Comparison of Grinding Characteristics of Converter Steel Slag with and without Pretreatment and Grinding Aids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
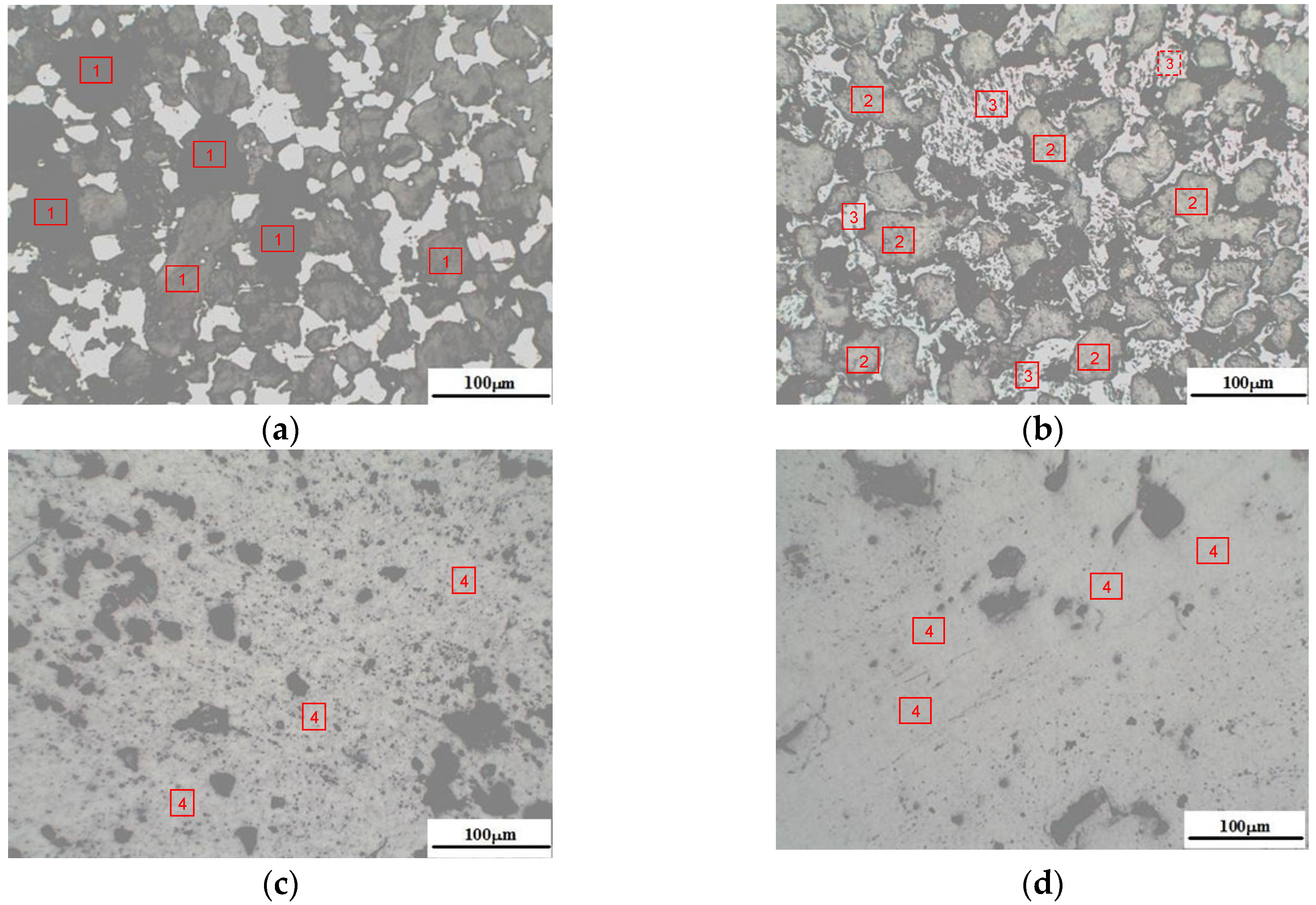
2.2.1. Observation and Identification of Mineral Phases for Converter Steel Slag
2.2.2. Measurement of Vickers Hardness of Mineral Phases for Converter Steel Slag
2.2.3. Procedure of Grinding Experiment for Steel Slag
2.2.4. Test Methods of Particle Size and Distribution of Ground Steel Slag Powder
2.2.5. Observation of Particle Morphology of Ground Steel Slag Powder
2.2.6. Test Methods of the Angle of Repose of Ground Steel Slag Powder
3. Results and Discussion
3.1. Mineral Phases’Characteristics of Converter Steel Slag
3.2. Determination of Hardly Grinding Phases (HGP) in Converter Steel Slag
3.2.1. Proportion of HGP in Converter Steel Slag
3.2.2. Morphology and Vickers Hardness of HGP in Converter Steel Slag
3.2.3. Chemical Compositions of HGP in Converter Steel Slag
3.3. Comparison of Grinding Characteristic between Untreated and Pre-treated Converter Steel Slag
3.3.1. Total Analysis of Iron Mineral Phases in Converter Steel Slag after Pre-treatment
3.3.2. Grinding Efficiency of Untreated and Pretreated Steel Slag
3.3.3. Particle Size Distributions of Ground Untreated and Pretreated Steel Slag
3.3.4. Particle Morphologies of Ground Untreated and Pretreated Steel Slag
3.4. Effect of Organic Grinding Aids on the Grinding Property of Converter Steel Slag
3.4.1. Effect of GA on the Sieving Residue of Steel Slag Powder
3.4.2. Effect of GA on the Particle Size Distribution of Steel Slag Powder
3.4.3. Effect of GA on the Angle of Repose of Steel Slag Powder
3.4.4. Effect of GA on the Particle Morphology of Steel Slag Powder
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yi, H.; Xu, G.P.; Cheng, H.G.; Wang, J.S.; Wan, Y.F.; Chen, H. An overview of utilization of steel slag. Proc. Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Zhang, T.S.; Yu, Q.J.; Wei, J.X.; Li, J.X.; Zhang, P.P. Preparation of high performance blended cements and reclamation of iron concentrate from basic oxygen furnace steel slag. Resour. Conserv. Recy. 2011, 56, 48–55. [Google Scholar] [CrossRef]
- Zhao, L.H.; Li, Y.; Zhou, Y.Y.; Cang, D.Q. Preparation of novel ceramics with high CaO content from steel slag. Mater. Des. 2014, 64, 608–613. [Google Scholar] [CrossRef]
- Li, Z.B.; Zhao, S.Y.; Zhao, X.G.; He, T.S. Cementitious property modification of basic oxygen furnace steel slag. Constr. Build. Mater. 2013, 48, 575–579. [Google Scholar] [CrossRef]
- Brand, A.S.; Roesler, J.R. Steel furnace slag aggregate expansion and hardened concrete properties. Cem. Concr. Comp. 2015, 60, 1–9. [Google Scholar] [CrossRef]
- Shi, C.J. Characteristics and cementitious properties of ladle slag fines from steel production. Cem. Concr. Res. 2002, 32, 459–462. [Google Scholar] [CrossRef]
- Shi, C.J. Steel slag- its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, D.M.; Wang, X.G.; Liao, S.C. Characteristics and mechanism of modified triethanolamine as cement grinding aids. J. Wuhan Univ. Technol. 2015, 30, 134–141. [Google Scholar] [CrossRef]
- Yan, P.Y.; Mi, G.D.; Wang, Q. A comparison of early hydration properties of cement-steel slag binder and cement-limestone powder binder. J. Therm. Anal. Calorim. 2014, 115, 193–200. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.E.; Papadimitriou, G.D.; Tsibouki, Z. Properties and hydration of blended cements with steel making slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, H.Y.; Wang, J.; Feng, Q.M. Preliminary investigation on the pozzolanic activity of superfine steel slag. Constr. Build. Mater. 2015, 82, 227–234. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, D.M.; Yan, P.Y.; Zhao, S.J.; Zhang, D.W. Particle characteristics and hydration activity of ground granulated blast furnace slag powder containing industrial crude glycerol-based grinding aids. Constr. Build. Mater. 2016, 104, 134–141. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, H.B.; Huang, X.Q.; Zhou, M.; Wang, W.X. Enhance hydration properties of steel slag using grinding aids by mechanochemical effect. Constr. Build. Mater. 2012, 29, 476–481. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, D.M.; Liao, S.C. Effect of mechanical grinding on physical and chemical characteristics of circulating fluidized bed fly ash from coal gangue power plant. Constr. Build. Mater. 2015, 101, 851–860. [Google Scholar] [CrossRef]
- Ghiasvand, E.; Ramezanianpour, A.A.; Ramezanianpour, A.M. Effect of grinding method and particle size distribution on the properties of Portland-pozzolan cement. Constr. Build. Mater. 2014, 53, 547–554. [Google Scholar] [CrossRef]
- Han, C.J.; Yang, X.J.; Zhou, H.Q.; Tang, Y. Steel slag and its application in cement industries. Mater. Rev. 2010, 24, 440–443. (In Chinese) [Google Scholar]
- Ghouleh, Z.; Guthrie, R.I.L.; Shao, Y.X. High-strength KOBM steel slag binder activated by carbonation. Constr. Build. Mater. 2015, 99, 175–183. [Google Scholar] [CrossRef]
- Zong, Y.B.; Cang, D.Q.; Zhen, Y.P.; Li, Y.; Bai, H. Component modification of steel slag in air quenching process to improve grindability. Tran. Nonferr. Met. Soc. 2009, 19, s834–s839. [Google Scholar] [CrossRef]
- Hou, G.H.; Li, W.F.; Wang, J.G. Difference of grindability and cementitious performance among minerals in steel slag. J. Chin. Ceram. Soc. 2009, 37, 1613–1617. (In Chinese) [Google Scholar]
- Kong, L.Z.; Wang, J.; Chen, L.Z. Research on mineral phase and grindability of steel slag by changing components contents. China Metall. 2013, 23, 56–59. (In Chinese) [Google Scholar]
- Zhou, Y.; Liu, H.B.; Dong, Y.C.; Chen, G.Y.; Liu, Y.L.; Wang, C. Research on grindability of steel slag by modifying. China Metall. 2010, 20, 38–41. (In Chinese) [Google Scholar]
- Zhao, F.C.; Ju, J.T.; Liao, J.L.; Kong, W.M.; Dang, Y.J. Analysis of comprehensive utilization and basic properties of converter slag processed. J. Iron Steel Res. 2013, 25, 23–28. (In Chinese) [Google Scholar]
- Choi, H.; Lee, W.; Kim, S. Effect of grinding aids on the kinetics of fine grinding energy consumed of calcite powders by a stirred ball mill. Adv. Powder Technol. 2009, 20, 350–354. [Google Scholar] [CrossRef]
- Choi, H.; Lee, W.; Kim, D.U.; Kumar, S.; Kim, S.S.; Chung, H.S.; Kim, J.H.; Ahn, Y.C. Effect of grinding aid on the grinding energy consumed during grinding of calcite in a stirred ball mill. Miner. Eng. 2010, 23, 54–57. [Google Scholar] [CrossRef]
- Gao, X.J.; Yang, Y.Z.; Deng, H.W. Utilization of beet molasses as a grinding aid in blended cements. Constr. Build. Mater. 2011, 25, 3782–3789. [Google Scholar] [CrossRef]
- Toraman, O.Y. Effect of chemical additive on stirred bead milling of calcite powder. Powder Technol. 2012, 221, 189–191. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.S.; Jung, S.S.; Hwang, J.I.; Choi, J.S.; Sohn, I. Valorization of electric arc furnace primary steelmaking slags for cement applications. Waste Manage. 2015, 41, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sadique, M.; Al-Nageim, H.; Atherton, W.; Seton, L.; Dempster, N. Mechano-chemical activation of high-Ca fly ash by cement free blending and gypsum aided grinding. Constr. Build. Mater. 2013, 43, 480–489. [Google Scholar] [CrossRef]
- Assaad, J.J.; Issa, C.A. Effect of clinker grinding aids on flow of cement-based materials. Cem. Concr. Res. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Katsioti, M.; Tsakiridis, P.E.; Giannatos, P.; Tsibouki, Z.; Marinos, J. Characterization of various cement grinding aids and their impact on grindability and cement performance. Constr. Build. Mater. 2009, 23, 1954–1959. [Google Scholar] [CrossRef]
- Altun, O.; Benzer, H.; Toprak, A.; Enderle, U. Utlization of grinding aids in dry horizontal stirred milling. Powder Technol. 2015, 286, 610–615. [Google Scholar] [CrossRef]













| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | SO3 | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|
| 39.67 | 21.71 | 1.58 | 25.52 | 4.92 | 0.02 | 0.18 | 1.70 | 0.32 |
| Region number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Mineral phases | Silicate phase | Calcium ferrite phase | RO phase | Metallic iron phase |
| Vickers hardness | 187.3 | 335.0 | 298.1 | 29.4 |
| Grinding time (min) | CaO | SiO2 | Al2O3 | Fe2O3 | Fe | MgO |
|---|---|---|---|---|---|---|
| 10 | 32.45 | 12.05 | 1.15 | 34.80 | 3.53 | 8.43 |
| 20 | 26.12 | 8.26 | 1.06 | 37.07 | 7.60 | 12.29 |
| 30 | 20.30 | 5.81 | 0.92 | 38.13 | 13.36 | 13.07 |
| 40 | 14.70 | 4.16 | 0.75 | 39.08 | 23.10 | 10.13 |
| 50 | 6.14 | 3.05 | 0.62 | 32.12 | 45.53 | 7.20 |
| 60 | 5.19 | 2.03 | 0.51 | 18.09 | 65.33 | 4.12 |
| 70 | 4.04 | 1.14 | 0.44 | 11.25 | 76.20 | 3.21 |
| Iron mineral phases | Proportions of iron mineral phases (%) | ||
|---|---|---|---|
| Untreated steel slag | PMS-steel slag | SMS-steel slag | |
| Metallic iron & magnetite | 2.38 | 1.18 | 0.45 |
| Hematite/limonite | 13.11 | 13.05 | 6.67 |
| Sulfide | 0.04 | 0.04 | 0.04 |
| Siderite | 2.52 | 2.26 | 1.98 |
| Iron silicate | 0.21 | 0.10 | 0.06 |
| Total | 18.26 | 16.63 | 9.20 |
| Grinding time (min) | Untreated steel slag | Pretreated steel slag | ||||||
|---|---|---|---|---|---|---|---|---|
| <3 μm | 3–32 μm | 32–65 μm | >65 μm | <3 μm | 3–32 μm | 32–65 μm | >65 μm | |
| 10 | 2.77 | 27.88 | 25.24 | 44.11 | 2.44 | 25.42 | 21.95 | 50.19 |
| 20 | 4.97 | 37.16 | 27.54 | 30.33 | 5.66 | 40.06 | 30.84 | 23.45 |
| 30 | 6.64 | 36.62 | 28.04 | 28.70 | 9.00 | 44.22 | 32.10 | 14.68 |
| 40 | 8.16 | 38.24 | 27.46 | 26.15 | 10.54 | 48.80 | 30.51 | 10.15 |
| 50 | 11.18 | 37.90 | 22.19 | 28.73 | 14.87 | 56.99 | 22.65 | 5.49 |
| 60 | 8.99 | 32.52 | 26.57 | 31.91 | 16.36 | 53.83 | 20.96 | 8.84 |
| 70 | 10.91 | 30.99 | 19.12 | 38.97 | 14.50 | 55.74 | 21.47 | 8.29 |
| Grinding time (min) | Without GA | With GA | ||||||
|---|---|---|---|---|---|---|---|---|
| <3 μm | 3–32 μm | 32–65 μm | >65 μm | <3 μm | 3–32 μm | 32–65 μm | >65 μm | |
| 10 | 2.44 | 25.42 | 21.95 | 50.19 | 2.83 | 23.83 | 22.80 | 50.54 |
| 20 | 5.66 | 40.06 | 30.84 | 23.45 | 4.81 | 37.44 | 34.14 | 23.62 |
| 30 | 9.00 | 44.22 | 32.10 | 14.68 | 7.63 | 47.08 | 32.74 | 12.55 |
| 40 | 10.54 | 48.80 | 30.51 | 10.15 | 10.44 | 56.04 | 28.47 | 5.04 |
| 50 | 14.87 | 56.99 | 22.65 | 5.49 | 14.32 | 64.21 | 21.10 | 0.38 |
| 60 | 16.36 | 53.83 | 20.96 | 8.84 | 15.73 | 64.46 | 18.61 | 1.20 |
| 70 | 14.50 | 55.74 | 21.47 | 8.29 | 15.05 | 57.33 | 19.49 | 8.13 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, D.; Yan, P.; Li, W. Comparison of Grinding Characteristics of Converter Steel Slag with and without Pretreatment and Grinding Aids. Appl. Sci. 2016, 6, 237. https://doi.org/10.3390/app6110237
Zhao J, Wang D, Yan P, Li W. Comparison of Grinding Characteristics of Converter Steel Slag with and without Pretreatment and Grinding Aids. Applied Sciences. 2016; 6(11):237. https://doi.org/10.3390/app6110237
Chicago/Turabian StyleZhao, Jihui, Dongmin Wang, Peiyu Yan, and Wenping Li. 2016. "Comparison of Grinding Characteristics of Converter Steel Slag with and without Pretreatment and Grinding Aids" Applied Sciences 6, no. 11: 237. https://doi.org/10.3390/app6110237






