Characterization of Transition Metal Oxide/Silicon Heterojunctions for Solar Cell Applications
Abstract
:1. Introduction
2. Experimental Details

3. Results and Discussion
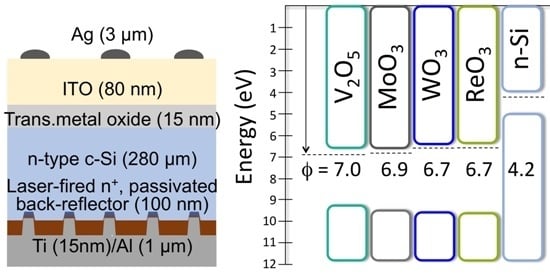
3.1. Transition Metal Oxide Characterization


| Oxide | Sheet Resistance (Rsh), Ω/sq | |
|---|---|---|
| Glass substrate | Silicon substrate | |
| V2Ox | 3.3 × 109 | 5.8 × 103 |
| MoOx | 5 × 1012 | 1.0 × 104 |
| WOx | 1.1 × 109 | 1.2 × 105 |

3.2. Solar Cell Characterization

| Oxide | PCE % | VOC mV | JSC mA/cm2 | FF % | RS Ωcm2 |
|---|---|---|---|---|---|
| V2O5 | 12.7 | 593 | 29.6 | 72.4 | 0.9 |
| MoO3 | 12.6 | 581 | 29.6 | 73.1 | 0.8 |
| WO3 | 11.8 | 570 | 29.1 | 71.0 | 1.0 |
| ReO3 | 3.1 | 245 | 28.9 | 44.4 | 1.1 |
| a-Si:H | 13.1 | 617 | 29.1 | 73.0 | 0.55 |


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alsema, E.A.; de Wild-scholten, M.J. Environmental impacts of crystalline silicon photovoltaic module production. In Proceedings of the 13th CIRP International Conference in Life Cycle Engineering, Leuven, Belgium, 31 May 2006.
- Thomas, J.P.; Leung, K.T. Defect-minimized PEDOT: PSS/planar-Si solar cell with very high efficiency. Adv. Funct. Mater. 2014, 24, 4978–4985. [Google Scholar]
- Weingarten, M.; Zweipfennig, T.; Vescan, A.; Kalisch, H. Low-temperature processed hybrid organic/silicon solar cells with power conversion efficiency up to 6.5%. MRS Proceedings 2015, 1771. mrss15-2133323. [Google Scholar] [CrossRef]
- Hussain, B.; Ebong, A.; Ferguson, I. Zinc oxide as an active n-layer and antireflection coating for silicon based heterojunction solar cell. Sol. Energy Mater. Sol. Cells 2015, 139, 95–100. [Google Scholar] [CrossRef]
- Nagamatsu, K.A.; Avasthi, S.; Sahasrabudhe, G.; Man, G.; Jhaveri, J.; Berg, A.H. Titanium dioxide/silicon hole-blocking selective contact to enable double-heterojunction crystalline silicon-based solar cell. Appl. Phys. Lett. 2015, 106, 123906. [Google Scholar] [CrossRef]
- Bullock, J.; Yan, D.; Cuevas, A.; Wan, Y.; Samundsett, C. n- and p-type silicon solar cells with molybdenum oxide hole contacts. Energy Procedia 2015, 77, 446–450. [Google Scholar] [CrossRef]
- Bivour, M.; Temmler, J.; Steinkemper, H.; Hermle, M. Alternative contact materials for induced junction silicon solar cells. Sol. Energy. Mater. Sol. Cells 2015, 142, 34–41. [Google Scholar] [CrossRef]
- Liu, R.; Lee, S.T.; Sun, B. 13.8% efficiency hybrid Si/organic heterojunction solar cells with MoO3 film as antireflection and inversion induced layer. Adv. Mater. 2014, 26, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Yu, X.; Xu, D.; Shen, X.; Xia, Z.; He, H. High efficiency organic/silicon hybrid solar cells with doping-free selective emitter structure induced by a WO3 thin interlayer. Nano Energy 2015, 16, 54–61. [Google Scholar] [CrossRef]
- Battaglia, C.; de Nicolás, S.M.; de Wolf, S.; Yin, X.; Zheng, M.; Ballif, C. Silicon heterojunction solar cell with passivated hole selective MoOx contact. Appl. Phys. Lett. 2014, 104, 113902. [Google Scholar] [CrossRef]
- Würfel, P. Physics of Solar Cells, 1st ed.; Wiley-VCH: Weinheim, Germany, 2005; pp. 113–114. [Google Scholar]
- Colina, M.; Morales-Vilches, A.; Voz, C.; Martin, I.; Ortega, P.R.; Alcubilla, R. Low surface recombination in silicon-heterojunction solar cells with rear laser-fired contacts from aluminum foils. IEEE J. Photovoltaics 2015, 5, 805–811. [Google Scholar] [CrossRef]
- Yoo, S.J.; Chang, J.H.; Lee, J.H.; Moon, C.K.; Wu, C.I.; Kim, J.J. Formation of perfect ohmic contact at indium tin oxide/N,N’-di(naphthalene-1-yl)-N,N’-diphenyl-benzidine interface using ReO3. Sci. Rep. 2014, 4, 3902. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition metal oxides for organic electronics: Energetics, device physics and applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef] [PubMed]
- Wafer Ray Tracer simulation tool, Version 1.6.4, PV Lighthouse Pty. Ltd., Australia. Available online: http://www.pvlighthouse.com.au (accessed on 1 June 2015).
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.-M.; Lu, Z.-H. Transition metal oxide work functions: The influence of cation oxidation state and oxygen vacancies. Adv. Funct. Mater. 2012, 22, 4557–4568. [Google Scholar] [CrossRef]
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.-M.; Lu, Z.-H. Metal/metal-oxide interfaces: How metal contacts affect the work function and band structure of MoO3. Adv. Funct. Mater. 2013, 23, 215–226. [Google Scholar] [CrossRef]
- Zilberberg, K.; Trost, S.; Meyer, J.; Kahn, A.; Behrendt, A.; Lützenkirchen-Hecht, D. Inverted organic solar cells with sol-gel processed high work-function vanadium oxide hole-extraction layers. Adv. Funct. Mater. 2011, 21, 4776–4783. [Google Scholar] [CrossRef]
- Irfan, I; Ding, H.; Gao, Y.; Small, C.; Kim, D.Y.; Subbiah, J.; So, F. Energy level evolution of air and oxygen exposed molybdenum trioxide films. Appl. Phys. Lett. 2010, 96, 243307. [Google Scholar]
- Shi, X.B.; Xu, M.F.; Zhou, D.Y.; Wang, Z.K.; Liao, L.S. Improved cation valence state in molybdenum oxides by ultraviolet-ozone treatments and its applications in organic light-emitting diodes. Appl. Phys. Lett. 2013, 102, 233304. [Google Scholar] [CrossRef]
- Meier, D.L.; Schroder, D.K. Contact resistance: Its measurement and relative importance to power loss in a solar cell. IEEE Trans. Electron Devices 1984, 31, 647–653. [Google Scholar] [CrossRef]
- Bullock, J.; Cuevas, A.; Allen, T.; Battaglia, C. Molybdenum oxide MoOx: A versatile hole contact for silicon solar cells. Appl. Phys. Lett. 2014, 105, 232109. [Google Scholar] [CrossRef]
- Lajaunie, L.; Boucher, F.; Dessapt, R.; Moreau, P. Strong anisotropic influence of local-field effects on the dielectric response of α-MoO3. Phys. Rev. B 2013, 88, 115141. [Google Scholar] [CrossRef]
- Illiberi, A.; Kudlacek, P.; Smets, A.H.M.; Creatore, M.; van de Sanden, M.C.M. Effect of ion bombardment on the a-Si:H based surface passivation of -Si surfaces. Appl. Phys. Lett. 2011, 98, 242115. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerling, L.G.; Mahato, S.; Voz, C.; Alcubilla, R.; Puigdollers, J. Characterization of Transition Metal Oxide/Silicon Heterojunctions for Solar Cell Applications. Appl. Sci. 2015, 5, 695-705. https://doi.org/10.3390/app5040695
Gerling LG, Mahato S, Voz C, Alcubilla R, Puigdollers J. Characterization of Transition Metal Oxide/Silicon Heterojunctions for Solar Cell Applications. Applied Sciences. 2015; 5(4):695-705. https://doi.org/10.3390/app5040695
Chicago/Turabian StyleGerling, Luis G., Somnath Mahato, Cristobal Voz, Ramon Alcubilla, and Joaquim Puigdollers. 2015. "Characterization of Transition Metal Oxide/Silicon Heterojunctions for Solar Cell Applications" Applied Sciences 5, no. 4: 695-705. https://doi.org/10.3390/app5040695