Indole-3-Acetic Acid Action in Outdoor and Indoor Cultures of Spirulina in Open Raceway Reactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga and Phytohormone
2.2. Culture Conditions
2.3. Evaluation of Cell Growth
2.4. Biomass Characterization
2.5. Cost Analysis
2.6. Statistical Analysis
3. Results
3.1. Biomass Growth and Productivity Evaluation
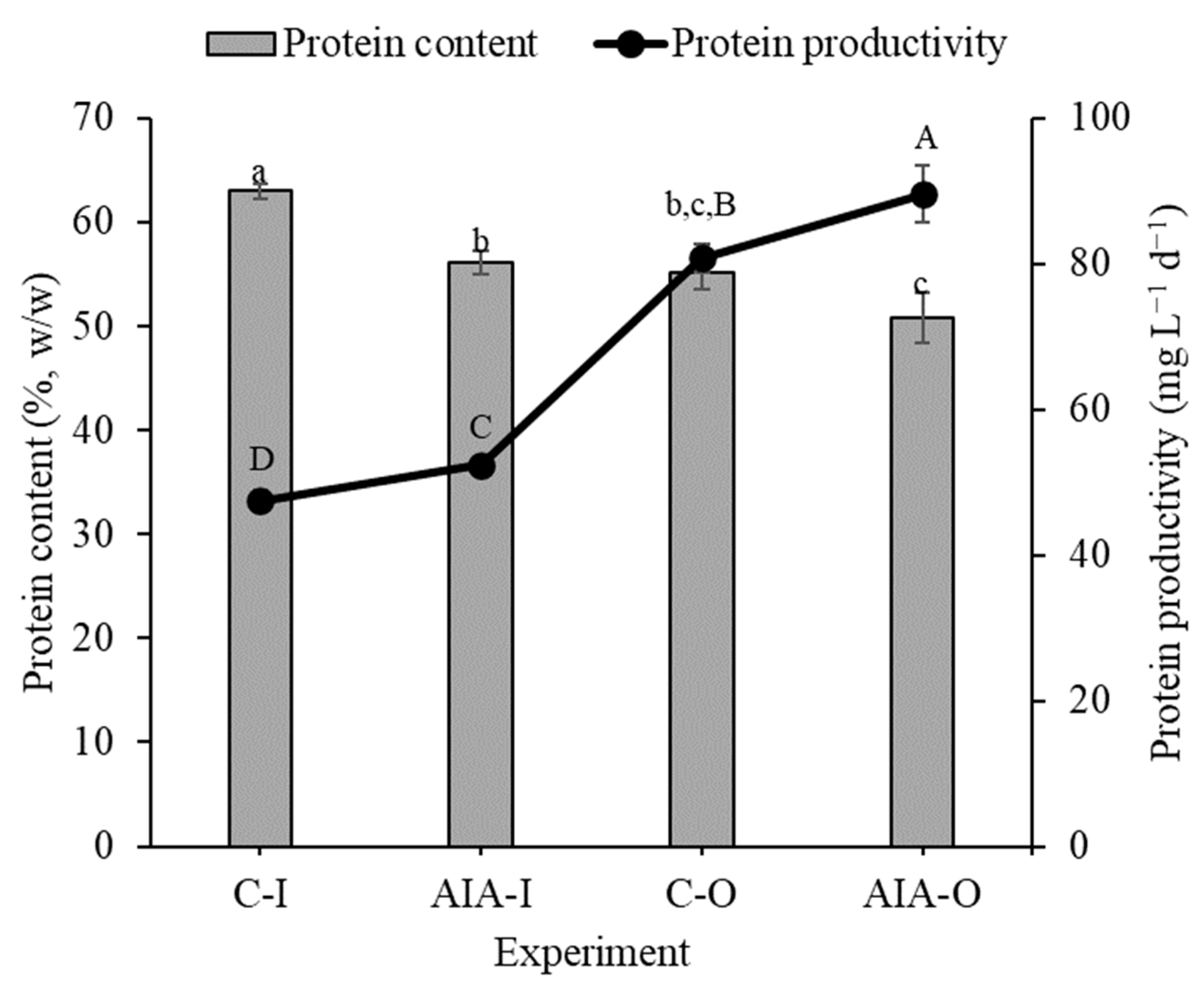
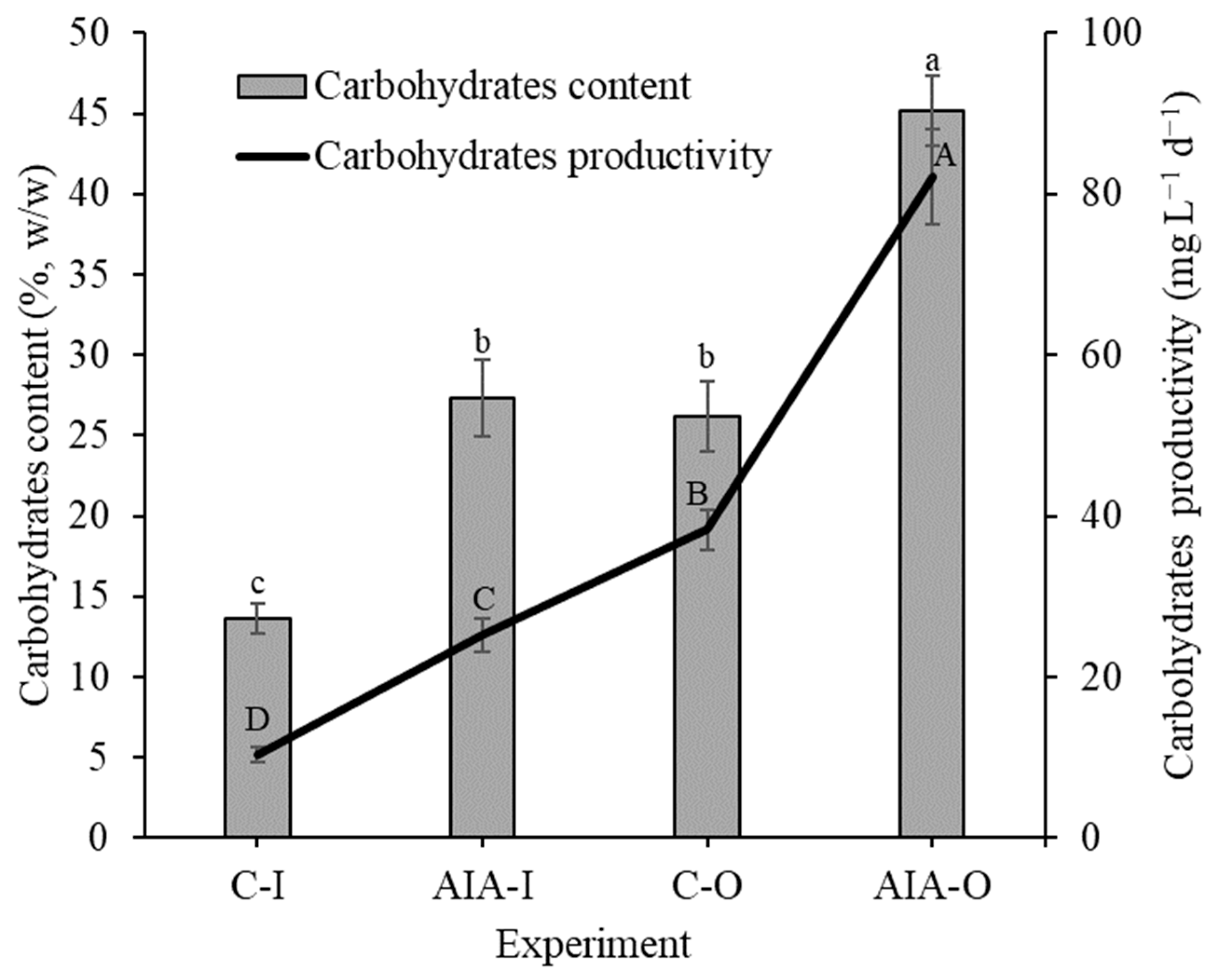
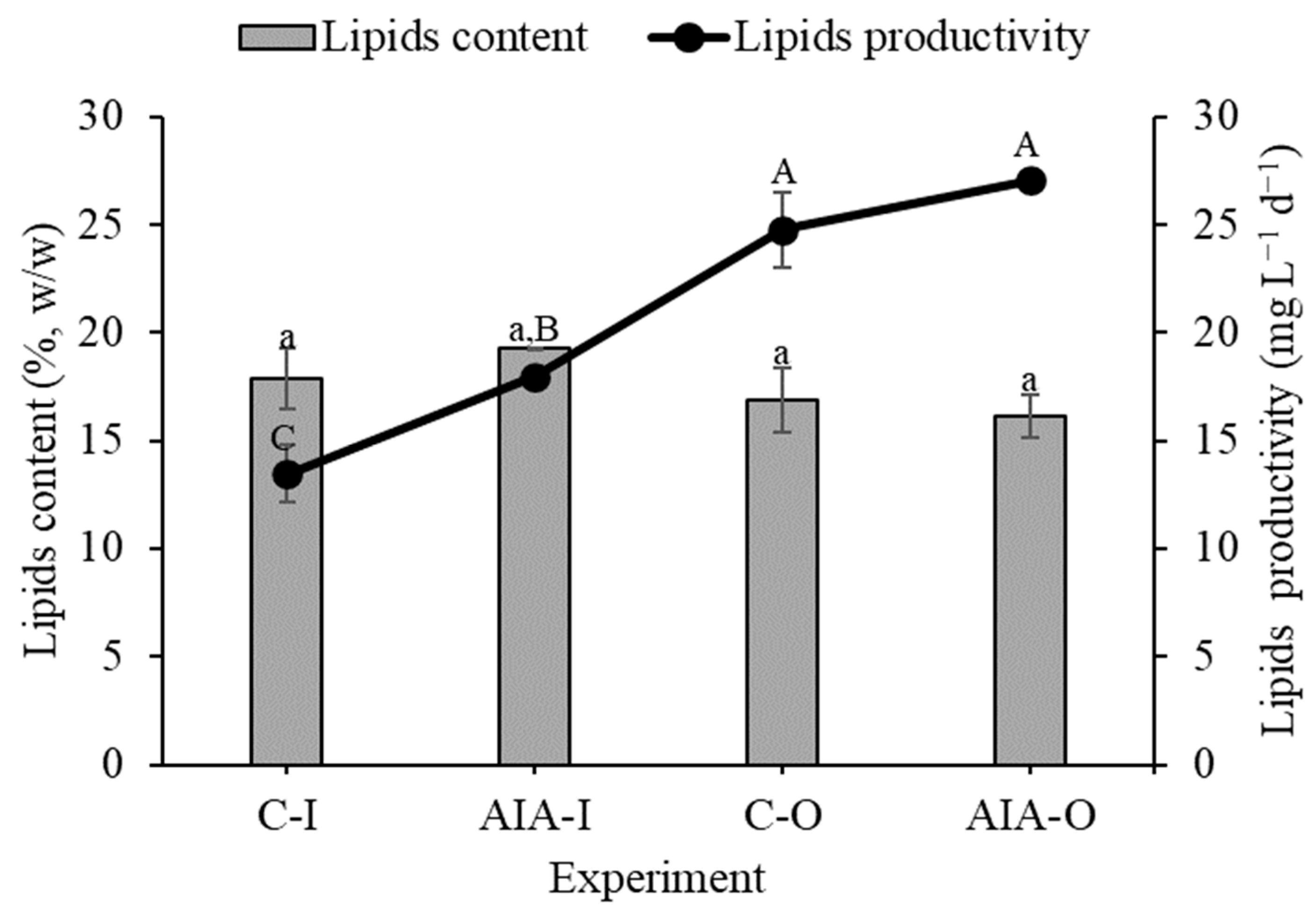
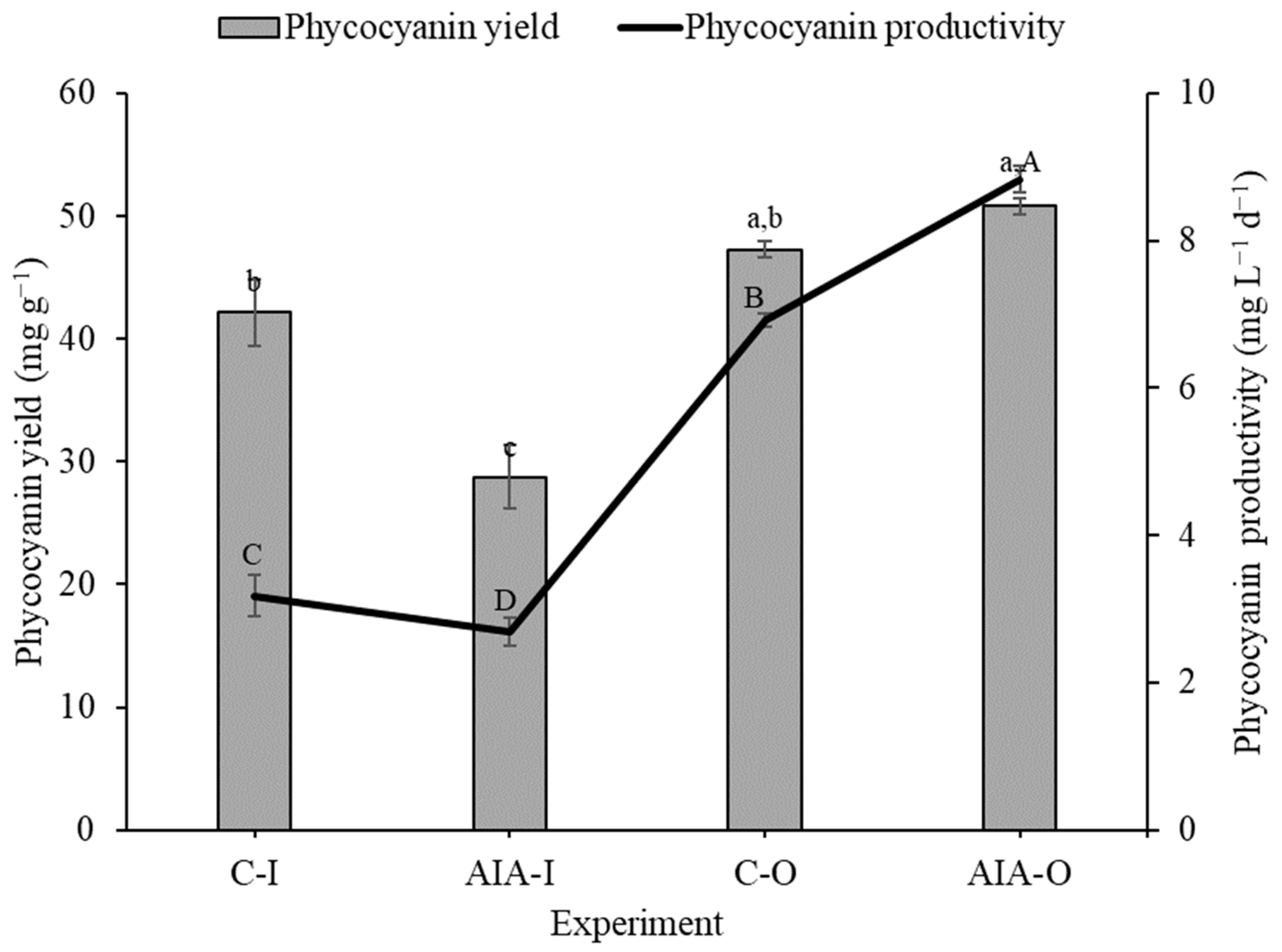
3.2. Biomass Characterization
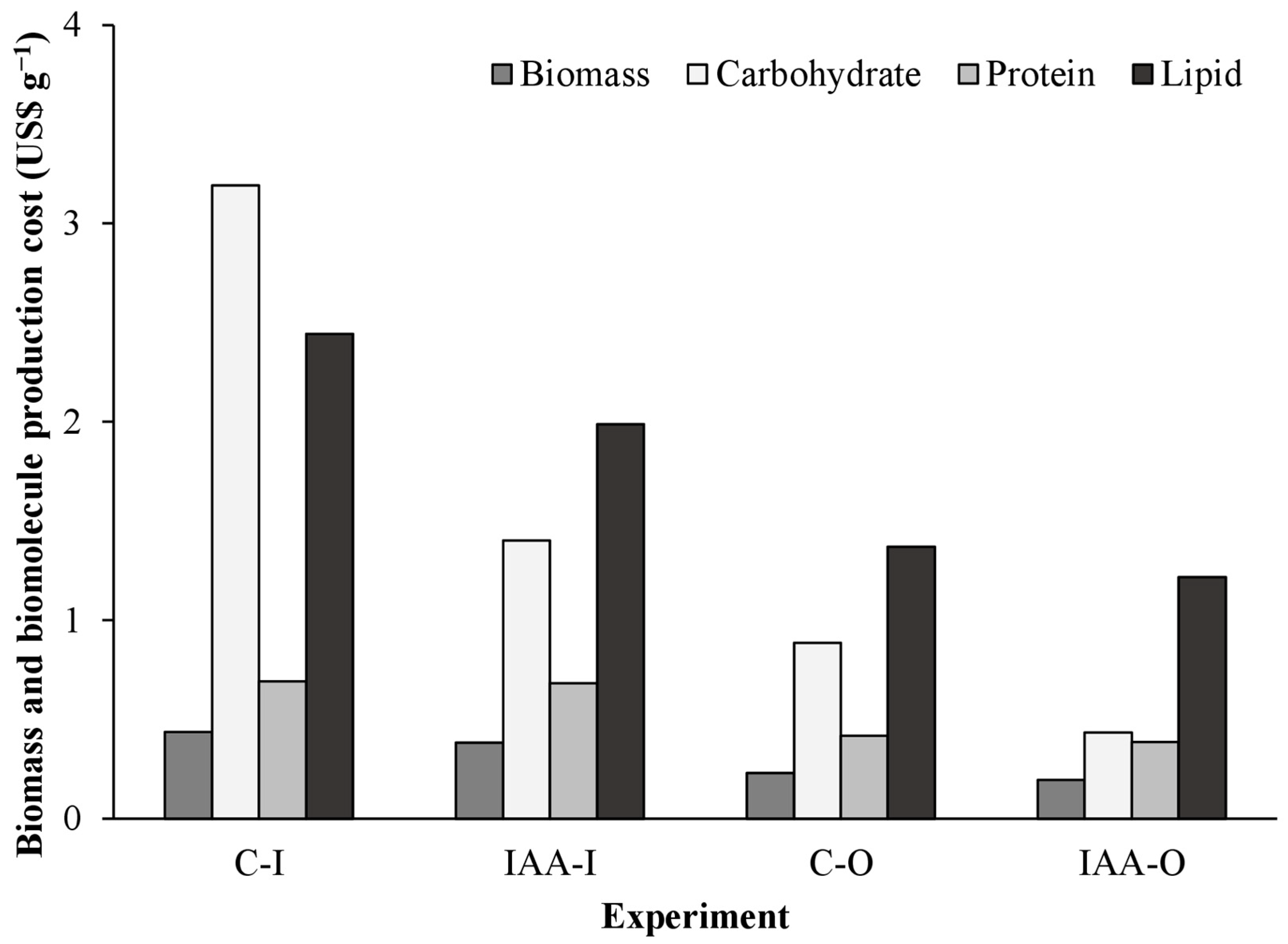
3.3. Biomass and Biomolecule Production Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esteves, A.F.; Pires, J.C.M.; Gonçalves, A.L. Current Utilization of Microalgae in the Food Industry beyond Direct Human Consumption. In Cultured Microalgae for the Food Industry; Lafarga, T., Acién, G., Eds.; Elsevier: London, UK, 2021; pp. 199–248. [Google Scholar]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The Role of Microalgae in the Bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae Research Worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up Microalgal Cultures to Commercial Scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Belay, A. Biology and Industrial Production of Arthrospira (Spirulina). In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Blackwell Science: Oxford, UK, 2013; pp. 339–358. [Google Scholar]
- Morais, M.G.; Radmann, E.M.; Andrade, M.R.; Teixeira, G.G.; Brusch, L.R.F.; Costa, J.A.V. Pilot Scale Semicontinuous Production of Spirulina Biomass in Southern Brazil. Aquaculture 2009, 294, 60–64. [Google Scholar] [CrossRef]
- Jiang, L.; Yu, S.; Chen, H.; Pei, H. Enhanced Phycocyanin Production from Spirulina Subsalsa via Freshwater and Marine Cultivation with Optimized Light Source and Temperature. Bioresour. Technol. 2023, 378, 129009. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.G.; Rosa, A.P.C.; Costa, J.A.V. Identification of the Phytohormones Indole-3-acetic Acid and trans-Zeatin in Microalgae. J. Chem. Technol. Biotechnol. 2023, 98, 1048–1056. [Google Scholar] [CrossRef]
- Ahmad, A.M.R.; Intikhab, A.; Zafar, S.; Farooq, U.; Shah, H.B.U.; Akram, S.; Abid, J.; Parveen, Z.; Iqbal, S. Spirulina, an FDA-Approved Functional Food: Worth the Hype? Cell Mol. Biol. 2023, 69, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Shao, W.; Ebaid, R.; El-Sheekh, M.; Abomohra, A.; Eladel, H. Pharmaceutical Applications and Consequent Environmental Impacts of Spirulina (Arthrospira): An Overview. Grasas Aceites 2019, 70, 292. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cheah, W.Y.; Ng, I.-S.; Chang, J.-S.; Zhao, M.; Show, P.L. Microalgae-Based Biotechnological Sequestration of Carbon Dioxide for Net Zero Emissions. Trends Biotechnol. 2022, 40, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.T.; Rosa, A.P.C.; Costa, J.A.V. Modulating Phytohormone Supplementation Can Efficiently Increase Biomass and Lipid Production in Spirulina (Arthrospira). Bioenergy Res. 2022, 15, 112–120. [Google Scholar] [CrossRef]
- Silveira, J.T.; Rosa, A.P.C.; Morais, M.G.; Costa, J.A.V. Cost Reduction in the Production of Spirulina Biomass and Biomolecules from Indole-3-Acetic Acid Supplementation in Different Growth Phases. Appl. Biochem. Biotechnol. 2023, 195, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Cooke, T.J.; Poli, D.; Sztein, A.E.; Cohen, J.D. Evolutionary Patterns in Auxin Action. Plant Mol. Biol. 2002, 49, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-M.; Xiong, J.-Q. Boosting the Yields of Microalgal Biomass and High-Value Added Products by Phytohormones: A Mechanistic Insight Using Transcriptomics. J. Clean. Prod. 2023, 393, 136175. [Google Scholar] [CrossRef]
- Chernyad’ev, I.I. The Protective Action of Cytokinins on the Photosynthetic Machinery and Productivity of Plants under Stress (Review). Appl. Biochem. Microbiol. 2009, 45, 351–362. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in Microalgae: A New Opportunity for Microalgal Biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Nitrogen Starvation-Induced Cellular Crosstalk of ROS-Scavenging Antioxidants and Phytohormone Enhanced the Biofuel Potential of Green Microalga Acutodesmus dimorphus. Biotechnol. Biofuels 2017, 10, 60. [Google Scholar] [CrossRef]
- Kozlova, T.A.; Hardy, B.P.; Krishna, P.; Levin, D.B. Effect of Phytohormones on Growth and Accumulation of Pigments and Fatty Acids in the Microalgae Scenedesmus quadricauda. Algal Res. 2017, 27, 325–334. [Google Scholar] [CrossRef]
- Chen, J.; Wei, D.; Lim, P.-E. Enhanced Coproduction of Astaxanthin and Lipids by the Green Microalga Chromochloris zofingiensis: Selected Phytohormones as Positive Stimulators. Bioresour. Technol. 2020, 295, 122242. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.K.; Salim, S.A.M.; Prabha, S.; George, B. Exogenous Carbon Source and Phytohormone Supplementation Enhanced Growth Rate and Metabolite Production in Freshwater Microalgae Scenedesmus Obtusus Meyen. Bioresour. Technol. Rep. 2021, 14, 100669. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Potential of Phytohormones as a Strategy to Improve Microalgae Productivity for Biotechnological Applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef] [PubMed]
- Slade, R.; Bauen, A. Micro-Algae Cultivation for Biofuels: Cost, Energy Balance, Environmental Impacts and Future Prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Morais, M.G.; da Reichert, C.; Dalcanton, F.; Durante, A.J.; Marins, L.F.; Costa, J.A.V. Isolation and Characterization of a New Arthrospira Strain. Z. Naturforschung C 2008, 63, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Colla, M.; Duarte Filho, P.; Kabke, K.; Weber, A. Modelling of Spirulina platensis Growth in Fresh Water Using Response Surface Methodology. World J. Microbiol. Biotechnol. 2002, 18, 603–607. [Google Scholar] [CrossRef]
- Zarrouk, C. Contributionà l Étuded Une Cyanophycée: Influence de Divers Facteurs Physiques et Chimiques Sur La Croissance et Photosynthese de Spirulina Maxima Geitler. Ph.D. Thesis, Faculté des Sciences de L’université de Paris, Paris, France, 1966. [Google Scholar]
- Andrade, M.R.; Costa, J.A.V. Outdoor and Indoor Cultivation of Spirulina platensis in the Extreme South of Brazil. Z. Naturforschung C 2008, 63, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.B.; Weinstein, D.B. Simple Charring Method for Determination of Lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Prates, D.F.; Radmann, E.M.; Duarte, J.H.; Morais, M.G.; Costa, J.A.V. Spirulina Cultivated under Different Light Emitting Diodes: Enhanced Cell Growth and Phycocyanin Production. Bioresour. Technol. 2018, 256, 38–43. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and Characterization of Phycocyanin from the Marine Cyanobacterium Synechococcus sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Park, W.-K.; Yoo, G.; Moon, M.; Kim, C.W.; Choi, Y.-E.; Yang, J.-W. Phytohormone Supplementation Significantly Increases Growth of Chlamydomonas reinhardtii Cultivated for Biodiesel Production. Appl. Biochem. Biotechnol. 2013, 171, 1128–1142. [Google Scholar] [CrossRef]
- Babu, A.G.; Wu, X.; Kabra, A.N.; Kim, D.-P. Cultivation of an Indigenous Chlorella sorokiniana with Phytohormones for Biomass and Lipid Production under N-Limitation. Algal Res. 2017, 23, 178–185. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone Addition Coupled with Nitrogen Depletion Almost Tripled the Lipid Productivities in Two Algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pei, H.; Li, Y.; Yang, Z.; Xie, Z.; Hou, Q.; Nie, C. Inclined Algal Biofilm Photobioreactor (IABPBR) for Cost-Effective Cultivation of Lipid-Rich Microalgae and Treatment of Seawater-Diluted Anaerobically Digested Effluent from Kitchen Waste with the Aid of Phytohormones. Bioresour. Technol. 2020, 315, 123761. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Han, B.; Yu, X. Coupling of Abiotic Stresses and Phytohormones for the Production of Lipids and High-Value by-Products by Microalgae: A Review. Bioresour. Technol. 2019, 274, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Han, B.; Zhao, Y.; Li, T.; Zhao, P.; Yu, X. Improvement in Lipid Production in Monoraphidium sp. QLY-1 by Combining Fulvic Acid Treatment and Salinity Stress. Bioresour. Technol. 2019, 294, 122179. [Google Scholar] [CrossRef]
- Chang, W.; Li, Y.; Qu, Y.; Liu, Y.; Zhang, G.; Zhao, Y.; Liu, S. Mixotrophic Cultivation of Microalgae to Enhance the Biomass and Lipid Production with Synergistic Effect of Red Light and Phytohormone IAA. Renew. Energy 2022, 187, 819–828. [Google Scholar] [CrossRef]
- Fierli, D.; Barone, M.E.; Donnell, A.M.; Conlon, T.; Touzet, N. Combined Application of Exogenous Phytohormones and Blue Light Illumination to the Marine Diatom Phaeodactylum tricornutum. Algal Res. 2023, 71, 103052. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of Culture Conditions on the Productivity and Lutein Content of the New Strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Pinto, R.F.; Sant’Anna, C. Low Light Intensity and Nitrogen Starvation Modulate the Chlorophyll Content of Scenedesmus dimorphus. J. Appl. Microbiol. 2016, 120, 661–670. [Google Scholar] [CrossRef]
- Ota, M.; Kato, Y.; Watanabe, H.; Watanabe, M.; Sato, Y.; Smith, R.L.; Inomata, H. Fatty Acid Production from a Highly CO2 Tolerant Alga, Chlorocuccum littorale, in the Presence of Inorganic Carbon and Nitrate. Bioresour. Technol. 2009, 100, 5237–5242. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, Y.; Goyal, A.; Tolbert, N.E. Alkalization of the Medium by Unicellular Green Algae during Uptake Dissolved Inorganic Carbon. Plant Cell Physiol. 1993, 34, 649–657. [Google Scholar] [CrossRef]
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Magnetic Field Action on Outdoor and Indoor Cultures of Spirulina: Evaluation of Growth, Medium Consumption and Protein Profile. Bioresour. Technol. 2018, 249, 168–174. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Perspectives on Microalgal CO2-Emission Mitigation Systems—A Review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Guldhe, A.; Singh, P.; Ansari, F.A.; Rawat, I.; Bux, F. Evaluating the Potential of Cytokinins for Biomass and Lipid Enhancement in Microalga Acutodesmus obliquus under Nitrogen Stress. Energy Convers. Manag. 2017, 140, 14–23. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Duarte, J.H.; Andrade, B.B.; Lemos, P.V.F.; Costa, J.A.V.; Druzian, J.I.; Chinalia, F.A. Spirulina sp. LEB 18 Cultivation in Outdoor Pilot Scale Using Aquaculture Wastewater: High Biomass, Carotenoid, Lipid and Carbohydrate Production. Aquaculture 2020, 525, 735272. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.L.; Monteiro, M.P.C.; Robbs, P.G.; Leite, S.G.F. Growth and Chemical Composition of Spirulina maxima and Spirulina platensis Biomass at Different Temperatures. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- Mata, S.N.; Santos, T.S.; Cardoso, L.G.; Andrade, B.B.; Duarte, J.H.; Costa, J.A.V.; Souza, C.O.; Druzian, J.I. Spirulina sp. LEB 18 Cultivation in a Raceway-Type Bioreactor Using Wastewater from Desalination Process: Production of Carbohydrate-Rich Biomass. Bioresour. Technol. 2020, 311, 123495. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.G.; Lombardi, A.T.; Silva, J.S.J.; Lemos, P.V.F.; Costa, J.A.V.; Souza, C.O.; Druzian, J.I.; Chinalia, F.A. Scaling-up Production of Spirulina sp. LEB 18 Grown in Aquaculture Wastewater. Aquaculture 2021, 544, 737045. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kabra, A.N.; Ji, M.-K.; Kim, J.R.; Min, B.; Jeon, B.-H. Enhancement of Microalgae Growth and Fatty Acid Content under the Influence of Phytohormones. Bioresour. Technol. 2014, 172, 97–103. [Google Scholar] [CrossRef]
- Zaparoli, M.; Ziemniczak, F.G.; Mantovani, L.; Costa, J.A.V.; Colla, L.M. Cellular Stress Conditions as a Strategy to Increase Carbohydrate Productivity in Spirulina platensis. Bioenergy Res. 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Chrismadha, T.; Satya, A.; Satya, I.A.; Rosidah, R.; Satya, A.D.M.; Pangestuti, R.; Harimawan, A.; Setiadi, T.; Chew, K.W.; Show, P.L. Outdoor Inclined Plastic Column Photobioreactor: Growth, and Biochemicals Response of Arthrospira Platensis Culture on Daily Solar Irradiance in a Tropical Place. Metabolites 2022, 12, 1199. [Google Scholar] [CrossRef]
- Ogbonda, K.H.; Aminigo, R.E.; Abu, G.O. Influence of Temperature and PH on Biomass Production and Protein Biosynthesis in a Putative Spirulina sp. Bioresour. Technol. 2007, 98, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.S.; Uebel, L.S.; Costa, S.S.; Miranda, A.L.; Morais, E.G.; Morais, M.G.; Costa, J.A.V.; Nunes, I.L.; Ferreira, E.S.; Druzian, J.I. Outdoor Pilot-Scale Cultivation of Spirulina sp. LEB-18 in Different Geographic Locations for Evaluating Its Growth and Chemical Composition. Bioresour. Technol. 2018, 256, 86–94. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques, 1st ed.; Academic Press: Burlington, NJ, USA, 2004. [Google Scholar]
- Pan, X.; Chang, F.; Kang, L.; Liu, Y.; Li, G.; Li, D. Effects of Gibberellin A3 on Growth and Microcystin Production in Microcystis aeruginosa (Cyanophyta). J. Plant Physiol. 2008, 165, 1691–1697. [Google Scholar] [CrossRef]
- Mansouri, H.; Talebizadeh, R. Effects of Indole-3-Butyric Acid on Growth, Pigments and UV-Screening Compounds in Nostoc linckia. Phycol. Res. 2017, 65, 212–216. [Google Scholar] [CrossRef]
- Mc Gee, D.; Archer, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. The Effect of Nutrient and Phytohormone Supplementation on the Growth, Pigment Yields and Biochemical Composition of Newly Isolated Microalgae. Process Biochem. 2020, 92, 61–68. [Google Scholar] [CrossRef]
- Tiwari, S.; Patel, A.; Prasad, S.M. Phytohormone Up-Regulates the Biochemical Constituent, Exopolysaccharide and Nitrogen Metabolism in Paddy-Field Cyanobacteria Exposed to Chromium Stress. BMC Microbiol. 2020, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Patel, A.; Prasad, S.M. Auxin and Cytokinin Alleviate Chromium-Induced Oxidative Stress in Nostoc muscorum and Anabaena sp. by Modulating Ascorbate–Glutathione Cycle. J. Plant Growth Regul. 2022, 41, 2743–2758. [Google Scholar] [CrossRef]
- Walter, A.; Carvalho, J.C.; Soccol, V.T.; Faria, A.B.B.; Ghiggi, V.; Soccol, C.R. Study of Phycocyanin Production from Spirulina platensis under Different Light Spectra. Braz. Arch. Biol. Technol. 2011, 54, 675–682. [Google Scholar] [CrossRef]
- Soni, B.; Kalavadia, B.; Trivedi, U.; Madamwar, D. Extraction, Purification and Characterization of Phycocyanin from Oscillatoria quadripunctulata—Isolated from the Rocky Shores of Bet-Dwarka, Gujarat, India. Process Biochem. 2006, 41, 2017–2023. [Google Scholar] [CrossRef]
- Deamici, K.M.; Costa, J.A.V.; Santos, L.O. Magnetic Fields as Triggers of Microalga Growth: Evaluation of Its Effect on Spirulina sp. Bioresour. Technol. 2016, 220, 62–67. [Google Scholar] [CrossRef]
- Olaizola, M.; Duerr, E.O. Effects of Light Intensity and Quality on the Growth Rate and Photosynthetic Pigment Content of Spirulina platensis. J. Appl. Phycol. 1990, 2, 97–104. [Google Scholar] [CrossRef]
- Salama, E.-S.; Jeon, B.-H.; Chang, S.W.; Lee, S.; Roh, H.-S.; Yang, I.-S.; Kurade, M.B.; El-Dalatony, M.M.; Kim, D.-H.; Kim, K.-H.; et al. Interactive Effect of Indole-3-Acetic Acid and Diethyl Aminoethyl Hexanoate on the Growth and Fatty Acid Content of Some Microalgae for Biodiesel Production. J. Clean. Prod. 2017, 168, 1017–1024. [Google Scholar] [CrossRef]
- Silvello, M.A.C.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-Based Carbohydrates: A Green Innovative Source of Bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef]
- Morais, E.G.; Silveira, J.T.; Schüler, L.M.; Freitas, B.C.B.; Costa, J.A.V.; Morais, M.G.; Ferrer, I.; Barreira, L. Biomass Valorization via Pyrolysis in Microalgae-Based Wastewater Treatment: Challenges and Opportunities for a Circular Bioeconomy. J. Appl. Phycol. 2023, 35, 2689–2708. [Google Scholar] [CrossRef]
- Moreira, J.B.; Vaz, B.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.; Costa, J.A.V.; Morais, M.G. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Moreira, J.B.; Santos, T.D.; Cruz, C.G.; Silveira, J.T.; Carvalho, L.F.; Morais, M.G.; Costa, J.A.V. Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields. Polysaccharides 2023, 4, 371–389. [Google Scholar] [CrossRef]
| Parameters | Indoor | Outdoor | ||
|---|---|---|---|---|
| C-I | IAA-I | C-O | IAA-O | |
| Xfinal (g L−1) | 2.44 c ± 0.09 | 2.78 c ± 0.29 | 4.60 b ± 0.01 | 5.43 a ± 0.07 |
| Pfinal (mg L−1 d−1) | 75.3 d ± 2.9 | 93.4 c ± 1.2 | 146.5 b ± 0.4 | 173.9 a ± 2.1 |
| Fv/Fm initial | 0.435 a ± 0.01 | 0.440 a ± 0.01 | 0.445 a ± 0.01 | 0.430 a ± 0.01 |
| Fv/Fm final | 0.385 b ± 0.01 | 0.385 b ± 0.01 | 0.450 a ± 0.01 | 0.440 a ± 0.01 |
| pH initial | 9.13 a ± 0.01 | 9.12 a ± 0.01 | 9.09 a ± 0.03 | 9.12 a ± 0.01 |
| pH final | 10.16 b ± 0.01 | 10.18 b ± 0.01 | 11.13 a ± 0.01 | 11.14 a ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, J.T.d.; Rosa, A.P.C.d.; Morais, M.G.d.; Costa, J.A.V. Indole-3-Acetic Acid Action in Outdoor and Indoor Cultures of Spirulina in Open Raceway Reactors. Appl. Sci. 2024, 14, 3715. https://doi.org/10.3390/app14093715
Silveira JTd, Rosa APCd, Morais MGd, Costa JAV. Indole-3-Acetic Acid Action in Outdoor and Indoor Cultures of Spirulina in Open Raceway Reactors. Applied Sciences. 2024; 14(9):3715. https://doi.org/10.3390/app14093715
Chicago/Turabian StyleSilveira, Jéssica Teixeira da, Ana Priscila Centeno da Rosa, Michele Greque de Morais, and Jorge Alberto Vieira Costa. 2024. "Indole-3-Acetic Acid Action in Outdoor and Indoor Cultures of Spirulina in Open Raceway Reactors" Applied Sciences 14, no. 9: 3715. https://doi.org/10.3390/app14093715






