SnCl4·5H2O: A Highly Efficient Catalyst for Hydration of Alkyne
Abstract
:1. Introduction

2. Results and Discussion
2.1. Effects of Varying the Catalyst
| Entry | Catalyst | Yield b % |
|---|---|---|
| 1 | CuCl | 15 |
| 2 | CuCl2·2H2O | 31 |
| 3 | Cu(NO3)2·3H2O | 26 |
| 4 | FeCl3 | 4 |
| 5 | SnCl2·2H2O | 66 |
| 6 | SnCl4·5H2O | 70 |
| 7 c | SnCl4·5H2O | 91 |
2.2. Optimum Reaction Conditions
| Entry | Solvent | Temperature °C | Time h | Yield b % |
|---|---|---|---|---|
| 1 | 1,4-dioxane | 120 | 18 | 1 |
| 2 | DMF | 120 | 18 | <1 |
| 3 | Toluene | 120 | 18 | 46 |
| 4 | DMSO | 120 | 18 | 49 |
| 5 | CH3CN | 120 | 18 | 37 |
| 6 | DCM | 120 | 18 | 42 |
| 7 | CH3COCH3 | 120 | 18 | 66 |
| 8 | EtOH | 120 | 18 | 90 |
| 9 | MeOH | 120 | 18 | 91 |
| 10 c | MeOH | 120 | 18 | 84 |
| 11 | MeOH | 110 | 18 | 60 |
| 12 | MeOH | 130 | 18 | 90 |
| 13 | MeOH | 120 | 14 | 80 |
| 14 | MeOH | 120 | 16 | 88 |
| 15 | MeOH | 120 | 20 | 90 |
2.3. Hydration of Other Alkynes
| Entry | Alkyne | Product | Yield b % |
|---|---|---|---|
| 1 | 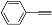 (1a) (1a) | 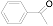 (2a) (2a) | 91 c |
| 2 |  (1b) (1b) | 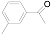 (2b) (2b) | 98 |
| 3 | 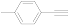 (1c) (1c) | 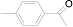 (2c) (2c) | 93 |
| 4 |  (1d) (1d) | 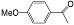 (2d) (2d) | >99 |
| 5 | 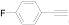 (1e) (1e) | 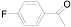 (2e) (2e) | 49 |
| 6 |  (1f) (1f) |  (2f) (2f) | 81 |
| 7 |  (1g) (1g) |  (2g) (2g) | - |
2.4. Possible Reaction Mechanism

3. Experimental Section
3.1. Chemicals
3.2. Hydration Reaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-metal-catalyzed addition of heteroatom-hydrogen bonds to alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Leyva-Pérez, A.; Sabater, M.J. Gold-catalyzed carbon-heteroatom bond-forming reactions. Chem. Rev. 2011, 111, 1657–1712. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, M.S.; Herzon, S.B. Broad-spectrum catalysts for the ambient temperature anti-markovnikov hydration of alkynes. Angew. Chem. Int. Ed. 2014, 53, 7892–7895. [Google Scholar] [CrossRef]
- Hintermann, L.; Labonne, A. Catalytic hydration of alkynes and its application in synthesis. Synthesis 2007. [Google Scholar] [CrossRef]
- Mello, R.; Alcalde-Aragonés, A.; González-Núñez, M.E. Silica-supported HgSO4/H2SO4: A convenient reagent for the hydration of alkynes under mild conditions. Tetrahedron Lett. 2010, 51, 4281–4283. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, J.H. Cerium (IV) sulfate-catalyzed hydration of alkynes. Chin. J. Org. Chem. 2006, 26, 1073–1078. [Google Scholar]
- Blum, J.; Huminer, H.; Alper, H. Alkyne hydration promoted by RhCl3 and quaternary ammonium salts. J. Mol. Catal. 1992, 75, 153–160. [Google Scholar] [CrossRef]
- Kanemitsu, H.; Uehara, K.; Fukuzumi, S.; Ogo, S. Isolation and crystal structures of both enol and keto tautomer intermediates in a hydration of an alkyne-carboxylic acid ester catalyzed by iridium complexes in water. J. Am. Chem. Soc. 2008, 130, 17141–17147. [Google Scholar] [CrossRef] [PubMed]
- Imi, K.; Imai, K.; Utimoto, K. Regioselective hydration of alkynones by palladium catalysis. Tetrahedron Lett. 1987, 28, 3127–3130. [Google Scholar] [CrossRef]
- Hartman, J.W.; Hiscox, W.C.; Jennings, P.W. Catalytic hydration of alkynes with platinum(II) complexes. J. Org. Chem. 1993, 58, 7613–7614. [Google Scholar] [CrossRef]
- Mizushima, E.; Sato, K.; Hayashi, T.; Tanaka, M. Highly efficient AuI-catalyzed hydration of alkynes. Angew. Chem. Int. Ed. 2002, 41, 4563–4565. [Google Scholar] [CrossRef]
- Casado, R.; Contel, M.; Laguna, M.; Romero, P.; Sanz, S. Organometallic gold(Ш) compounds as catalysts for the addition of water and methanol to terminal alkynes. J. Am. Chem. Soc. 2003, 125, 11925–11935. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Zheng, A.M.; Zhao, P.Q.; Xia, C.G.; Li, F.W. Au-NHC@Porous organic polymers: Synthetic control and its catalytic application in alkyne hydration reactions. ACS Catal. 2014, 4, 321–327. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Estiri, H.; Azad, M. Au anchored to (α-Fe2O3)-MCM-41-HS as a novel magnetic nanocatalyst for water-medium and solvent-free alkyne hydration. Catal. Commun. 2014, 57, 29–35. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Q.J.; Lv, W.F.; Pang, Q.Y.; Ben, R.; Qian, Y.; Zhao, J. Indazolin-s-ylidene-N-heterocyclic carbene complexes of rhodium, palladium, and gold: Synthesis, characterization, and catalytic hydration of alkynes. Organometallics 2013, 32, 3753–3759. [Google Scholar] [CrossRef]
- Xu, X.Y.; Kim, S.H.; Zhang, X.; Das, A.K.; Hirao, H.; Hong, S.H. Abnormal N-heterocyclic carbene gold(i) complexes: Synthesis, structure, and catalysis in hydration of alkynes. Organometallics 2013, 32, 164–171. [Google Scholar] [CrossRef]
- Sayantani, S.; Abir, K.B. Bulky, spherical, and fluorinated anion BArF4 induces ‘on-water’ activity of silver salt for the hydration of terminal alkynes. Tetrahedron Lett. 2014, 55, 1444–1447. [Google Scholar] [CrossRef]
- Thuong, M.B.T.; Mann, A.; Wagner, A. Mild chemo-selective hydration of terminal alkynes catalysed by AgSbF6. Chem. Commun. 2012, 48, 434–436. [Google Scholar] [CrossRef]
- Wu, X.F.; Bezier, D.; Darcel, C. Development of the first iron chloride-catalyzed hydration of terminal alkynes. Adv. Synth. Catal. 2009, 351, 367–370. [Google Scholar] [CrossRef]
- Tachinami, T.; Nishimura, T.; Ushimaru, R.; Noyori, R.; Naka, H. Hydration of terminal alkynes catalyzed by water-soluble cobalt porphyrin complexes. J. Am. Chem. Soc. 2013, 135, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Breit, B.; Gellrich, U.; Li, T.; Lynam, J.M.; Milner, L.M.; Pridmore, N.E.; Slattery, J.M.; Whitwood, A.C. Mechanistic insight into the ruthenium-catalysed anti-Markovnikov hydration of alkynes using a self-assembled complex: A crucial role for ligand-assisted proton shuttle processes. Dalton Trans. 2014, 43, 11277–11285. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.S.; Li, L.; Herzon, S.B. A highly active and air-stable ruthenium complex for the ambient temperature anti-Markovnikov reductive hydration of terminal alkynes. J. Am. Chem. Soc. 2014, 136, 7058–7067. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimoto, T.; Joya, T.; Shirakawa, E.; Kawakami, Y. Brønsted acid-catalyzed hydration of alkynes: A convenient route to diverse carbonyl compounds. Synlett 2000, 1777–1778. [Google Scholar]
- Allen, A.D.; Chiang, Y.; Kresge, A.J.; Tidwell, T.T. Substituent effects on the acid hydration of acetylenes. J. Org. Chem. 1982, 47, 775–779. [Google Scholar] [CrossRef]
- Menashe, N.; Shvo, Y. Hydration of alkynes in anhydrous medium with formic acid as water donor. J. Org. Chem. 1993, 58, 7434–7439. [Google Scholar] [CrossRef]
- Maud, J.; Olivier, P.; Jean-François, P.; Abdallah, H.; Jean-Daniel, B.; Mouad, A. A ring-closing metathesis based synthesis of bicyclic nucleosides locked in S-type conformations by hydroxyl functionalised 3′,4′-trans linkages. Tetrahedron 2010, 66, 3775–3786. [Google Scholar] [CrossRef]
- Gao, Q.; Li, S.Y.; Pan, Y.M.; Xu, Y.L.; Wang, H.S. p-Toluenesulfonic acid-promoted selective functionalization of unsymmetrical arylalkynes: A regioselective access to various arylketones and heterocycles. Tetrahedron 2013, 69, 3775–3778. [Google Scholar] [CrossRef]
- Mauro, B.; Samuele, C.; Federico, L.; Chiara, P. Hydration of aromatic terminal alkynes catalyzed by iron(III) sulfate hydrate under chlorine-free conditions. Tetrahedron Lett. 2014, 55, 1608–1612. [Google Scholar] [CrossRef]
- Park, J.; Yeon, J.; Lee, P.H.; Lee, K. Iron-catalyzed indirect hydration of alkynes in presence of methanesulfonic acid. Tetrahedron Lett. 2013, 54, 4414–4417. [Google Scholar] [CrossRef]
- Chen, Z.W.; Ye, D.N.; Ye, M.; Liu, L.X. Highly efficient AgBF4-catalyzed synthesis of methyl ketones from terminal alkynes. Tetrahedron 2013, 69, 6116–6120. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, D. AgOTf catalyzed hydration of terminal alkynes. Appl. Organomet. Chem. 2012, 26, 722–726. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Leyva-Pérez, A.; Corma, A. Regioselective hydration of alkynes by iron(III) Lewis/Brønsted catalysis. Chem. Eur. J. 2012, 18, 11107–11114. [Google Scholar] [CrossRef] [PubMed]
- Cabrero-Antonino, J.R.; Leyva-Pérez, A.; Corma, A. Iron(III) Triflimide as a catalytic substitute for gold(I) in hydroaddition reactions to unsaturated carbon-carbon bonds. Chem. Eur. J. 2013, 19, 8627–8633. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, L.; Singhamahapatra, A.; Paul, K.J.V.; Loganathan, D. SnCl4/Sn catalyzed chemoselective reduction of glycopyranosyl azides for the synthesis of diversely functionalized glycopyranosyl chloroacetamides. Tetrahedron Lett. 2013, 54, 5361–5365. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, D.; Wang, B.; Liu, Q.F.; Liu, T.X.; Zhang, W.; Yuan, B.B.; Zhao, Z.J.; Han, D.D.; Zhang, G.S. SnCl4 mediated synthesis of γ-amino ketones derivatives via the ring-opening reaction of 4, 5-dihydropyrroles. Tetrahedron 2013, 69, 9063–9067. [Google Scholar] [CrossRef]
- Hu, S.Q.; Zhang, Z.F.; Song, J.L.; Zhou, Y.X.; Han, B.X. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Loh, T.P. Bio-inspired polyene cyclization: Synthesis of tetracyclic terpenoids promoted by steroidal acetal-SnCl4. Chem. Commun. 2008, 1434–1436. [Google Scholar] [CrossRef]
- Toman, L.; Lukas, R.; Spevacek, J. Isobutylene polymerization in the presence of t-BuCl/SnCl4. Polym. Bull. 1992, 28, 175–180. [Google Scholar] [CrossRef]
- Han, J.K.; Lee, K.W.; Lee, C.E. Proton dynamics in SnCl4·5H2O. J. Korean Phys. Soc. 2000, 37, L487–L489. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Wang, D.; Wu, W.; Xiao, L. SnCl4·5H2O: A Highly Efficient Catalyst for Hydration of Alkyne. Appl. Sci. 2015, 5, 114-121. https://doi.org/10.3390/app5020114
Chen D, Wang D, Wu W, Xiao L. SnCl4·5H2O: A Highly Efficient Catalyst for Hydration of Alkyne. Applied Sciences. 2015; 5(2):114-121. https://doi.org/10.3390/app5020114
Chicago/Turabian StyleChen, Dongxue, Dantong Wang, Wei Wu, and Linfei Xiao. 2015. "SnCl4·5H2O: A Highly Efficient Catalyst for Hydration of Alkyne" Applied Sciences 5, no. 2: 114-121. https://doi.org/10.3390/app5020114





