Barley Malt as a Binder for Moulding Sands—Gas Evolution and Surface Quality of Iron Castings
Abstract
:1. Introduction
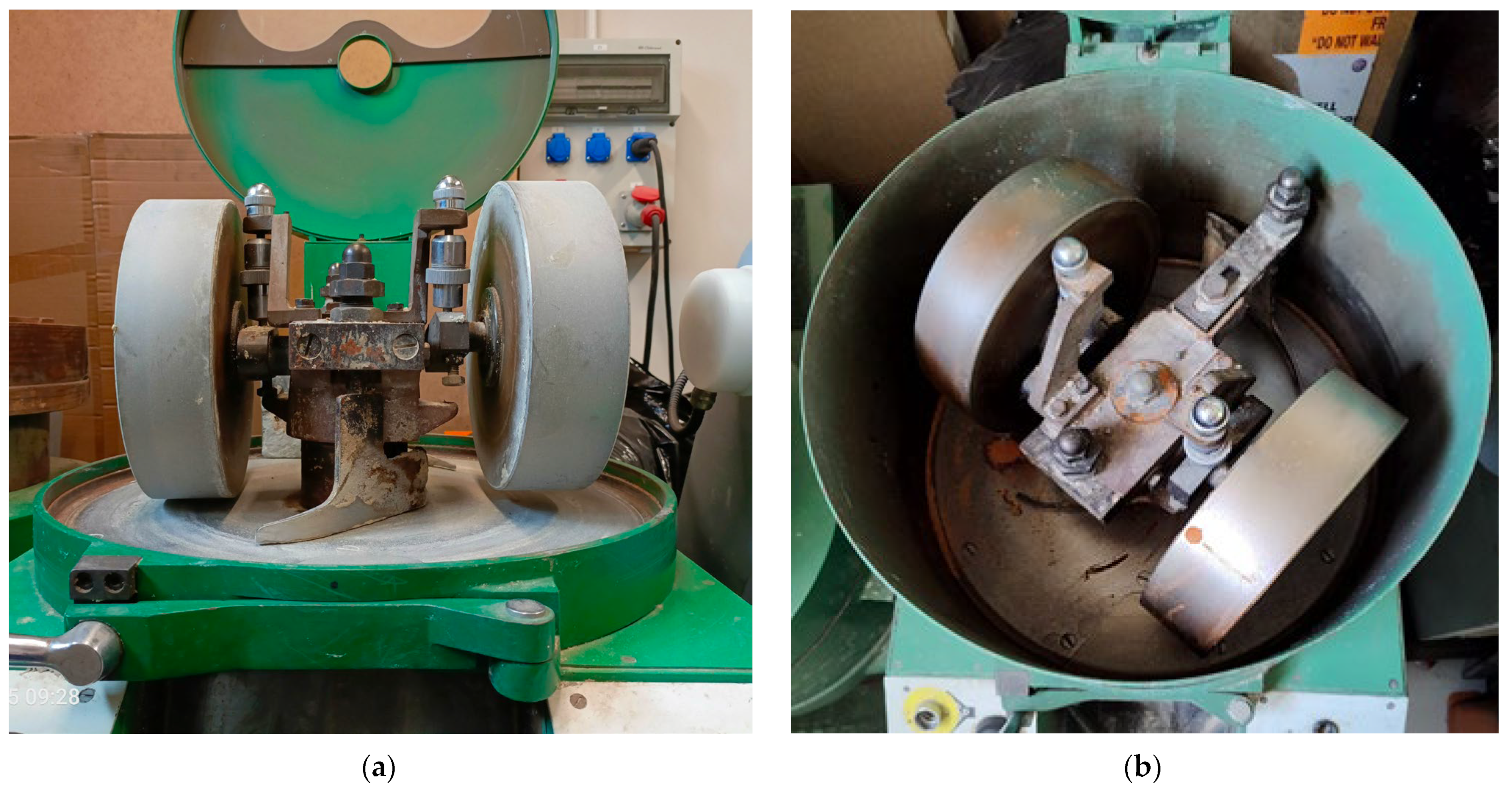
2. Materials and Methods
- ▪
- pH of 5.6;
- ▪
- a colour with a colour unit of 2.4 EBC;
- ▪
- extractivity of 88.5% (this is a value indicating the amount of substance that can be extracted into an aqueous solution, the so-called wort, in the mashing process without the use of enzyme preparations).

- -
- hub height—10 mm;
- -
- hub diameter—200 mm;
- -
- tooth height—15 mm;
- -
- tooth width—10 mm;
- -
- inner diameter—100 mm.
3. Results and Discussion
3.1. Volume and Rate of Gas Emission
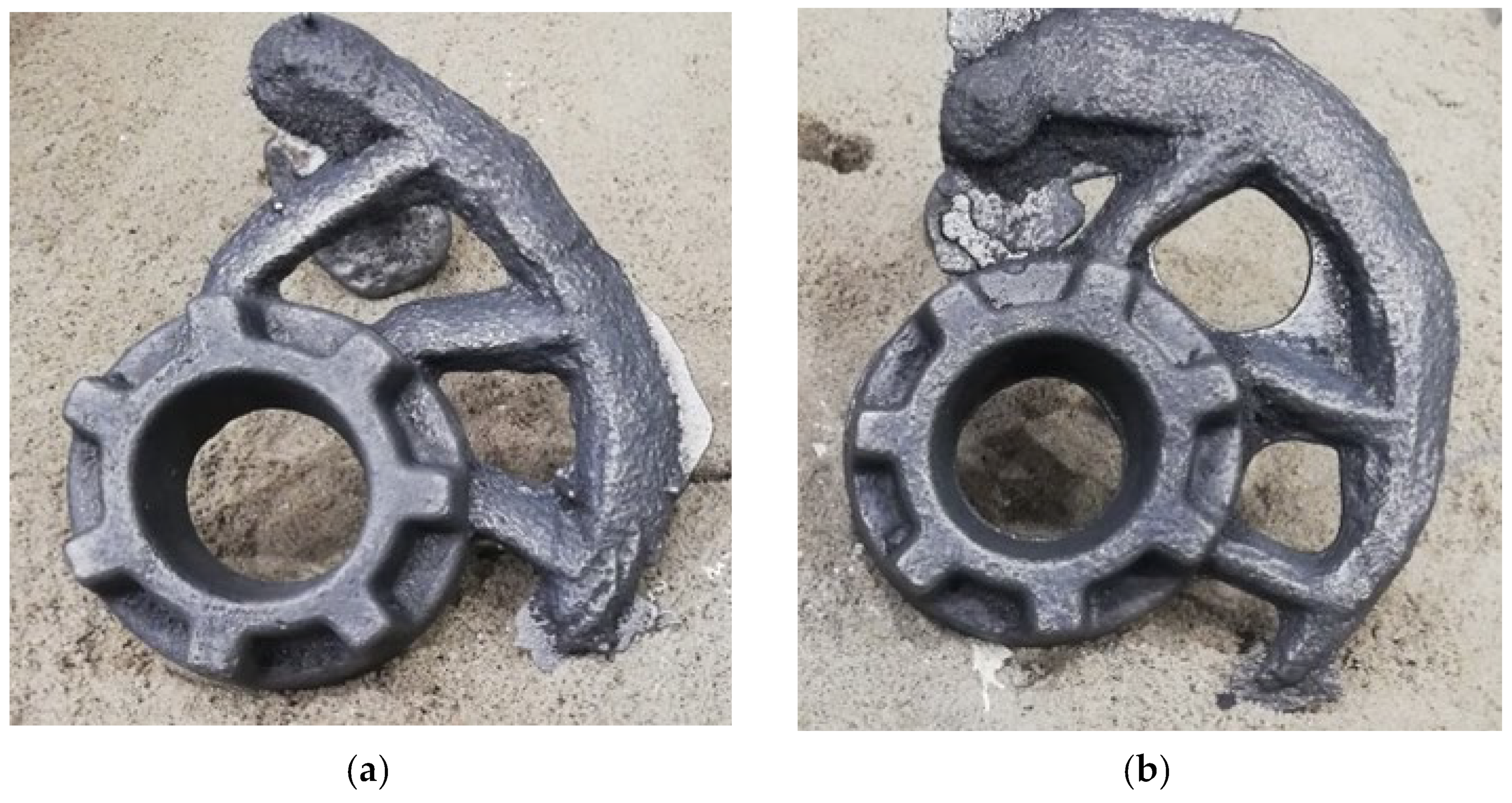
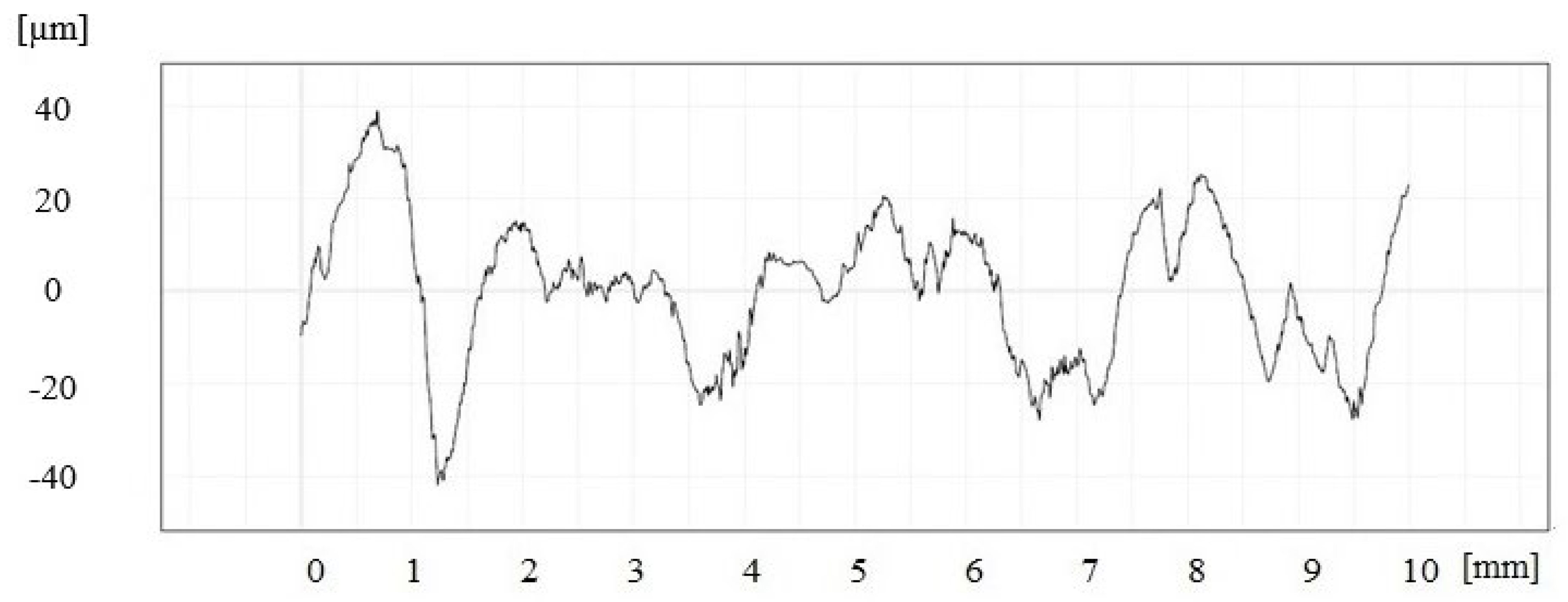
3.2. Macroscopic Examination of Castings
4. Conclusions
- -
- in terms of emissions of BTEX compounds, sands with a barley malt binder do not differ from the currently widely used sands with a phenolic binder (Alphaset technology), and at the same time they are approximately 50% less harmful than moulding sands with bentonite and a WB carrier;
- -
- sands with barley malt have a similar tendency to create defects of gaseous origin (total volume of gases from the moulding sand sample) to popular sands with a phenolic binder;
- -
- total volume of gases produced and the kinetics of their release indicate that making castings in this type of moulding sand should not cause technological inconveniences and result in a tendency to create casting defects of gas origin, which was confirmed by the castings made;
- -
- the use of a binder in the amount of 2% allowed the preparation of moulding sand, which could be used in industrial conditions to prepare a casting mould;
- -
- experimental moulding sand made it possible to make castings that showed no shape or surface defects;
- -
- the casting was characterized by surface roughness, which was classified as accurate sand casting and ordinary die casting;
- -
- barley malt is a renewable raw material through agricultural production, which is important from the point of view of sustainable development of humanity and the Earth, i.e., the preservation of non-renewable resources [23].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastas, P.; Warneren, J. The Environmental Protection Agency. Available online: https://www.epa.gov/greenchemistry/basics-green-chemistry#twelve (accessed on 4 March 2024).
- Sanderson, K. It’s not easy being being green. Nature 2011, 469, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 91/156/EEC of 18 March 1991 Amending Directive 75/442/EEC on Waste. Available online: https://www.prawo.pl/akty/dz-u-ue-l-1991-78-32,67513615.html (accessed on 4 March 2024).
- Major-Gabryś, K. Environmentally Friendly Foundry Molding and Core Sands. J. Mater. Eng. Perform. 2019, 28, 3905–3911. [Google Scholar] [CrossRef]
- Holtzer, M. Trends in sand moulds and cores with organic binder. Arch. Foundry 2003, 3, 189–196. [Google Scholar]
- Tackes, G. Core binders: A look into the future. Mod. Cast. 2001, 91, 24. [Google Scholar]
- Ochulorl, E.F.; Ugboaja, J.O.; Olowomeye, O.A. Performance of kaolin and cassavastarch as replacements for bentonite in moulding sand used in thin wall ductile iron castings. Niger. J. Technol. 2019, 38, 947–956. [Google Scholar] [CrossRef]
- Dobosz, S.M.; Jelinek, P.; Major-Gabryś, K. Development tendencies of moulding and core sands. China Foundry 2011, 8, 438–446. [Google Scholar]
- Lynch, P.; Hasbrouck, C.; Wilck, J.; Kay, M.; Manogharan, G. Challenges and opportunities to integrate the oldest and newest manufacturing processes: Metal casting and additive manufacturing. Rapid Prototyp. J. 2020, 26, 1145–1154. [Google Scholar] [CrossRef]
- Holtzer, M.; Grabowska, B.; Żymankowska-Kumon, S.; Kwaśniewska-Królikowska, D.; Dańko, R.; Solarski, W.; Bobrowski, A. Harmfulness of moulding sands with bentonite and lustrous carbon carriers. Metalurgija 2012, 51, 437–440. [Google Scholar]
- PN-H-11001:1985; Foundry Molding Materials—Quartz Foundry Sands; Polish Version; Polski Komitet Normalizacyjny (PKN): Warszawa, Poland, 1985.
- Lewandowski, J.L. Tworzywa na Formy Odlewnicze; Akapit, Kraków: Kraków, Poland, 1997. [Google Scholar]
- Holtzer, M.; Harabasz, M. Water Quality as One of the Factors Determining the Properties of Bentonite Moulding Sands in Iron Foundry. Arch. Foundry Eng. 2013, 13, 45–50. [Google Scholar]
- Loch, J.; Grabowska, B.; Kaczmarska, K. BTEX emissions from bioco2 bonded moulding sands. Metall. Foundry Eng. 2013, 39, 25–31. [Google Scholar] [CrossRef]
- Bobrowski, A.; Żymankowska-Kumon, S.; Drozyński, D.; Grabowska, B.; Kaczmarska, K. TG/DTG/DTA, FTIR and GC/MS Studies of Oil Sand for Artistic and Precision Foundry with the Emission of Gases Assessment. Arch. Foundry Eng. 2017, 17, 25–30. [Google Scholar] [CrossRef]
- Łągiewka, M.; Konopka, Z.; Zyska, A. Studies of the effect of negative pressure in the mould on the cast surface roughness. Pr. Inst. Odlew. 2018, 58, 3–12. [Google Scholar] [CrossRef]
- Nwaogu, U.C.; Tiedje, N.S.; Hansen, H.N. A non-contact 3D method to characterize the surface roughness of castings. J. Mater. Process. Technol. 2013, 213, 59–68. [Google Scholar] [CrossRef]
- Franciszek, P.; Izdebska-Szanda, I.; Smoluchowska, E.; Świder, R.; Pysz, A. Application of moulding sands with geopolymer binder in the manufacture of castings from aluminium alloys. Pr. Inst. Odlew. 2011, 50, 23–34. [Google Scholar] [CrossRef]
- Sakwa, W.; Wachelko, T. Materiały na Formy i Rdzenie Odlewnicze; Wydawnictwo Śląsk: Katowice, Poland, 1980. [Google Scholar]
- Stachowicz, M.; Granat, K.; Nowak, D. Bending strength measurement as a method of binder quality assessment on the example of water-glass containing moulding sands. Arch. Foundry Eng. 2012, 12, 175–178. [Google Scholar] [CrossRef]
- Popoola, A.P.I.; Abdulwahab, M.; Fayomi, O.S.I. Synergetic performance of palm oil (Elaeis guineensis) and pine oil (Pinus sylvestris) as binders on foundry core strength. Int. J. Phys. Sci. 2012, 7, 3062–3066. [Google Scholar] [CrossRef]
- Atanda, P.O.; Akinlosotu, O.C.; Oluwole, O.O. Effect of Some Polysaccharide Starch Extracts on Binding Characteristics of Foundry Moulding Sand. Int. J. Sci. Eng. Res. 2014, 5, 362–367. [Google Scholar]
- Rogers, C.W.; Hu, G.; Mikkelsen, R. Grain Yield, Quality, and Nutrient Concentrations of Feed, Food, and Malt Barley. Commun. Soil Sci. Plant Anal. 2017, 48, 2678–2686. [Google Scholar] [CrossRef]







| Sample | Mass of Sample [g] | Volume of Gases [dm3/kg m.s.] | Emission of BTEX [mg/kg m.s.] | |||
|---|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | Xylenes | |||
| D1 | 142.37 | 17.62 | 287.63 | 16.71 | 0.21 | 7.51 |
| D2 | 134.03 | 17.97 | 254.47 | 14.18 | 0.23 | 7.20 |
| Mean | 138.20 | 17.79 | 271.05 | 15.44 | 0.22 | 7.36 |
| Marking of a Moulding Sand Sample | Volume of Gases [dm3/kg m.s.] | Gas Emissions [mg/kg.s] | |||
|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | Xylenes | ||
| Oil | 112.100 | 2646.22 | 61.05 | 4.87 | 22.15 |
| Green sand with lustrous carbon carrier | 19.11 | 386.73 | 9.76 | 0.13 | 1.19 |
| Phenolic | 17.37 | 325.89 | 29.33 | 0.22 | 1.86 |
| Alphaset technology | 11.303 | 246.7 | 2.1 | 0.3 | 0.02 |
| Geopol (inorganic) | 9.90 | 37.00 | 0.58 | 0.01 | 0.00 |
| Industrial Castings | Raav | Rzav |
|---|---|---|
| Average value from measurements | 12.33 | 54.12 |
| Type of Casting | Precision | Ra Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.3 | 3.2 | 1.6 | 0.8 | 0.4 | 0.2 | 0.1 | 0.05 | ||
| Sand casting | ordinary | x | x | x | |||||||||
| accurate | x | x | x | ||||||||||
| Die casting | ordinary | x | x | x | |||||||||
| accurate | x | x | |||||||||||
| Injection casting | ordinary | x | x | ||||||||||
| accurate | x | x | |||||||||||
| Qualitative and Quantitative Composition | Compressive Strength [MPa] | Tensile Strength [MPa] | Bending Strength [MPa] | Permeability [10−8 m2/Pa∙s] | Wear Resistance [%] | Flowability [%] | Reference |
|---|---|---|---|---|---|---|---|
| * High-silica sand (98%), Malted barley binder (2%), and Distilled water (2%) | 4.5 | 0.56 | 0.55 | 317 | 6.25 | 90.8 | own research |
| ** High-silica sand (~80%), Bentonite (10%), Humidity (3.65%), Dextrin (2%), and Coal dust (5%) | 0.060 | 0.0052 | - | 200 | 15.5 | 79.9 | [18] |
| *** High-silica sand (94%), Bentonite (8%), and Distilled water (4%) | 0.55 | - | - | - | 18 | - | [19] |
| High-silica sand (96%), Water glass 145 (3.5%), and Distilled water (0.5%) | - | 2.4 | - | - | - | - | [20] |
| High-silica sand (90%), Cassava Starch (6%), and Water (14 mL—4%) | 0.51 | - | - | 126 | - | - | [7] |
| High-silica sand (87%), Cassava Starch (6%), Palm oil (6%), Pine oil (2%), and Water (3%) | 1.35 | - | - | 85 | - | - | [21] |
| High-silica sand (80%), Rice Starch (20%), and Water (40 cm3) | 0.035 | - | - | 122 vol./min | - | - | [22] |
| High-silica sand (80%), Maize starch (20%), and Water (40 cm3) | 0.050 | - | - | 156 vol./min | - | - | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, D.; Bobrowski, A.; Samociuk, B.; Żymankowska-Kumon, S.; Medyński, D. Barley Malt as a Binder for Moulding Sands—Gas Evolution and Surface Quality of Iron Castings. Appl. Sci. 2024, 14, 3560. https://doi.org/10.3390/app14093560
Nowak D, Bobrowski A, Samociuk B, Żymankowska-Kumon S, Medyński D. Barley Malt as a Binder for Moulding Sands—Gas Evolution and Surface Quality of Iron Castings. Applied Sciences. 2024; 14(9):3560. https://doi.org/10.3390/app14093560
Chicago/Turabian StyleNowak, Daniel, Artur Bobrowski, Bartłomiej Samociuk, Sylwia Żymankowska-Kumon, and Daniel Medyński. 2024. "Barley Malt as a Binder for Moulding Sands—Gas Evolution and Surface Quality of Iron Castings" Applied Sciences 14, no. 9: 3560. https://doi.org/10.3390/app14093560





