Antibacterial Agents Used in Modifications of Dental Resin Composites: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Research Strategy
- −
- The keyword combinations used were as follows: “dental AND materials AND antibacterial modifier” OR “dental AND materials AND antibacterial agent” OR “antibacterial polymer” OR “antibacterial dental resin” OR “resin composites AND antibacterial modification AND antibacterial agent” OR “antimicrobial monomers AND dental resin composite” OR “dental AND composite AND antibacterial” OR “dental AND composite AND antibacterial AND properties”.
- −
- Only English-language publications were analyzed.
- −
- Publications published between 2017 and 2024.
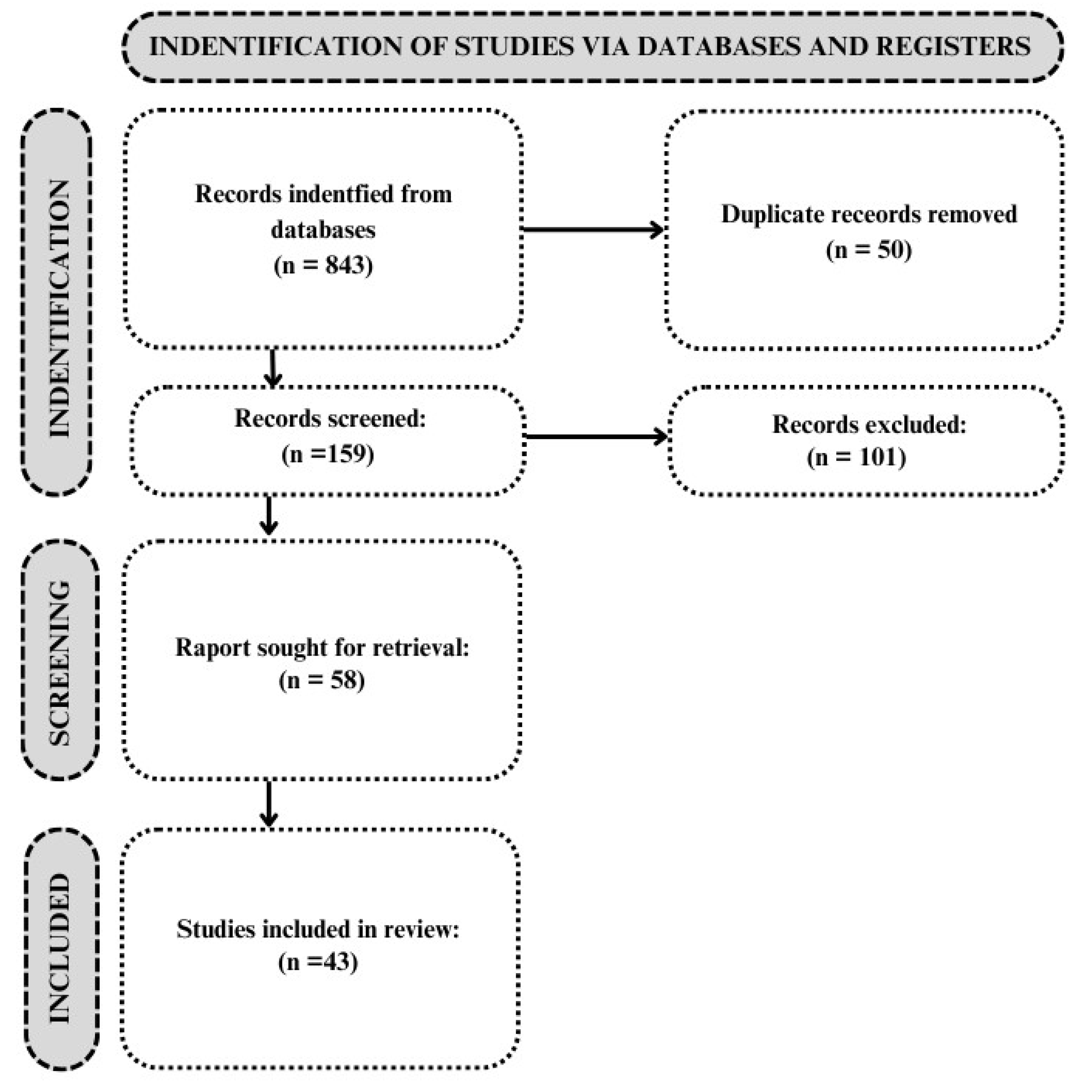
2.2. Article Selection
3. Results
4. Discussion
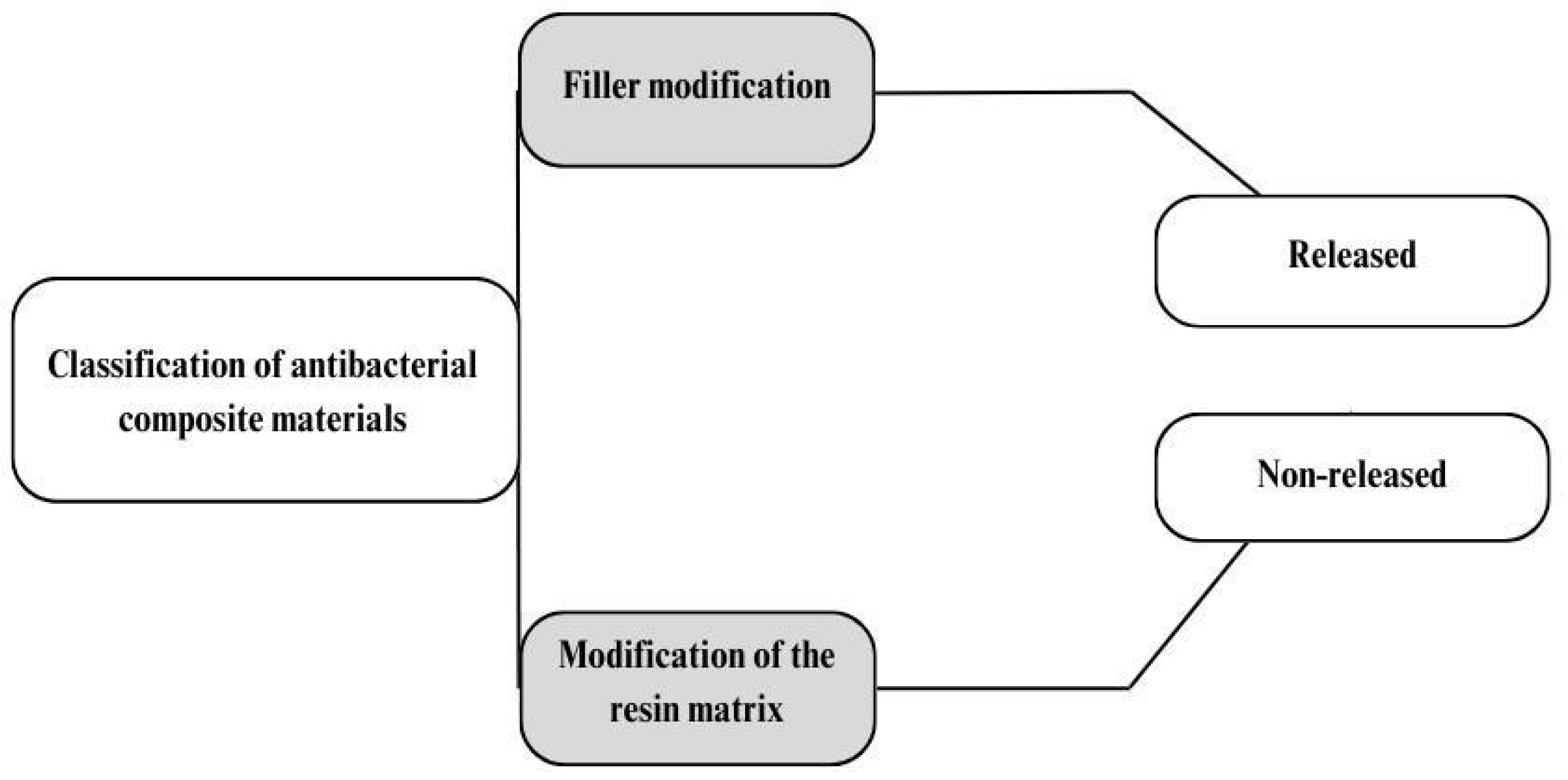
4.1. Modification with Released Agents
4.2. Modification of RBC Compositions
4.2.1. Organic Compounds and Monomers
4.2.2. Other Additives
4.3. Limitation of the Review and Future Insights
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, M.; Xuan, S.; Wang, Z. Oral Microbiota: A New View of Body Health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is Secondary Caries with Composites a Material-Based Problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef] [PubMed]
- Mjör, I.A.; Toffenetti, F. Secondary Caries: A Literature Review with Case Reports. Quintessence Int. 2000, 31, 165–179. [Google Scholar] [PubMed]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus Mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef]
- Al Sunbul, H.; Silikas, N.; Watts, D.C. Polymerization Shrinkage Kinetics and Shrinkage-Stress in Dental Resin-Composites. Dent. Mater. 2016, 32, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial Dental Restorative Materials: A Review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar]
- Alansy, A.S.; Saeed, T.A.; Guo, Y.; Yang, Y.; Liu, B.; Fan, Z. Antibacterial Dental Resin Composites: A Narrative Review. Open J. Stomatol. 2022, 12, 147–165. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Ferracane, J.; Powers, J. (Eds.) Chapter 9—Restorative Materials: Resin Composites and Polymers. In Craig’s Restorative Dental Materials, 14th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 135–170. ISBN 978-0-323-47821-2. [Google Scholar]
- Combe, E.C.; Purzyński, M. Wstęp Do Materiałoznawstwa Stomatologicznego; Wydawnictwo Medyczne Sanmedica: Warszawa, Poland, 1997; ISBN 8386516321. [Google Scholar]
- Fabiano, F.; Calabrese, L.; Proverbio, E. Chapter 9—Mechanical Behavior of Hydroxyapatite-Based Dental Resin Composites. In Materials for Biomedical Engineering; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 251–295. ISBN 978-0-12-816909-4. [Google Scholar]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of Filler Characteristics on the Performance of Dental Composites: A Comprehensive Review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Ludovichetti, F.S.; Lucchi, P.; Zambon, G.; Pezzato, L.; Bertolini, R.; Zerman, N.; Stellini, E.; Mazzoleni, S. Depth of Cure, Hardness, Roughness and Filler Dimension of Bulk-Fill Flowable, Conventional Flowable and High-Strength Universal Injectable Composites: An In Vitro Study. Nanomaterials 2022, 12, 1951. [Google Scholar] [CrossRef]
- Beyth, N.; Farah, S.; Domb, A.J.; Weiss, E.I. Antibacterial Dental Resin Composites. React. Funct. Polym. 2014, 75, 81–88. [Google Scholar] [CrossRef]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef]
- Ren, J.; Guo, X. The Germicidal Effect, Biosafety and Mechanical Properties of Antibacterial Resin Composite in Cavity Filling. Heliyon 2023, 9, e19078. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa-Pacher, A. Research Questions with PICO: A Universal Mnemonic. Publications 2022, 10, 21. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.A.; Rodrigues, N.S.; Souza, L.C.; Lomonaco, D.; Rodrigues, F.P.; Degrazia, F.W.; Collares, F.M.; Sauro, S.; Saboia, V.P.A. Physicochemical and Microbiological Assessment of an Experimental Composite Doped with Triclosan-Loaded Halloysite Nanotubes. Materials 2018, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, X.; Yu, Q.; Baker, S.N.; Li, H.; Larm, N.E.; Baker, G.A.; Chen, L.; Tan, J.; Chen, M. Incorporation of Antibacterial Agent Derived Deep Eutectic Solvent into an Active Dental Composite. Dent. Mater. 2017, 33, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial Resin-Based Composite Containing Chlorhexidine for Dental Applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.; Wang, X.; Chen, Y.; Li, Q.; Xing, X.; Xiao, Y.; Peng, X.; Ye, Z. Development of a Novel Resin-Based Dental Material with Dual Biocidal Modes and Sustained Release of Ag+ Ions Based on Photocurable Core-Shell AgBr/Cationic Polymer Nanocomposites. J. Mater. Sci. Mater. Med. 2017, 28, 103. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, C.; Liu, H.; Sun, Y.; Zhang, J. Investigating the Antibacterial Activity of Thyme Oil/TiO2 Modified Resins against Oral Pathogenic Bacteria. Alex. Eng. J. 2024, 89, 195–201. [Google Scholar] [CrossRef]
- Fugolin, A.P.; Dobson, A.; Huynh, V.; Mbiya, W.; Navarro, O.; Franca, C.M.; Logan, M.; Merritt, J.L.; Ferracane, J.L.; Pfeifer, C.S. Antibacterial, Ester-Free Monomers: Polymerization Kinetics, Mechanical Properties, Biocompatibility and Anti-Biofilm Activity. Acta Biomater. 2019, 100, 132–141. [Google Scholar] [CrossRef]
- Fanfoni, L.; Marsich, E.; Turco, G.; Breschi, L.; Cadenaro, M. Development of Di-Methacrylate Quaternary Ammonium Monomers with Antibacterial Activity. Acta Biomater. 2021, 129, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Cherchali, F.Z.; Mouzali, M.; Tommasino, J.B.; Decoret, D.; Attik, N.; Aboulleil, H.; Seux, D.; Grosgogeat, B. Effectiveness of the DHMAI Monomer in the Development of an Antibacterial Dental Composite. Dent. Mater. 2017, 33, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Huang, S.; Liang, X.; Qin, W.; Liu, F.; Lin, Z.; He, J. The Antibacterial, Cytotoxic, and Flexural Properties of a Composite Resin Containing a Quaternary Ammonium Monomer. J. Prosthet. Dent. 2018, 120, 609–616. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ye, L.; He, R.; He, J.; Ouyang, S.; Zhang, J. Antibacterial Dental Resin Composites (DRCs) with Synthesized Bis-Quaternary Ammonium Monomethacrylates as Antibacterial Agents. J. Mech. Behav. Biomed. Mater. 2022, 135, 105487. [Google Scholar] [CrossRef] [PubMed]
- Chrószcz-Porębska, M.; Kazek-Kęsik, A.; Chladek, G.; Barszczewska-Rybarek, I. Novel Mechanically Strong and Antibacterial Dimethacrylate Copolymers Based on Quaternary Ammonium Urethane-Dimethacrylate Analogues. Dent. Mater. 2023, 39, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Chrószcz-Porębska, M.W.; Barszczewska-Rybarek, I.M.; Chladek, G. Characterization of the Mechanical Properties, Water Sorption, and Solubility of Antibacterial Copolymers of Quaternary Ammonium Urethane-Dimethacrylates and Triethylene Glycol Dimethacrylate. Materials 2022, 15, 5530. [Google Scholar] [CrossRef] [PubMed]
- Alhussein, A.; Alsahafi, R.; Wang, X.; Mitwalli, H.; Filemban, H.; Hack, G.D.; Oates, T.W.; Sun, J.; Weir, M.D.; Xu, H.H.K. Novel Dental Low-Shrinkage-Stress Composite with Antibacterial Dimethylaminododecyl Methacrylate Monomer. J. Funct. Biomater. 2023, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shan, T.; Ren, B.; Zhang, L.; Xu, H.H.K.; Wang, N.; Zhou, X.; Li, H.; Cheng, L. Dimethylaminododecyl Methacrylate-Incorporated Dental Materials Could Be the First Line of Defense against Helicobacter Pylori. Int. J. Mol. Sci. 2023, 24, 13644. [Google Scholar] [CrossRef] [PubMed]
- Saiprasert, P.; Tansakul, C.; Pikulngam, A.; Promphet, P.; Naorungroj, S.; Ratanasathien, S.; Aksornmuang, J.; Talungchit, S. Novel Hydrolytic Resistant Antibacterial Monomers for Dental Resin Adhesive. J. Dent. 2023, 135, 104597. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.M.; Garcia, I.M.; Nunes, J.; Visioli, F.; Leitune, V.C.B.; Melo, M.A.; Collares, F.M. Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus Mutans Grown over Dental Resins: An in Vitro Study. J. Funct. Biomater. 2020, 11, 9. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Rücker, V.B.; Nunes, J.; Visioli, F.; Collares, F.M. Physicochemical and Biological Evaluation of a Triazine-Methacrylate Monomer into a Dental Resin. J. Dent. 2021, 114, 103818. [Google Scholar] [CrossRef]
- Stenhagen, I.S.R.; Rukke, H.V.; Dragland, I.S.; Kopperud, H.M. Effect of Methacrylated Chitosan Incorporated in Experimental Composite and Adhesive on Mechanical Properties and Biofilm Formation. Eur. J. Oral Sci. 2019, 127, 81–88. [Google Scholar] [CrossRef]
- Burujeny, S.B.; Yeganeh, H.; Atai, M.; Gholami, H.; Sorayya, M. Bactericidal Dental Nanocomposites Containing 1,2,3-Triazolium-Functionalized POSS Additive Prepared through Thiol-Ene Click Polymerization. Dent. Mater. 2017, 33, 119–131. [Google Scholar] [CrossRef]
- He, J.; Stenhagen, I.S.R.; Dragland, I.S.; Kopperud, H.M. Preparation of a Fluorinated Dental Resin System and Its Anti-Adhesive Properties against S. Mutans. Dent. Mater. 2023, 39, 402–409. [Google Scholar] [CrossRef]
- Zajdowicz, S.; Song, H.B.; Baranek, A.; Bowman, C.N. Evaluation of biofilm formation on novel copper-catalyzed azide-alkyne cycloaddition (CuAAC)-based resins for dental restoratives. Dent. Mater. 2018, 34, 657–666. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Wang, L.; Xu, H.H.K. Novel Rechargeable Calcium Phosphate Nanocomposite with Antibacterial Activity to Suppress Biofilm Acids and Dental Caries. J. Dent. 2018, 72, 44–52. [Google Scholar] [CrossRef]
- Bhadila, G.; Wang, X.; Zhou, W.; Menon, D.; Melo, M.A.S.; Montaner, S.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Novel Low-Shrinkage-Stress Nanocomposite with Remineralization and Antibacterial Abilities to Protect Marginal Enamel under Biofilm. J. Dent. 2020, 99, 103406. [Google Scholar] [CrossRef]
- Mohamed, N.I.; Safy, R.K.; Elezz, A.F.A. Microtensile Bond Strength, Marginal Leakage, and Antibacterial Effect of Bulk Fill Resin Composite with Alkaline Fillers versus Incremental Nanohybrid Composite Resin. Eur. J. Dent. 2021, 15, 425–432. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with Enhanced Physico-Mechanical and Biological Properties for Dental Applications. J. Compos. Sci. 2020, 4, 81. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Guo, Y.; Zhang, H.; Qiu, Y.; Li, J.; Ma, D.; Li, Z.; Zhen, P.; Liu, B.; et al. Novel Core–Shell CHX/ACP Nanoparticles Effectively Improve the Mechanical, Antibacterial and Remineralized Properties of the Dental Resin Composite. Dent. Mater. 2021, 37, 636–647. [Google Scholar] [CrossRef]
- Tian, J.; Wu, Z.; Wang, Y.; Han, C.; Zhou, Z.; Guo, D.; Lin, Y.; Ye, Z.; Fu, J. Multifunctional Dental Resin Composite with Antibacterial and Remineralization Properties Containing NMgO-BAG. J. Mech. Behav. Biomed. Mater. 2023, 141, 105783. [Google Scholar] [CrossRef]
- Ai, M.; Du, Z.; Zhu, S.; Geng, H.; Zhang, X.; Cai, Q.; Yang, X. Composite Resin Reinforced with Silver Nanoparticles–Laden Hydroxyapatite Nanowires for Dental Application. Dent. Mater. 2017, 33, 12–22. [Google Scholar] [CrossRef]
- Tanaka, C.B.; Lopes, D.P.; Kikuchi, L.N.T.; Moreira, M.S.; Catalani, L.H.; Braga, R.R.; Kruzic, J.J.; Gonçalves, F. Development of Novel Dental Restorative Composites with Dibasic Calcium Phosphate Loaded Chitosan Fillers. Dent. Mater. 2020, 36, 551–559. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Bauab, T.M.; Hernandes, A.C.; de Souza Rastelli, A.N. Titanium Dioxide and Modified Titanium Dioxide by Silver Nanoparticles as an Anti Biofilm Filler Content for Composite Resins. Dent. Mater. 2019, 35, e36–e46. [Google Scholar] [CrossRef]
- Chambers, C.; Stewart, S.B.; Su, B.; Jenkinson, H.F.; Sandy, J.R.; Ireland, A.J. Silver Doped Titanium Dioxide Nanoparticles as Antimicrobial Additives to Dental Polymers. Dent. Mater. 2017, 33, e115–e123. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Lefkelidou, A.; Papadopoulou, L.; Pavlidou, E.; Paraskevopoulos, K.M.; Christopher Fenno, J.; Flannagan, S.; González-Cabezas, C.; Kotsanos, N.; Papagerakis, P. Bactericidal and Bioactive Dental Composites. Front. Physiol. 2018, 9, 103. [Google Scholar] [CrossRef]
- Arif, W.; Rana, N.F.; Saleem, I.; Tanweer, T.; Khan, M.J.; Alshareef, S.A.; Sheikh, H.M.; Alaryani, F.S.; AL-Kattan, M.O.; Alatawi, H.A.; et al. Antibacterial Activity of Dental Composite with Ciprofloxacin Loaded Silver Nanoparticles. Molecules 2022, 27, 7182. [Google Scholar] [CrossRef]
- Srivastava, R.; Sun, Y. Silver Sulfadiazine Immobilized Glass as Antimicrobial Fillers for Dental Restorative Materials. Mater. Sci. Eng. C 2017, 75, 524–534. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Ramos, M.A.D.S.; Trevisan, T.C.; Bauab, T.M.; Hernandes, A.C.; de Souza Rastelli, A.N. Zinc Oxide 3D Microstructures as an Antimicrobial Filler Content for Composite Resins. Microsc. Res. Tech. 2017, 80, 634–643. [Google Scholar] [CrossRef]
- Wang, Y.; Hua, H.; Li, W.; Wang, R.; Jiang, X.; Zhu, M. Strong Antibacterial Dental Resin Composites Containing Cellulose Nanocrystal/Zinc Oxide Nanohybrids. J. Dent. 2019, 80, 23–29. [Google Scholar] [CrossRef]
- Chen, H.; Wang, R.; Zhang, J.; Hua, H.; Zhu, M. Synthesis of Core-Shell Structured ZnO@m-SiO2 with Excellent Reinforcing Effect and Antimicrobial Activity for Dental Resin Composites. Dent. Mater. 2018, 34, 1846–1855. [Google Scholar] [CrossRef]
- Qin, L.; Yao, S.; Meng, W.; Zhang, J.; Shi, R.; Zhou, C.; Wu, J. Novel Antibacterial Dental Resin Containing Silanized Hydroxyapatite Nanofibers with Remineralization Capability. Dent. Mater. 2022, 38, 1989–2002. [Google Scholar] [CrossRef]
- Yang, S.Y.; Han, A.R.; Choi, J.W.; Kim, K.M.; Kwon, J.S. Novel Antibacterial and Apatite Forming Restorative Composite Resin Incorporated with Hydrated Calcium Silicate. Biomater. Res. 2023, 27, 1–15. [Google Scholar] [CrossRef]
- Go, H.B.; Lee, M.J.; Seo, J.Y.; Byun, S.Y.; Kwon, J.S. Mechanical Properties and Sustainable Bacterial Resistance Effect of Strontium-Modified Phosphate-Based Glass Microfiller in Dental Composite Resins. Sci. Rep. 2023, 13, 17763. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhang, T.; Yao, S.; Wang, Z.; Zhou, C.; Wu, J. Novel Low-Shrinkage Dental Resin Containing Microcapsules with Antibacterial and Self-Healing Properties. J. Mech. Behav. Biomed. Mater. 2023, 148, 106212. [Google Scholar] [CrossRef]
- Adeeb, S.; Adeeb, S.; Chladek, G.; Pakieła, W.; Mertas, A. Influence of Silver-Containing Filler on Antibacterial Properties of Experimentaresin Composites against Enterococcus Faecalis. J. Achiev. Mater. Manuf. Eng. 2021, 109, 59–67. [Google Scholar] [CrossRef]
- Lapinska, B.; Szram, A.; Zarzycka, B.; Grzegorczyk, J.; Hardan, L.; Sokolowski, J.; Lukomska-Szymanska, M. An in Vitro Study on the Antimicrobial Properties of Essential Oil Modified Resin Composite against Oral Pathogens. Materials 2020, 13, 4383. [Google Scholar] [CrossRef]
- Kim, K.H.; Mai, H.N.; Hyun, D.C.; Lee, D.H. New Autonomous Water-Enabled Self-Healing Coating Material with Antibacterial-Agent-Releasing Properties. Pharmaceutics 2022, 14, 1005. [Google Scholar] [CrossRef]
- Mai, H.N.; Kim, D.Y.; Hyun, D.C.; Park, J.H.; Lee, S.M.; Lee, D.H. A New Antibacterial Agent-Releasing Polydimethylsiloxane Coating for Polymethyl Methacrylate Dental Restorations. J. Clin. Med. 2019, 8, 1831. [Google Scholar] [CrossRef]
- ul Haque, S.; Nasar, A.; Inamuddin. 27—Montmorillonite Clay Nanocomposites for Drug Delivery. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing Series in Biomaterials; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 633–648. ISBN 978-0-12-813741-3. [Google Scholar]
- Altaie, A.; Bubb, N.; Franklin, P.; German, M.J.; Marie, A.; Wood, D.J. Development and Characterisation of Dental Composites Containing Anisotropic Fluorapatite Bundles and Rods. Dent. Mater. 2020, 36, 1071–1085. [Google Scholar] [CrossRef]
- Zalega, M.; Nowak, J.; Bociong, K. The Influence of Quaternary Ammonium Salts on Mechanical Properties of Light-Cured Resin Dental Composites. Polimery 2023, 68, 195–205. [Google Scholar] [CrossRef]
- Fujimura, Y.; Weerasinghe, D.; Kawashima, M. Development of an Antibacterial Bioactive Dental Adhesive: Simplicity and Innovation. Am. J. Dent. 2018, 31, 13B–16B. [Google Scholar] [PubMed]
- Xue, J.; Wang, J.; Feng, D.; Huang, H.; Wang, M. Application of Antimicrobial Polymers in the Development of Dental Resin Composite. Molecules 2020, 25, 4738. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.R.; Frukhtbeyn, S.A.; Giuseppetti, A.A.; Okeke, U.C.; Pires, R.M.; Antonucci, J.M.; Skrtic, D. Ionic Dimethacrylates for Antimicrobial and Remineralizing Dental Composites. Ann. Dent. Oral Disord. 2018, 2, 108. [Google Scholar] [PubMed]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Published since 2017 Publication in English Containing the following keywords “dental AND materials AND antibacterial modifier” OR “dental AND materials AND antibacterial agent” OR “antibacterial polymer” OR “antibacterial dental resin” OR “resin composites AND antibacterial modification AND antibacterial agent OR “antimicrobial monomers AND dental resin composite” OR “dental AND composite AND antibacterial” OR “dental AND composite AND antibacterial AND properties”. | Published before 2017 Publication not in English Publications without keywords |
| Modification |
Microorganisms Tested | Results | References |
|---|---|---|---|
| Triclosan-encapsulated halloysite nanotubes | Streptococcus mutans | No significant antimicrobial effect was observed between the groups for CFUs and similarly for dry mass | [18] |
| Deep eutectic solvent (DES) with benzalkonium chloride (BC) and acrylic acid (AA) | Streptococcus mutans, Staphylococcus aureus | BC-C produced a larger inhibitory halo | [19] |
| MMT loaded with chlorhexidine (CHX) | Staphylococcus aureus, Streptococcus mutans, Porphyromonas gingivalis | Among the three tested bacteria, modified composites exhibited growth inhibition at all concentrations with one exception (2.5%)—no growth inhibition of Porphyromonas gingivalis was observed | [20] |
| Core–shell AgBr/cationic polymer nanocomposite (AgBr/BHPVP) | Streptococcus mutans | Strong bactericidal effect | [21] |
| Thyme oil/TiO2 nanoparticle filler | Streptococcus mutans, Lactobacillus acidophilus, Candida albicans | The resin containing 2 wt% thyme/TiO2 exhibited the most significant inhibition zone against Streptococcus mutans. Similar trends were observed against Lactobacillus acidophilus and Candida albicans | [22] |
| Tertiary quaternary ammonium acrylamides (AMs) and methacrylamides (MAMs) with alkyl side chain lengths of 9 and 14 carbons (C9 and C14) | Streptococcus mutans | All the C14 versions demonstrated potent antibacterial properties | [23] |
| Nine monomers based on bis-quaternary ammonium salts were prepared | Streptococcus mutans, Escherichia coli, Staphylococcus aureus, Streptococcus sanguinis, Streptococcus mitis | All bis-QAMs were capable of inhibiting Streptococcus mutans biofilm formation | [24] |
| Quaternary ammonium dimethyl-hexadecyl-methacryloxyethyl-ammonium iodide (DHMAI), methacryloyloxyethylphosphorylcholine (MPC) | Streptococcus mutans | Reduction in biofilm | [25] |
| Urethane dimethacrylate quaternary ammonium compound (UDMQA-12) | Streptococcus mutans | Significant antibacterial activity | [26] |
| bi-quaternary ammonium methacrylates (biQAMA-12, biQAMA-14, and biQAMA-16) | Streptococcus mutans | BiQAMA-12 showed the greatest antimicrobial efficacy | [27] |
| Quaternary ammonium urethane-dimethacrylate derivative (QAUDMA-m (m—number of carbon atoms in the N-alkyl substituent—8, 10, 12, 14, 16, 18)) | Staphylococcus aureus, Escherichia coli | Three copolymers, BG:QA8:TEG, BG:QA10:TEG, and BG:QA12:TEG, demonstrated high antibacterial activity against both bacterial strains | [28,29] |
| Dimethylaminododecyl methacrylate (DMADDM) | Streptococcus mutans | The incorporation of 3% and 5% DMADDM resulted in a significant decrease in Streptococcus mutans biofilm colony-forming units (CFUs). Modification with DMADDM led to notable reductions in biofilm biomass and lactic acid levels | [30] |
| Dimethylaminododecyl methacrylate (DMADDM) | Helicobacter pylori | DMADDM reduced H. pylori colonization of oral origin in the stomach, thereby alleviating both local and systemic gastritis | [31] |
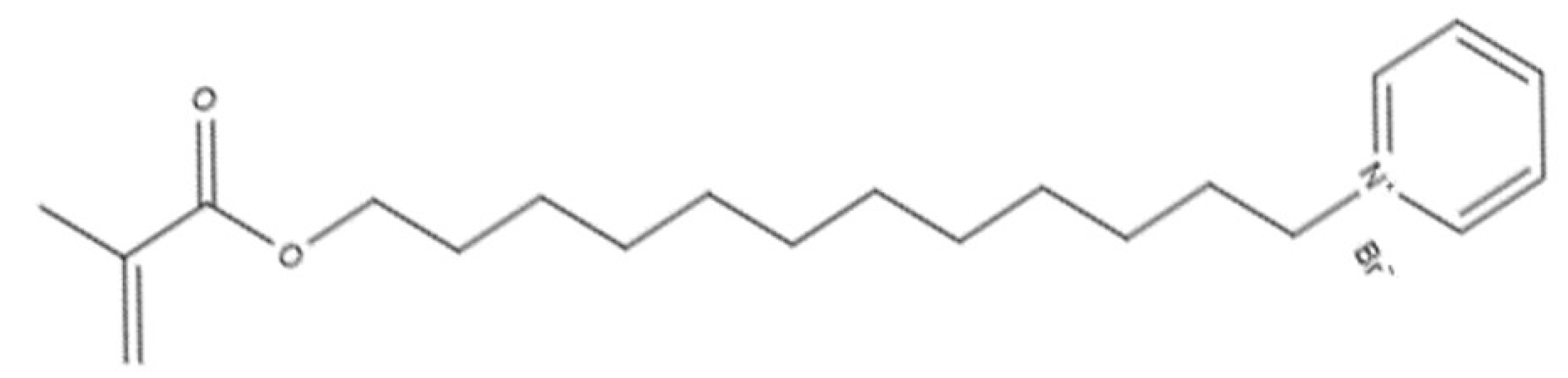
| Methacryloyloxydodecylpyridinium bromide (MDPB) | Enterococcus faecalis | Significant antibacterial activity | [32] |
| Myristyltrimethylammonium Bromide (MYTAB) | Streptococcus mutans | Antimicrobial activity against biofilm formation was obtained with 0.5 wt%, and activity against planktonic bacteria was obtained with 1 wt% | [33] |
| 2.5 or 5 wt % of the methacrylate monomer 1,3,5-triacryloylhexahydro-1,3,5-triazine (TAT) | Streptococcus mutans | The higher the TAT concentration, the higher the antibacterial activity | [34] |
| Methacrylated chitosan (CH-MA) | Streptococcus mutans | Reduction in biofilm formation | [35] |
| 1,2,3-triazolium-functionalized POSS | Streptococcus mutans | The nanocomposite incorporating Triazolium/POSS exhibited markedly elevated bactericidal activity | [36] |
| Fluorinated dimethacrylate FDMA was mixed with triethylene-glycol dimethacrylate (TEGDMA) and fluorinated diluent 1 H,1 H-heptafluorobutyl methacrylate (FBMA) | Streptococcus mutans | The use of fluorinated methacrylate monomers reduced the adhesion of Streptococcus mutans | [37] |
| Copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) | Streptococcus mutans | Reduction in luciferase activity and the number of viable bacteria recovered from biofilms on CuAAC-based resins | [38] |
| Nanoparticles of amorphous calcium phosphate (NACP) with dimethylaminohexadecyl methacrylate (DMAHDM) | Streptococcus mutans | Reduction in biofilm growth | [39] |
| Triethylene glycol divinylbenzyl ether (TEG-DVBE), and 3% dimethylaminohexadecyl methacrylate (DMAHDM), and 20% calcium phosphate nanoparticles (NACP) | Streptococcus mutans | Exhibited remineralizing and antibacterial properties | [40] |
| Alkaline fillers (alkasite—alkaline fillers such as barium aluminum silicate glass and ytterbium trifluoride) | Streptococcus mutans | The antibacterial effect of alkasite was lesser than that of the control group | [41] |
| Development of Chlorhexidine-loaded halloysite nanotube | Streptococcus mutans | Significant antibacterial activity | [42] |
| Core–shell chlorhexidine/amorphous calcium phosphate (CHX/ACP) nanoparticles | Streptococcus mutans | 5 wt% and more of CHX/ACP nanoparticles effectively inhibited the growth of Streptococcus mutans | [43] |
| Magnesium oxide nanoparticles (nMgO) | Streptococcus mutans | Very good antibacterial properties | [44] |
| Silver nanoparticle (AgNP)-laden HA (HA–PDA–Ag) nanowires | Streptococcus mutans | High antibacterial activity | [45] |
| Chitosan or chitosan loaded with dibasic calcium phosphate anhydrous (DCPA) particles | Streptococcus mutans | The modified composites contained about 20% less biofilm | [46] |
| TiO2 and TiO2/Ag nanoparticles | Streptococcus mutans | Modification with 2% TiO2/Ag nanoparticles significantly reduced biofilm accumulation | [47] |
| Ag–TiO2 filler particles | Streptococcus mutans | Bactericidal effect (small quantities) | [48] |
| Ag–doped sol–gel–derived bioactive glass (Ag–BG) | Streptococcus mutans | As the concentration of Ag–BG in BGCOMP increases, the number of dead bacteria in the biofilm increases | [49] |
| Ciprofloxacin–loaded silver nanoparticles (CIP–AgNPs) | Streptococcus mutans, Enterococcus faecalis, Saliva microorganism | Antimicrobial activity after CIP–AgNP modification was increased | [50] |
| Commercial barium borosilicate-based glass powders immobilized with silver sulfadiazine filler | Streptococcus mutans | Strong and effective action | [51] |
| Zinc oxide 3D microstructures filler | Streptococcus mutans, Escherichia coli, Staphylococcus aureus, Candida albicans | Low quantities of ZnO microparticles significantly inhibited the growth of Streptococcus mutans on the resin surface | [52] |
| Cellulose nanocrystal/zinc oxide nanohybrids | Streptococcus mutans | A 78% reduction in bacterial counts was achieved with the addition of 2% CNC/ZnO nanohybrids | [53] |
| Core–shell-structured ZnO@m-SiO2 | Streptococcus mutans | Superior antimicrobial activity (>99.9%) | [54] |
| Hydroxyapatite nanofibers loaded with erythromycin (s-HAFs@EM) | Streptococcus mutans | Modification with 15–20% s-HAFs@EM showed a high antibacterial rate (>85%) | [55] |
| Hydrated calcium silicate (hCS) | Streptococcus mutans | The adhesion of Streptococcus mutans decreased as the amount of hCS increased. It was found that the relative survival rates of Streptococcus mutans decreased after the addition of hCS | [56] |
| Strontium-modified phosphate-based glass (Sr–PBG) | Streptococcus mutans | A sustained in vitro bacterial resistance effect was achieved | [57] |
| Nanoparticle–modified MC with a nano–antibacterial inorganic filler (NIF) containing a quaternary ammonium salt | Streptococcus mutans | High antimicrobial activity and a corresponding self-healing efficiency of 71% were found | [58] |
| Silver sodium hydrogen zirconium phosphate (S–P) | Enterococcus faecalis | Bacterial colonies were found to be reduced when S–P concentrations ranging from 7% to 13% were used | [59] |
| Essential oils (EOs)—anise, cinnamon, citronella, clove, geranium, lavender, limette, mint, rosemary thyme | Streptococcus mutans, Lactobacillus acidophilus,
Candida albicans | Cinnamon and thyme oils showed the highest antibacterial activity against Streptococcus mutans and Lactobacillus acidophilus. The composite with 2 µL of cinnamon oil showed the best antimicrobial properties against Streptococcus mutans and Candida albicans and that with 1 µL the best antimicrobial properties against Lactobacillus acidophilus | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalega, M.; Bociong, K. Antibacterial Agents Used in Modifications of Dental Resin Composites: A Systematic Review. Appl. Sci. 2024, 14, 3710. https://doi.org/10.3390/app14093710
Zalega M, Bociong K. Antibacterial Agents Used in Modifications of Dental Resin Composites: A Systematic Review. Applied Sciences. 2024; 14(9):3710. https://doi.org/10.3390/app14093710
Chicago/Turabian StyleZalega, Maja, and Kinga Bociong. 2024. "Antibacterial Agents Used in Modifications of Dental Resin Composites: A Systematic Review" Applied Sciences 14, no. 9: 3710. https://doi.org/10.3390/app14093710






