MoSe2 with Ultra-Fine Pt Decoration for Efficient Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparations
2.2. Characterizations
2.3. Photodegradation Measurements
3. Results and Discussion
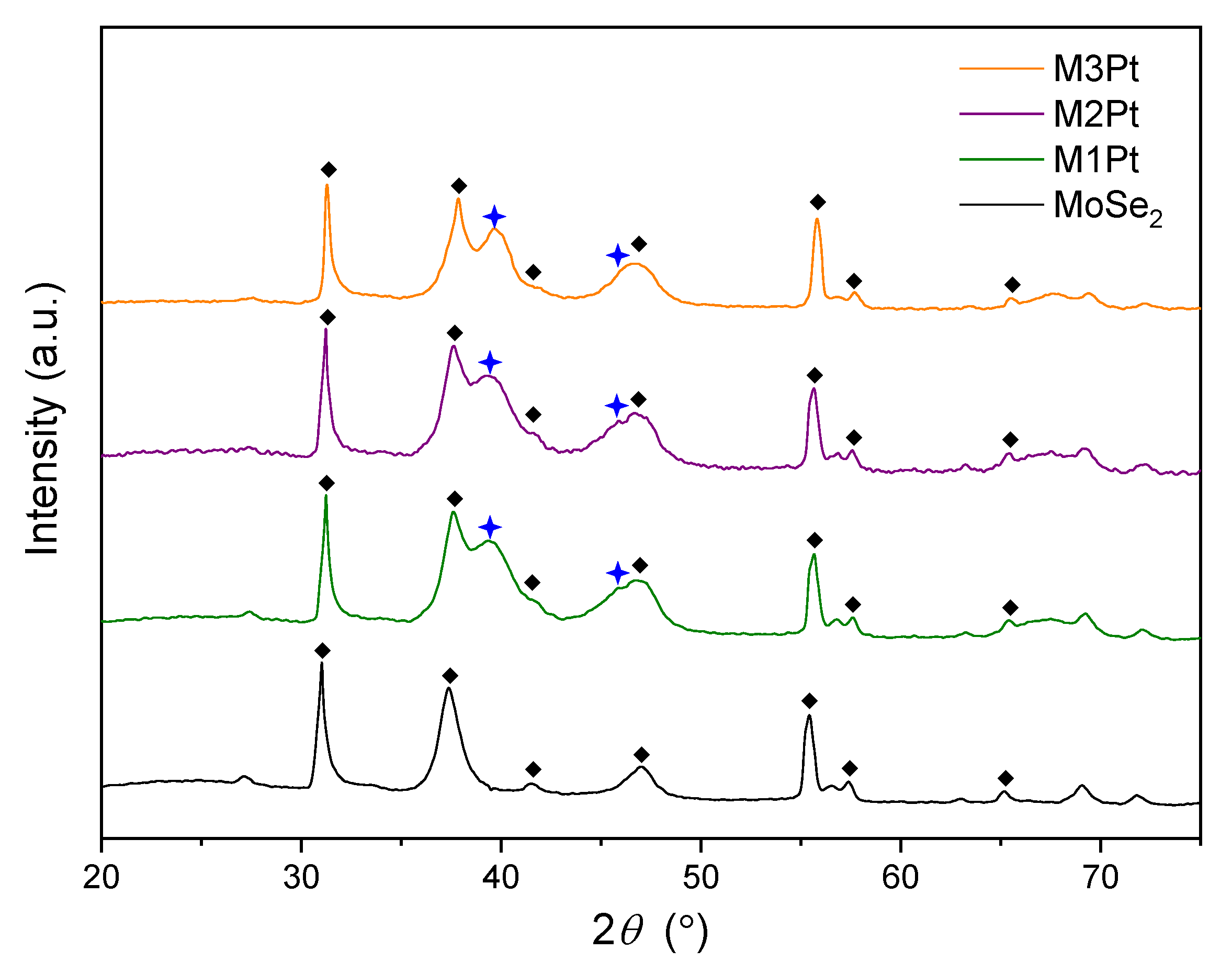
3.1. MoSe2-Pt Morphologies and Crystal Structures
3.2. Chemical States, Mechanism of Efficient Photocatalytic Reactions
3.3. Optical Properties of the Catalysts
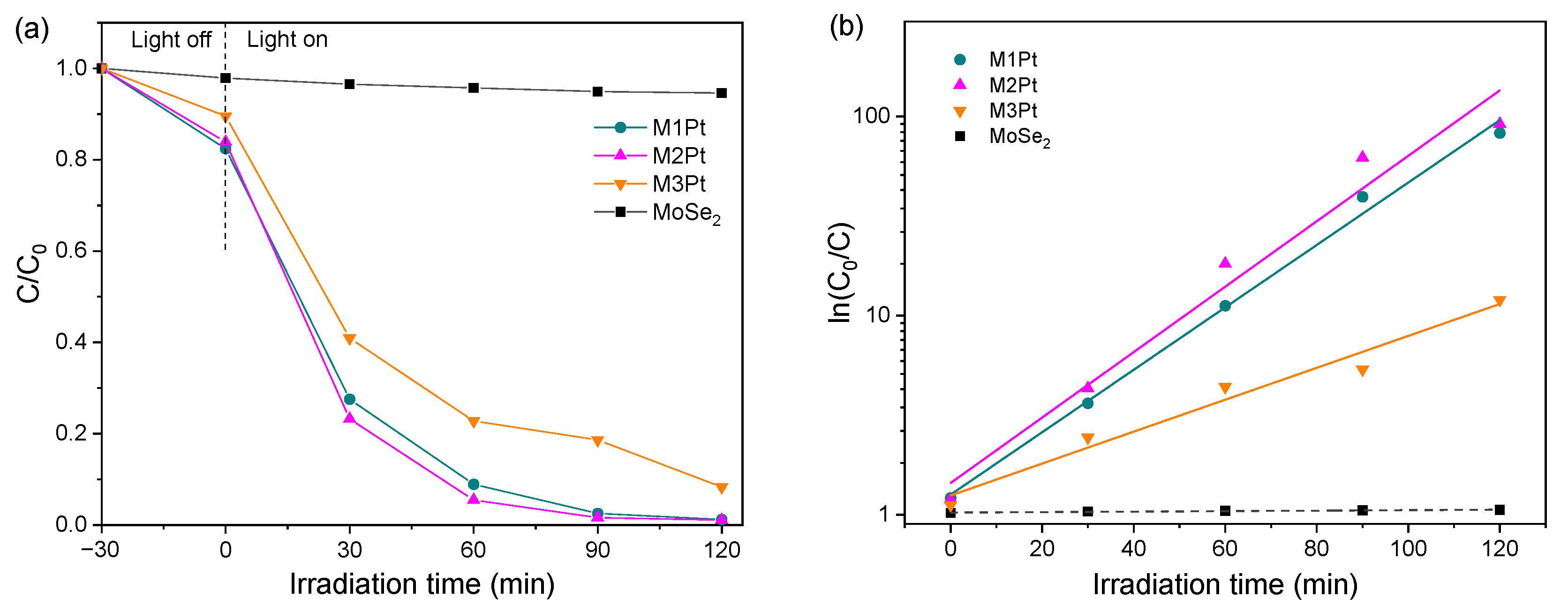
3.4. Photodegradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sellers, P.; Kelly, C.A.; Rudd, J.W.M.; MacHutchon, A.R. Photodegradation of methylmercury in lakes. Nature 1996, 380, 694–697. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lu, Y.; Huang, Z.; Hu, J. Facile solvent-thermal synthesis of ultrathin MoSe2 nanosheets for hydrogen evolution and organic dyes adsorption. Appl. Surf. Sci. 2017, 402, 277–285. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Krasnok, A.; Lepeshov, S.; Alú, A. Nanophotonics with 2D transition metal dichalcogenides. Opt. Express 2018, 26, 15972. [Google Scholar] [CrossRef]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-dimensional transition metal dichalcogenide (TMD) nanosheets. Chem. Soc. Rev. 2015, 44, 2584. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Shi, X.; Posysaev, S.; Huttula, M.; Pankratov, V.; Hoszowska, J.; Dousse, J.C.; Zeeshan, F.; Niu, Y.; Zakharov, A.; Li, T.; et al. Metallic contact between MoS2 and Ni via Au nanoglue. Small 2018, 14, 1704526. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, M.; Wang, X.; Kistanov, A.A.; Li, T.; Cao, W.; Huttula, M. Nickel nanoparticle-activated MoS2 for efficient visible light photocatalytic hydrogen evolution. Nanoscale 2022, 14, 8601–8610. [Google Scholar] [CrossRef]
- Shim, G.W.; Yoo, K.; Seo, S.B.; Shin, J.; Jung, D.Y.; Kang, I.S.; Ahn, C.W.; Cho, B.J.; Choi, S.Y. Large-area single-layer MoSe2 and its van der Waals heterostructures. ACS Nano 2014, 8, 6655–6662. [Google Scholar] [PubMed]
- Mathew, S.; Sai Chandu, K.K.; Halder, S.; Polumati, G.; Chakraborty, C.; Sahatiya, P.; Pal, S. Band alignment and charge transport study of Au nanoparticles decorated over MoS2/MoSe2 hybrid heterostructure for plasmon enhanced photodetection. Mater. Sci. Semicond. Process. 2023, 156, 107302. [Google Scholar] [CrossRef]
- Kumar, S.; Mirzaei, A.; Kumar, A.; Lee, M.H.; Ghahremani, Z.; Kim, T.U.; Kim, J.Y.; Kwoka, M.; Kumar, M.; Kim, S.S.; et al. Nanoparticles anchored strategy to develop 2D MoS2 and MoSe2 based room temperature chemiresistive gas sensors. Coord. Chem. Rev. 2024, 503, 215657. [Google Scholar] [CrossRef]
- Nisar, S.; Dastgeer, G.; Shahzadi, M.; Shahzad, Z.M.; Elahi, E.; Irfan, A.; Eom, J.; Kim, H.; Kim, D.K. Gate-assisted MoSe2 transistor to detect the streptavidin via supporter molecule engineering. Mater. Today Nano 2023, 24, 100405. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Li, M.; Wang, Y.; Wang, D. Nanoflower-like 1T/2H mixed-phase MoSe2 as an efficient electrocatalyst for hydrogen evolution. J. Alloys Compd. 2021, 878, 160381. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, T.R.; Zhou, B.; Cui, Y.T.; Yan, H.; Liu, Z.; Schmitt, F.; Lee, J.; Moore, R.; Chen, Y.; et al. Direct observation of the transition from indirect to direct bandgap in atomically thin epitaxial MoSe2. Nat. Nanotech. 2014, 9, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Das, B.; Samanta, M.; Das, B.K.; Ghosh, S.; Chattopadhyay, K.K. MoSe2-Amorphous CNT Hierarchical Hybrid Core–Shell Structure for Efficient Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 5067–5076. [Google Scholar] [CrossRef]
- Hwang, Y.; Shin, N. Colloidal Synthesis of MoSe2/WSe2 Heterostructure Nanoflowers via Two-Step Growth. Materials 2021, 14, 7294. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Nie, Z.; Feng, Y.; Hu, X.; Li, H.; Zhao, Z.; Zan, S.; Qi, S. Hollow core–shell structure Co/C@MoSe2 composites for high-performance microwave absorption. Compos. Part A Appl. Sci. Manuf. 2022, 162, 107140. [Google Scholar] [CrossRef]
- Rao, B.G.; Matte, H.S.S.R.; Rao, C.N.R. Decoration of few-layer graphene-like MoS2 and MoSe2 by noble metal nanoparticles. J. Clust. Sci. 2012, 23, 929–937. [Google Scholar]
- Upadhyay, S.N.; Pakhira, S. Nanostructured Pt-doped 2D MoSe2: An efficient bifunctional electrocatalyst for both hydrogen evolution and oxygen reduction reactions. Phys. Chem. Chem. Phys. 2022, 37, 22823–22844. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.D.; Lilley, L.M.; Cain, J.D.; Hao, S.; Palacios, E.; Aydin, K.; Wolverton, C.; Meade, T.; Dravid, V.P. Phase engineering and optical properties of 2D MoSe2: Promise and pitfalls. Mater. Chem. Phys. 2019, 225, 219–226. [Google Scholar] [CrossRef]
- Wang, F.; Lu, T.; Cheng, X.; Zhang, Y.; Xiao, X. Fabrication of MoSe2/Bi3O4Br Z-scheme heterojunction with enhanced photocatalytic performance. J. Environ. Chem. Eng. 2024, 12, 111991. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Li, S.; Meng, A.; Li, Z. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qiao, W.; Yu, L.; Wang, S.; Feng, L. Support engineering modulated Pt/hierarchical MoSe2@mesoporous hollow carbon spheres for efficient methanol-assisted water splitting. Chem. Eng. J. 2024, 483, 149055. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Bao, S.; Wang, M.; Qi, X.; Fan, Z.; Zhang, H. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 2013, 4, 1444. [Google Scholar] [CrossRef]
- Lin, C.S.; Khan, M.R.; Lin, S.D. Platinum states in citrate sols by EXAFS. J. Colloid Interface Sci. 2005, 287, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Chan, K.; Abild-Pedersenb, F.; Nørskov, J.K. Active edge sites in MoSe2 and WSe2 catalysts for the hydrogen evolution reaction: A density functional study. Phys. Chem. Chem. Phys. 2014, 16, 13156–13164. [Google Scholar] [CrossRef]
- Shelke, N.T.; Late, D.J. Hydrothermal growth of MoSe2 nanoflowers for photo- and humidity sensor applications. Sens. Actuators A Phys. 2019, 295, 160–168. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Qiao, W.; Yu, L.; Chang, J.; Yang, F.; Feng, L. Efficient bi-functional catalysis of coupled MoSe2 nanosheet/Pt nanoparticles for methanol-assisted water splitting. Chin. J. Catal. 2023, 51, 113–123. [Google Scholar]
- Avsar, A.; Ciarrocchi, A.; Pizzochero, M.; Unuchek, D.; Yazyev, O.V.; Kis, A. Defect induced, layer-modulated magnetism in ultrathin metallic PtSe2. Nat. Nanotech. 2019, 14, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liao, Y.; Liu, Y.; Zeng, W.; Zhou, Q. Room temperature detection of nitrogen dioxide gas sensor based on Pt-modified MoSe2 nanoflowers: Experimental and theoretical analysis. Appl. Surf. Sci. 2023, 610, 155527. [Google Scholar] [CrossRef]
- Dong, N.; Li, Y.; Feng, Y.; Zhang, S.; Zhang, X.; Chang, C.; Fan, J.; Zhang, L.; Wang, J. Optical limiting and theoretical modelling of layered transition metal dichalcogenide nanosheets. Sci. Rep. 2015, 5, 14646. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, C.; Li, F.; Li, T.; Zhang, M.; Cao, W. Improved photocatalytic Bi2WO6/BiOCl heterojunctions: One-step synthesis via an ionic-liquid assisted ultrasonic method and first-principles calculations. Mol. Catal. 2017, 435, 33–48. [Google Scholar]
- Sidorowicz, A.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Concas, A.; Orrù, R.; Cao, G.; Rupprechter, G. Microalgae-derived Co3O4 nanomaterials for catalytic CO oxidation. RSC Adv. 2024, 14, 4575–4586. [Google Scholar]



| Samples | MoSe2 | K2PtCl4 | Conditions |
|---|---|---|---|
| M1Pt | 0.1 mg/mL × 10 mL | 41.5 mg | 50 °C, 150 min |
| M2Pt | 0.2 mg/mL × 10 mL | 41.5 mg | 50 °C, 150 min |
| M3Pt | 0.3 mg/mL × 10 mL | 41.5 mg | 50 °C, 200 min |
| Samples | 2θ/° | FWHM/° | d (111)/nm | D (111)/nm |
|---|---|---|---|---|
| M1Pt | 39.40 | 12.4217 | 0.2285 | 0.6793 |
| M2Pt | 39.30 | 12.4017 | 0.2291 | 0.6802 |
| M3Pt | 39.60 | 12.0752 | 0.2274 | 0.6992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shao, D.; Xu, F.; Huang, Z.; Shi, X. MoSe2 with Ultra-Fine Pt Decoration for Efficient Photodegradation. Appl. Sci. 2024, 14, 3592. https://doi.org/10.3390/app14093592
Chen Y, Shao D, Xu F, Huang Z, Shi X. MoSe2 with Ultra-Fine Pt Decoration for Efficient Photodegradation. Applied Sciences. 2024; 14(9):3592. https://doi.org/10.3390/app14093592
Chicago/Turabian StyleChen, Yong, Dawei Shao, Fupeng Xu, Zhongjia Huang, and Xinying Shi. 2024. "MoSe2 with Ultra-Fine Pt Decoration for Efficient Photodegradation" Applied Sciences 14, no. 9: 3592. https://doi.org/10.3390/app14093592






