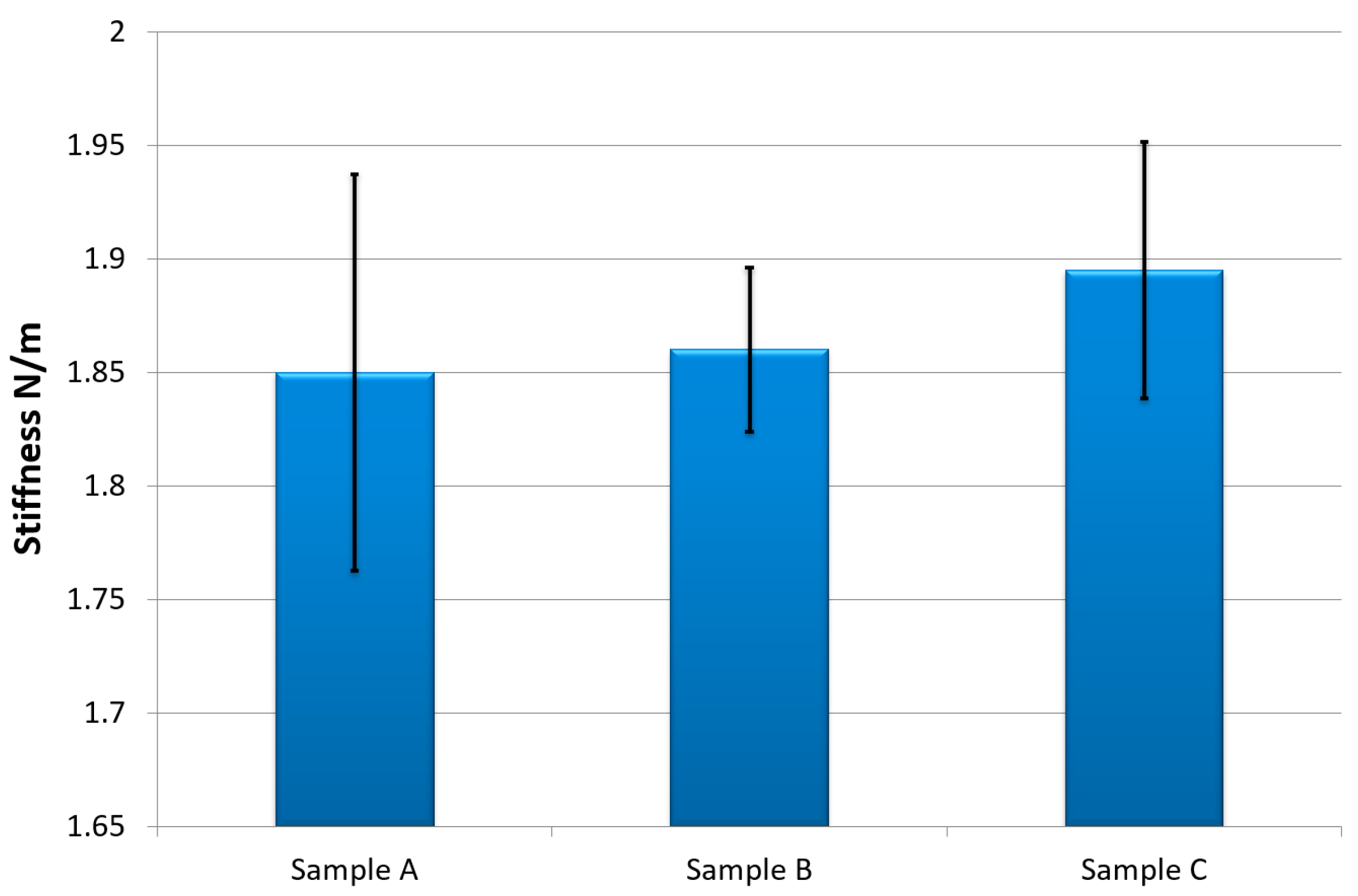
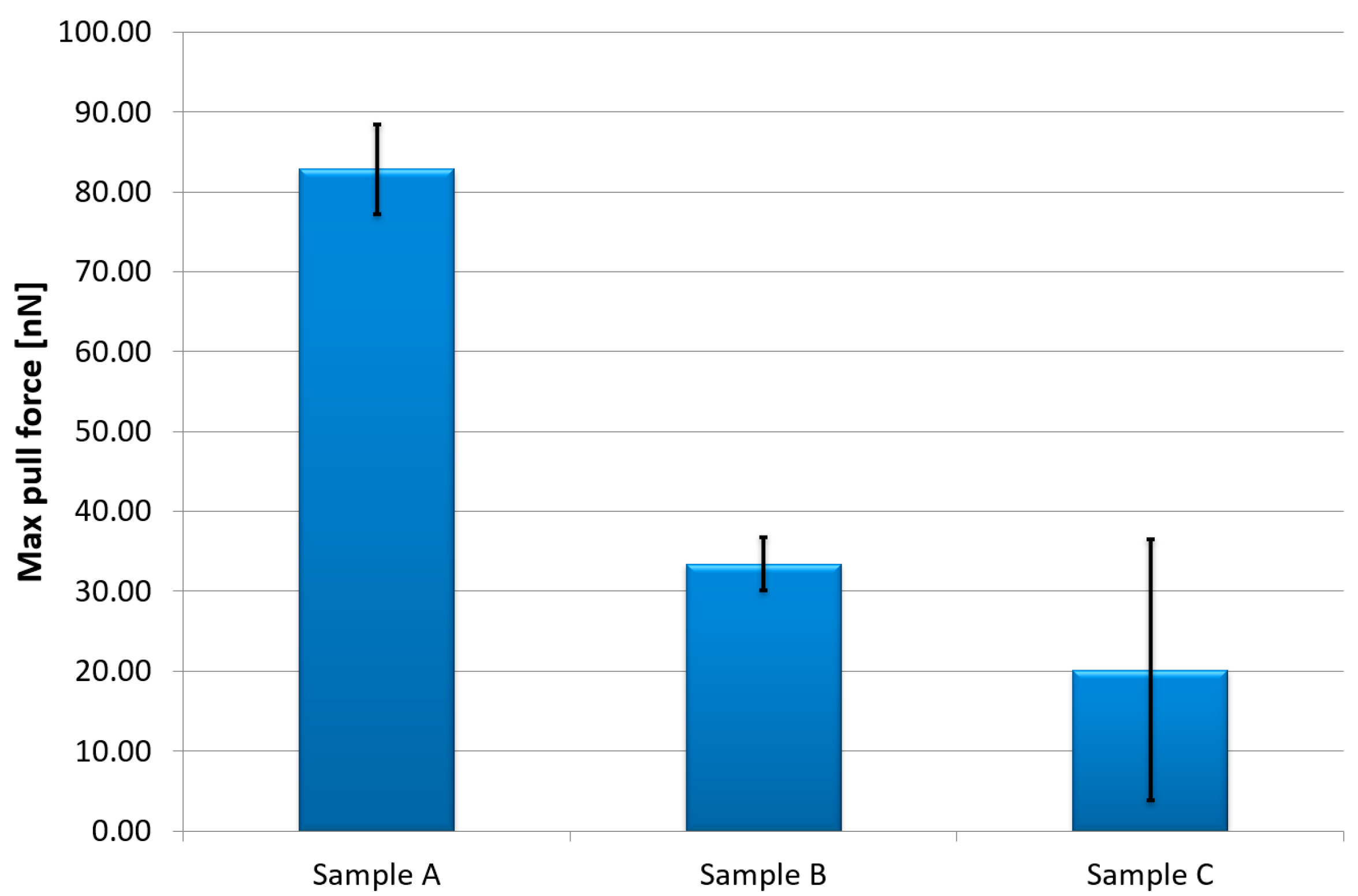
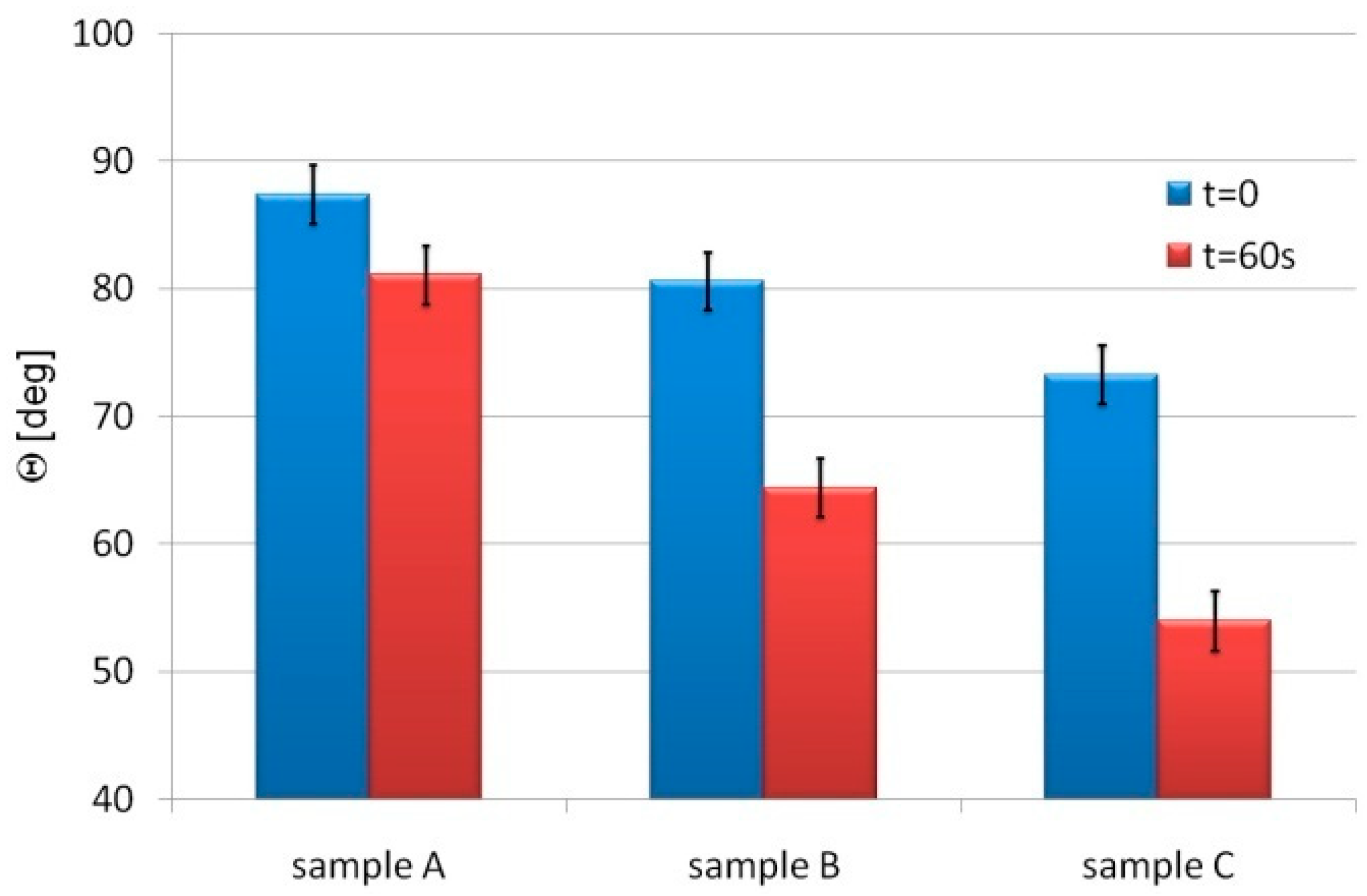
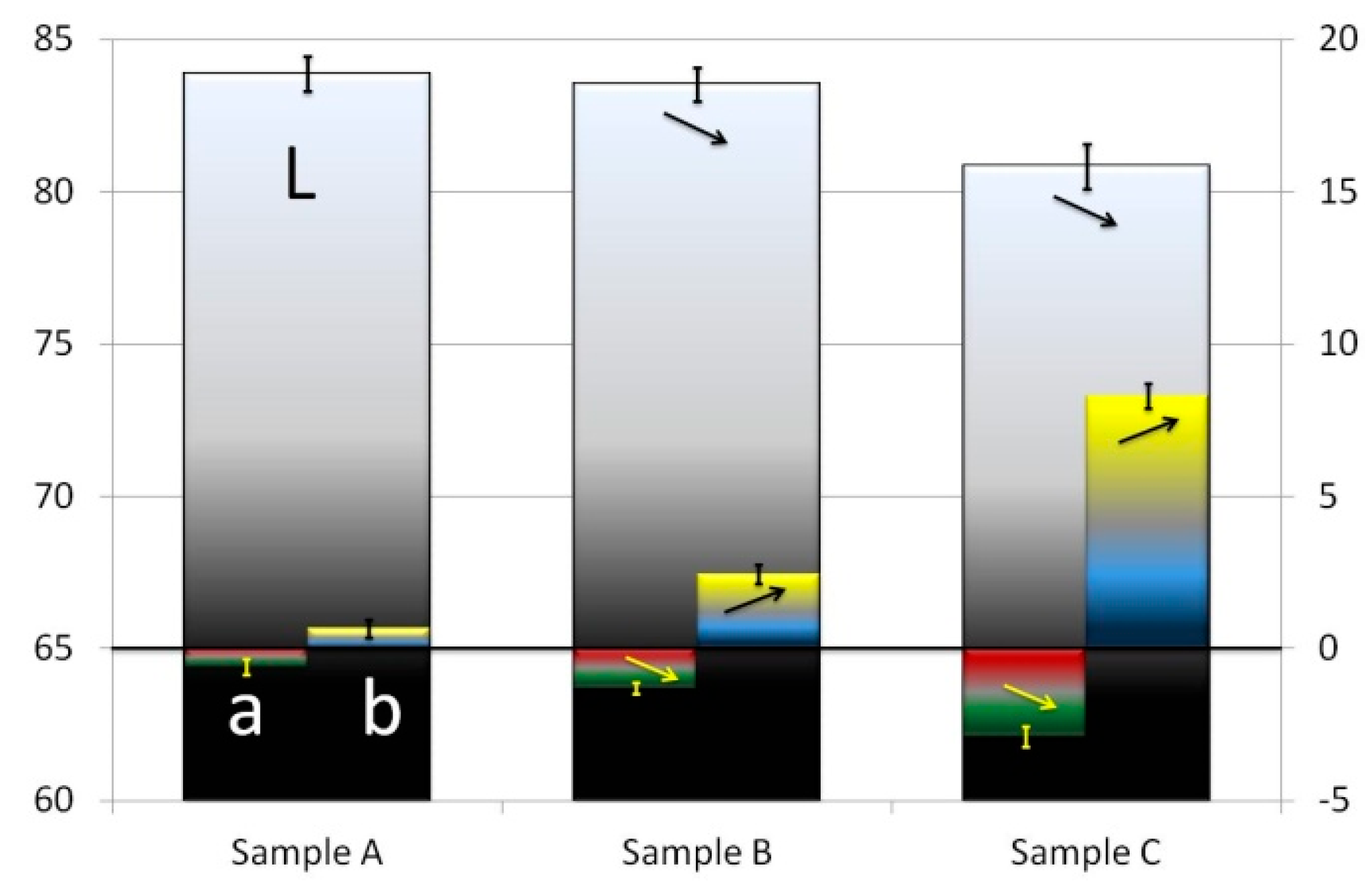
3. Experimental Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hospital Acquired Infections Infographic. Available online: http://chgbeds.blogspot.ca/2012/11/hospital-acquired-infections-infographic.html (accessed on 20 January 2024).
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic Resistance in Hospital-Acquired ESKAPE-E Infections in Low- and Lower-Middle-Income Countries: A Systematic Review and Meta-Analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Sevin, T.; Daniau, C.; Alfandari, S.; Piednoir, E.; Dumartin, C.; Blanchard, H.; Simon, L.; Berger-Carbonne, A.; Le Vu, S. Patterns of Antibiotic Use in Hospital-Acquired Infections. J. Hosp. Infect. 2021, 114, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Despotovic, A.; Milosevic, B.; Milosevic, I.; Mitrovic, N.; Cirkovic, A.; Jovanovic, S.; Stevanovic, G. Hospital-Acquired Infections in the Adult Intensive Care Unit—Epidemiology, Antimicrobial Resistance Patterns, and Risk Factors for Acquisition and Mortality. Am. J. Infect. Control 2020, 48, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Robertson, C.; Pan, J.; Kennedy, S.; Dancer, S.; Haahr, L.; Manoukian, S.; Mason, H.; Kavanagh, K.; Cook, B.; et al. Epidemiology of Healthcare-Associated Infection Reported from a Hospital-Wide Incidence Study: Considerations for Infection Prevention and Control Planning. J. Hosp. Infect. 2021, 114, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Malpiedi, P.; Walia, K.; Srikantiah, P.; Gupta, S.; Lohiya, A.; Chakrabarti, A.; Ray, P.; Biswal, M.; Taneja, N.; et al. Health-Care-Associated Bloodstream and Urinary Tract Infections in a Network of Hospitals in India: A Multicentre, Hospital-Based, Prospective Surveillance Study. Lancet Glob. Health 2022, 10, e1317–e1325. [Google Scholar] [CrossRef] [PubMed]
- Chapa González, C.; González García, L.I.; Burciaga Jurado, L.G.; Carrillo Castillo, A. Bactericidal Activity of Silver Nanoparticles in Drug-Resistant Bacteria. Braz. J. Microbiol. 2023, 54, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Yang, H.H.; Lv, J.Y.; Fan, S.G.; Xie, M.H.; Wang, Z.J.; Gao, J.H. Study on the Bactriostasis of Nano-Silver against Four Strains of Bacteria. Adv. Mater. Res. 2014, 1051, 3–11. [Google Scholar] [CrossRef]
- Kaiser, K.G.; Delattre, V.; Frost, V.J.; Buck, G.W.; Phu, J.V.; Fernandez, T.G.; Pavel, I.E. Nanosilver: An Old Antibacterial Agent with Great Promise in the Fight against Antibiotic Resistance. Antibiotics 2023, 12, 1264. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic Antibacterial Activity of Silver Nanoparticles Biosynthesized by Carbapenem-Resistant Gram-Negative Bacilli. Sci. Rep. 2022, 12, 15254. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, P.; Majeed, M.A.; Ibrahim, S.; Asif, S.; Kalsoom, R.; Hussain, I. Polymeric Coating Doped with Nanomaterials for Functional Impact on Different Substrates. Sci. Rep. 2024, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- ISO 22196:2011; Plastics–Measurement of Antibacterial Activity on Plastics Surfaces. Standard Webpage. Available online: https://www.iso.org/standard/54431.html (accessed on 11 April 2024).
- ISO 4892-2:2013; Plastics—Methods of Exposure to Laboratory Light Sources—Part 2: Xenon-Arc Lamps. Standard Webpage. Available online: https://www.iso.org/standard/55481.html (accessed on 11 April 2024).
- Rollet, F.; Morlat-Thérias, S.; Gardette, J.L. AFM analysis of CD-R photoageing. Polym. Degrad. Stab. 2009, 94, 877–885. [Google Scholar] [CrossRef]
- Suresh, B.; Maruthamuthu, S.; Khare, A.; Palanisamy, N.; Muralidharan, V.S.; Ragunathan, R.; Kannan, M.; Pandiyaraj, K.N. Influence of thermal oxidation on surface and thermo-mechanical properties of polyethylene. J. Polym. Res. 2011, 18, 2175–2184. [Google Scholar] [CrossRef]
- Yang, X.F.; Li, J.; Croll, S.G.; Tallman, D.E.; Bierwagen, G.P. Degradation of low gloss polyurethane aircraft coatings under UV and prohesion alternating exposures. Polym. Degrad. Stab. 2003, 80, 51–58. [Google Scholar] [CrossRef]
- Sikora, A.; Bednarz, L.; Fałat, T.; Wałecki, M.; Adamowska, M. The investigation of the simulated solar radiation impact on the micro- and nanoscale morphology and mechanical properties of the sheet moulded composite surface. Mater. Sci. 2016, 34, 641–649. [Google Scholar] [CrossRef]
- Wang, J.H.; Chang, C.-L.; Zhang, Z.W.; EL-Mahdy, A.F.M. Facile Metal-Free Synthesis of Pyrrolo[3,2-b]Pyrrolyl-Based Conjugated Microporous Polymers for High-Performance Photocatalytic Degradation of Organic Pollutants. Polym. Chem. 2022, 13, 5300–5308. [Google Scholar] [CrossRef]
- Baumann, F.; Raga, S.R.; Lira-Cantú, M. Monitoring the Stability and Degradation Mechanisms of Perovskite Solar Cells by in Situ and Operando Characterization. APL Energy 2023, 1, 011501. [Google Scholar] [CrossRef]
- Pinto, J.; Dias, M.; Amaral, J.; Ivanov, M.; Paixão, J.A.; Coimbra, M.A.; Ferreira, P.; Pereira, E.; Gonçalves, I. Influence of UV Degradation of Bioplastics on the Amplification of Mercury Bioavailability in Aquatic Environments. Mar. Pollut. Bull. 2022, 180, 113806. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Grabarek, A.; Moroń, L.; Wałecki, M.; Kryla, P. The investigation of the light radiation caused polyethylene based materials deterioration by means of atomic force microscopy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 113, 012016. [Google Scholar] [CrossRef]
- Nowicki, M.; Richter, A.; Wolf, B.; Kaczmarek, H. Nanoscale mechanical properties of polymers irradiated by UV. Polymer 2003, 44, 6599–6606. [Google Scholar] [CrossRef]
- Gryta, M.; Grzechulska-Damszel, J.; Markowska, A.; Karakulski, K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J. Membr. Sci. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Kamińska, A.; Sawczak, M.; Ciepliński, M.; Śliwiński, G.; Kosmowski, B. Colorimetric study of the post-processing effect due to pulsed laser cleaning of paper. Opt. Appl. 2004, 34, 121–132. [Google Scholar]
- Ekwińska, M.; Ekwiński, G.; Rymuza, Z. Calibration of normal force in atomic force microscope. In Recent Advantages in Mechatronics; Springer: Berlin/Heidelberg, Germany, 2007; pp. 505–510. [Google Scholar]
- Ekwińska, M.; Rymuza, Z. Normal Force Calibration Method Used for Calibration of Atomic Force Microscope. Acta Phys. Pol. A 2009, 116, 78–81. [Google Scholar] [CrossRef]
- Image Metrology Webpage. Available online: https://www.imagemet.com/ (accessed on 20 January 2024).
- Czylkowski, D.; Hrycak, B.; Sikora, A.; Moczała-Dusanowska, M.; Dors, M.; Jasiński, M. Plasma surface modification of PMMA polymer and its composites with PCBM fullerene using an atmospheric pressure microwave argon plasma sheet. Appl. Surf. Sci. 2021, 11, 9270. [Google Scholar]
- ISO 19606:2017; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics) Test Method for Surface Roughness of Fine Ceramic Films by Atomic Force Microscopy. Standard Webpage. Available online: https://www.iso.org/standard/65457.html (accessed on 10 April 2024).
- ISO 23729:2022; Surface Chemical Analysis Atomic Force Microscopy Guideline for Restoration Procedure for Atomic Force Microscopy Images Dilated by Finite Probe Size. Standard Webpage. Available online: https://www.iso.org/standard/79844.html (accessed on 10 April 2024).
- Lochyński, P.; Sikora, A.; Szczygieł, B. Surface morphology and passive film composition after pickling and electropolishing. Surf. Eng. 2017, 33, 395–403. [Google Scholar] [CrossRef]
- Ho, T.M.; Abik, F.; Mikkonen, K.S. An Overview of Nanoemulsion Characterization via Atomic Force Microscopy. Crit. Rev. Food Sci. Nutr. 2022, 62, 4908–4928. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Jo, A.; Lee, J.; Cho, S.-J. Automatic Defect Review of a Patterned Wafer Using Hybrid Metrology. In Proceedings of the 2021 IEEE International Symposium on the Physical and Failure Analysis of Integrated Circuits (IPFA), Singapore, 15 September–15 October 2021; pp. 1–4. [Google Scholar]
- EN ISO/CIE 11664-4:2019; Colorimetry Part 4: CIE 1976 L*a*b* Colour Space. Standard Webpage. Available online: https://www.iso.org/standard/74166.html (accessed on 11 April 2024).




| Device | Atlas Ci65 |
|---|---|
| Basic exposure time | 100 h |
| Type of the lamp | Xenon |
| Spraying of the samples | 102 min (no spraying) 18 min (spraying) |
| Black plate’s temperature | 65.1 °C (during dry periods) |
| Dry thermometer temperature | 47.0 °C (average value during the dry periods) |
| Relative humidity during the exposure | 48% (average value during the dry periods) |
| The radiation intensity of full spectra of the xenon lamp (290 nm < λ < 800 nm) | |
| The radiation intensity of UV spectra of the xenon lamp (290 nm < λ < 800 nm) | |
| The radiation intensity of the xenon lamp λ = 340 nm |
| Basic Exposure Time | 500 h |
|---|---|
| Type of lamp | evanescent |
| Spraying of the samples | no spraying |
| Black plate’s temperature | 37.1 °C |
| Relative humidity during the exposure | 48% (average value during the dry periods) |
| The radiation intensity of the evanescent lamp λ = 253 nm |
| The Results Obtained for Test and Control Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Determined Parameter | Tested Sample Directly at Start Moment | Control Sample after 24 h | Tested Sample after 24 h | ||||||
| Amount of living cells on sample | 9.9 × 103 | 1.0 × 104 | 9.2 × 103 | 7.2 × 106 | 6.0 × 106 | 4.3 × 106 | 3.8 × 101 | 1.3 × 101 | 3.8 × 101 |
| Average | 9.8 × 103 | 5.8 × 106 => 6.77 log | 2.9 × 101 => 1.46 log | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, A.; Witos, Ł. The Implementation of AFM-Based Nanoscale Diagnostic Methods in the Investigation of the Degradation Process of Bacteriostatic Acrylic Film with Silver Nanoparticles. Appl. Sci. 2024, 14, 3503. https://doi.org/10.3390/app14083503
Sikora A, Witos Ł. The Implementation of AFM-Based Nanoscale Diagnostic Methods in the Investigation of the Degradation Process of Bacteriostatic Acrylic Film with Silver Nanoparticles. Applied Sciences. 2024; 14(8):3503. https://doi.org/10.3390/app14083503
Chicago/Turabian StyleSikora, Andrzej, and Łukasz Witos. 2024. "The Implementation of AFM-Based Nanoscale Diagnostic Methods in the Investigation of the Degradation Process of Bacteriostatic Acrylic Film with Silver Nanoparticles" Applied Sciences 14, no. 8: 3503. https://doi.org/10.3390/app14083503





