Green High-Yielding One-Pot Approach to Biginelli Reaction under Catalyst-Free and Solvent-Free Ball Milling Conditions
Abstract
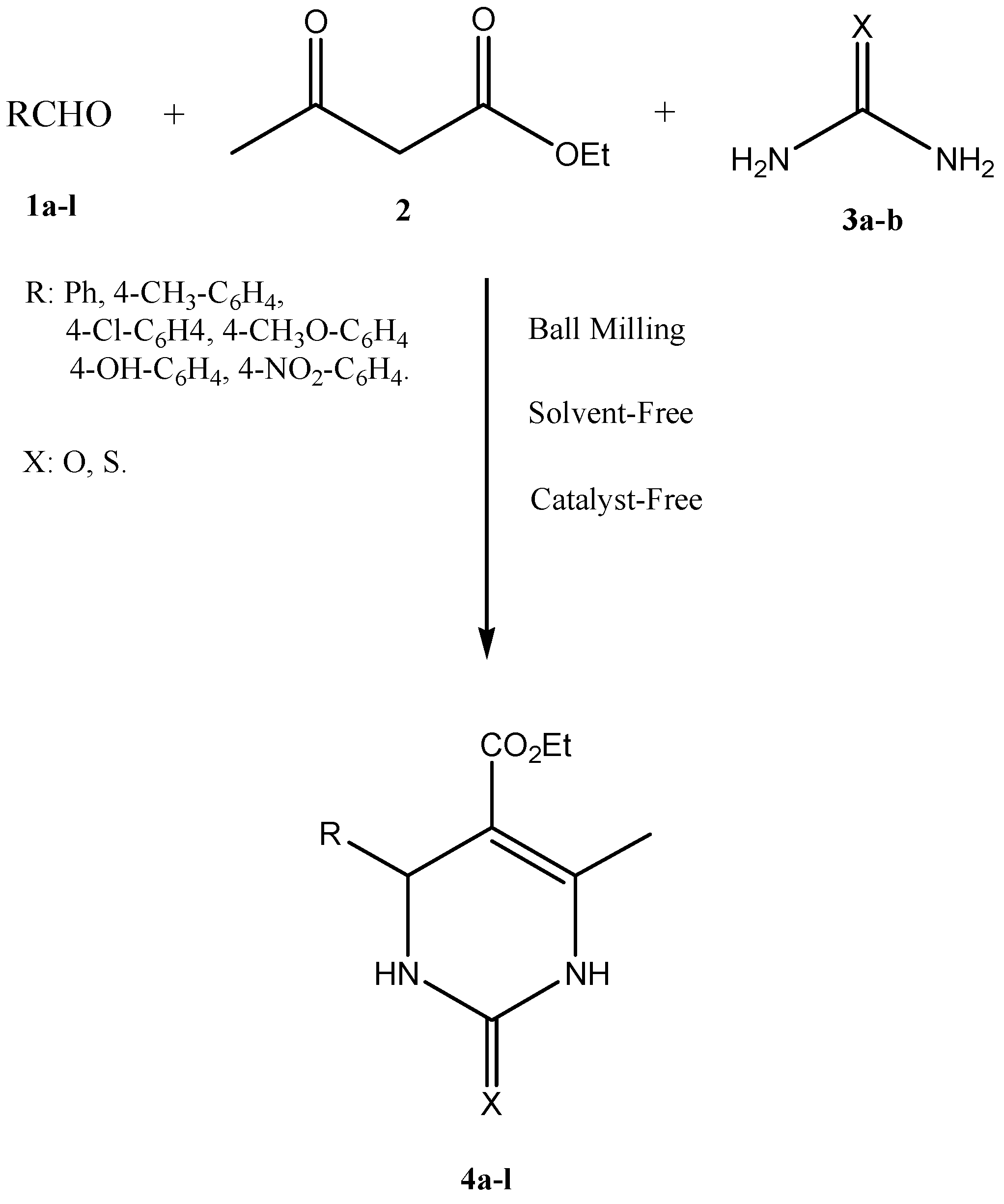
:1. Introduction
2. Experimental Section
2.1. Materials and Techniques
2.2. General Procedure for Synthesis of 3,4-Dihydropyrimidine Compound 4a
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, J.; Bienayme, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005; pp. 33–75. [Google Scholar]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, D.; Garcia-Tellado, F. Chimo-differentiating ABB’ multicomponent reactions. Previleged building blocks. Chem. Soc. Rev. 2007, 36, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.J.J. Multicomponent reactions. Beilstein J. Org. Chem. 2011, 7, 960–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.G.; Turberg, M.; Schiffers, I.; Bolm, C. Mechanochemical strecker reaction: Access to α-aminonitriles and tetrahydroisoquinolines under ball-milling conditions. Chem. Eur. J. 2016, 22, 14513–14517. [Google Scholar] [CrossRef] [PubMed]
- Polindara-García, L.A.; Juaristi, E. Synthesis of ugi 4-CR and passerini 3-CR adducts under mechanochemical activation. Eur. J. Org. Chem. 2016, 1095–1102. [Google Scholar] [CrossRef]
- Biginelli, P. Aldehyde-urea derivatives of aceto-and oxaloacetic acids. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Kidwai, M.; Venkatramanan, R.; Garg, R.K.; Bhushan, K.R. Novel one pot synthesis of new pyranopyrimidines using microwaves. J. Chem. Res(s). 2000, 586–587. [Google Scholar] [CrossRef]
- Tabern, D.L.; Volwiler, E.H. Sulfur-containing barbiturate hypnotics. J. Am. Chem. Soc. 1935, 57, 1961–1963. [Google Scholar] [CrossRef]
- Atwal, K.S.; Rovnyak, G.C.; O’Reilly, B.C.; Schwartz, J. Substituted1,4-dihydropyrimidines: Synthesis of selectively functionalized 2-hetero-1,4- dihydropyrimidines. J. Org. Chem. 1989, 54, 5898–5907. [Google Scholar] [CrossRef]
- Kappe, C.O.; Fabian, W.M.F.; Semones, M.A. Synthetic applications of furan Diels-Alder chemistry. Tetrahedron 1997, 53, 2803–2816. [Google Scholar]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.I.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 286, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Hurst, E.W.; Hull, R. Two new synthetic substances active against viruses of the psittacosis-lymphogranuloma-trachoma group. J. Med. Pharm. Chem. 1961, 3, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. Japan Kokai Tokkyo Koho. JP Patent 59,190,974, 1984. [Google Scholar]
- Suguira, K.; Schmid, F.A.; Schmid, M.M.; Brown, G.F. Effect of compounds on a spectrum of rat tumors. Cancer Chemother. Rep. Part 2 1973, 3, 231–238. [Google Scholar]
- Regnier, G.L.; Canevar, R.J.; Le Douarec, J.C.; Halstop, S.; Daussy, J. Triphenylpropylpiperazine derivatives as new potent analgetic substances. J. Med. Chem. 1972, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Pershin, G.N.; Sherbakova, L.I.; Zykova, T.N.; Sakolova, V.N. Antibacterial activity of pyrimidine and pyrrolo (3,2-d)pyrimidine derivatives. Farmakol. Taksikol. 1972, 35, 466–471. [Google Scholar]
- Metolcsy, G. Structure-activity correlations and mode of action of some selected types of antifungal compounds. World Rev. Pest Control 1971, 10, 50–59. [Google Scholar]
- Suresh; Sandhu, J.S. Past, present and future of the Biginelli reaction: A critical perspective. ARKIVOV 2012, 66–133. [Google Scholar]
- Deyanira, A.-B.; Leticia, L.-R.; Victor, H.L.-C.; Eduardo, G.-Z.; Guillermo, N.-S. Sulfated zirconia-catalyzed synthesis of 3,4-dihydro-pyrimidin-2(1h)-ones (dhpms) under solventless conditions: Competitive multicomponent Biginelli vs. Hantzsch reactions. Molecules 2006, 11, 731–738. [Google Scholar]
- Srinivasa, R.J.; Divya, V.; Shubha, J. One-pot three-component biginelli-type reaction to synthesize 5-carboxanilide-dihydropyrimidinones catalyzed by ionic liquids in aqueous media. J. Chem. Tech. Res. 2012, 4, 1720–1727. [Google Scholar]
- Gopalakrishnan, A.; Yeon, T.J. A convenient one-pot Biginelli reaction catalyzed by Y(OAc)3: An improved protocol for the synthesis of 3,4-dihydropyrimidin-2(1h)-ones and their sulfur analogues. Bull. Korean. Chem. Soc. 2010, 31, 863–868. [Google Scholar]
- Haline, G.O.; Alvim, T.B.; de Lima, H.C.B.; de Oliveira, F.C.; Gozzo, J.L.; de Macedo, P.V.; Abdelnur, W.A.S.; Brenno, A.D.N. Ionic liquid effect over the biginelli reaction under homogeneous and heterogeneous catalysis. ACS Catal. 2013, 3, 1420–1430. [Google Scholar]
- Qu, H.; Li, X.; Mo, F.; Lin, X. Efficient synthesis of dihydropyrimidinones via a three-component Biginelli-type reaction of urea, alkylaldehyde and arylaldehyde. Beilstein J. Org. Chem. 2013, 9, 2846–2851. [Google Scholar] [CrossRef] [PubMed]
- Pasunooti, K.K.; Chai, H.; Jensen, C.N.; Gorityala, B.K.; Wang, S.; Liu, X.W. A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions. Tetrahedron Lett. 2011, 52, 80–84. [Google Scholar] [CrossRef]
- Ding, D.; Zhao, C.G. Primary amine-catalyzed biginelli reaction for the enantioselective synthesis of 3,4-dihydropyrimidin-2(1h)-ones. Eur. J. Org. Chem. 2010, 20, 3802–3805. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Xu, X.Y.; Liu, H.; Cun, L.F.; Gong, L.Z. Highly enantioselective organocatalytic biginelli reaction. J. Am. Chem. Soc. 2006, 128, 14802–14803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J.; Miao, Z.; Chen, R. Bifunctional primary amine-thiourea–TfOH(BPAT·TfOH) as a chiral phase-transfer catalyst: The asymmetric synthesis of dihydropyrimidines. Org. Biomol. Chem. 2011, 9, 3050–3054. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of Biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Hajra, A.; Jana, U. Indium(III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: An improved procedure for the Biginelli reaction. J. Org. Chem. 2000, 65, 6270–6272. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qian, C.; Wang, L.; Yang, M. Lanthanide triflate catalyzed Biginelli reaction. one-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 2000, 65, 3864–3868. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, H. Iron(III)-catalyzed synthesis of dihydropyrimidinones. Improved conditions for the Biginelli reaction. Synlett 2000, 1, 63–64. [Google Scholar]
- Litvic, M.; Vecenaj, I.; Ladisic, Z.M.; Lovric, M.; Vinkovic, V.; Filipan-Litvic, M. First application of hexaaquaaluminium(III) tetrafluoroborate as a mild, recyclable, non-hygroscopic acid catalyst in organic synthesis: A simple and efficient protocol for the multigram scale synthesis of 3,4-dihydropyrimidinones by Biginelli reaction. Tetrahedron 2010, 66, 3463–3471. [Google Scholar] [CrossRef]
- Dharma Rao, G.B.; Acharya, B.N.; Verma, S.K.; Kaushik, M.P. N,N′-Dichlorobis(2,4,6-trichlorophenyl)urea(CC-2) as a new reagent for the synthesis of pyrimidone and pyrimidine derivatives via Biginelli reaction. Tetrahedron Lett. 2011, 52, 809–812. [Google Scholar]
- Narahari, S.R.; Reguri, B.R.; Gudaparthi, O.; Mukkanti, K. Synthesis of dihydropyrimidinones via Biginelli multi-component reaction. Tetrahedron Lett. 2012, 53, 1543–1545. [Google Scholar] [CrossRef]
- Konkala, K.; Sabbavarapu, N.M.; Katla, R.; Durga, N.Y.V.; Reddy, T.V.K.; Devi, B.; Prasad, R.B.N. Revisit to the Biginelli reaction: A novel and recyclable bioglycerol-based sulfonic acid functionalized carbon catalyst for one-pot synthesis of substituted 3,4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett. 2012, 53, 1968–1973. [Google Scholar] [CrossRef]
- Da Silva, D.L.; Fernandes, S.A.; Sabino, A.A.; de Fatima, A. p-Sulfonic acid calixarenes as efficient and reusable organocatalysts for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/-thiones. Tetrahedron Lett. 2011, 52, 6328–6330. [Google Scholar] [CrossRef]
- Javad, S.G.; Raheleh, T.; Abolfazl, Z. A green synthesis of 3,4-dihydropyrimidine-2(1H)-one/thione derivatives using nanosilica-supported tin(II) chloride as a heterogeneous nanocatalyst. Monatsh. Chem. 2013, 144, 1865–1870. [Google Scholar]
- Zhang, Y.; Wang, B.; Zhang, X.; Huang, J.; Liu, C. An efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones catalyzed by a novel brønsted acidic ionic liquid under solvent-free conditions. Molecules 2015, 20, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Bose, A.; Mal, P. Solvent-free ball-milling Biginelli reaction by subcomponent synthesis. Eur. J. Org. Chem. 2015, 32, 6994–6998. [Google Scholar] [CrossRef]
- Ould M’hamed, M.; Alduaij, O.K. An efficient one-pot synthesis of new 2-thioxo and 2-oxo-pyrimidine-5-carbonitriles in ball-milling under solvent-free and catalyst-free conditions. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 235–241. [Google Scholar] [CrossRef]
| Entry * | BRR (Balls-to-Reagents Weight Ratio) | Time (min) | Conversion (%) |
|---|---|---|---|
| 1 | 1:1 | 720 | 00 |
| 2 | 2:1 | 720 | 00 |
| 3 | 3:1 | 720 | 20 |
| 4 | 4:1 | 720 | 40 |
| 5 | 5:1 | 300 | 70 |
| 6 | 6:1 | 360 | 100 |
| 7 | 7:1 | 180 | 100 |
| 8 | 8:1 | 30 | 100 |
| Entry | R | X | Product | Yield (%) | M.P. (°C) |
|---|---|---|---|---|---|
| Found Reported References | |||||
| 1 | C6H5– | O | 4a | >98 | 200–202 (202–204) [40] |
| 2 | 4-CH3–C6H4– | O | 4b | >98 | 214–216 (215–217) [39] |
| 3 | 4-Cl–C6H4– | O | 4c | >98 | 208–210 (207–210) [40] |
| 4 | 4-CH3O–C6H4– | O | 4d | >98 | 204–207 (203–205) [40] |
| 5 | 4-OH–C6H4– | O | 4e | >98 | 221–223 (224–227) [40] |
| 6 | 4-O2N–C6H4– | O | 4f | >98 | 208–210 (206–208) [39] |
| 7 | C6H5– | S | 4g | >98 | 209–211 (206–208) [39] |
| 8 | 4-CH3–C6H4– | S | 4h | >98 | 187–189 (186–188) [39] |
| 9 | 4-Cl–C6H4– | S | 4i | >98 | 182–184 (181–183) [39] |
| 10 | 4-CH3O–C6H4– | S | 4j | >98 | 155–157 (151–153) [39] |
| 11 | 4-OH–C6H4– | S | 4k | >98 | 206–208 (200–202) [40] |
| 12 | 4-O2N–C6H4– | S | 4l | >98 | 197–199 (191–193) [39] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ould M’hamed, M.; Alshammari, A.G.; Lemine, O.M. Green High-Yielding One-Pot Approach to Biginelli Reaction under Catalyst-Free and Solvent-Free Ball Milling Conditions. Appl. Sci. 2016, 6, 431. https://doi.org/10.3390/app6120431
Ould M’hamed M, Alshammari AG, Lemine OM. Green High-Yielding One-Pot Approach to Biginelli Reaction under Catalyst-Free and Solvent-Free Ball Milling Conditions. Applied Sciences. 2016; 6(12):431. https://doi.org/10.3390/app6120431
Chicago/Turabian StyleOuld M’hamed, Mohamed, Abdulrahman G. Alshammari, and O. M. Lemine. 2016. "Green High-Yielding One-Pot Approach to Biginelli Reaction under Catalyst-Free and Solvent-Free Ball Milling Conditions" Applied Sciences 6, no. 12: 431. https://doi.org/10.3390/app6120431






