Distinguishing Bovine Fecal Matter on Spinach Leaves Using Field Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Field Spectroscopy
2.3. Spectroscopic Data Analysis
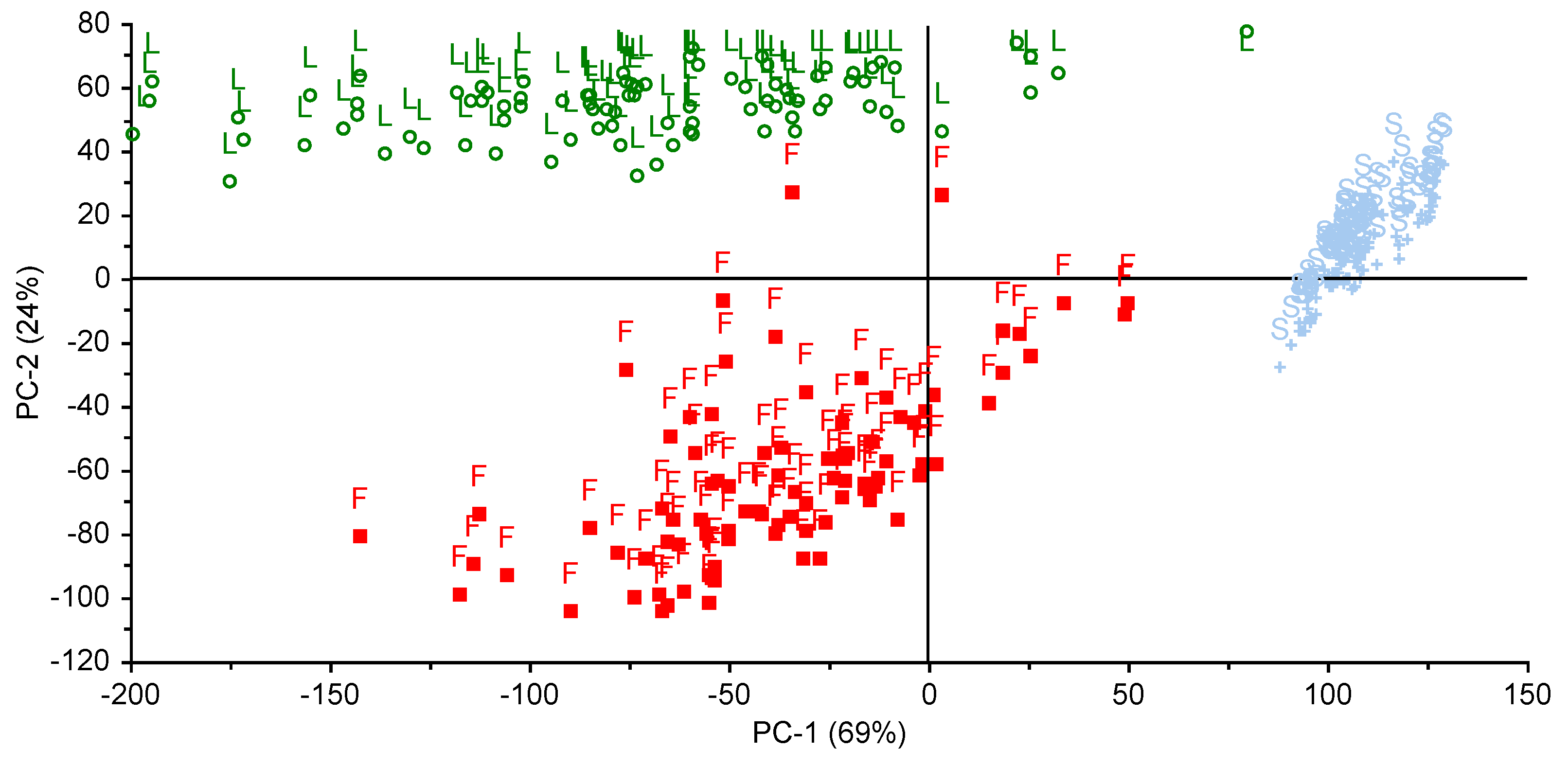
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Everard, C.D.; Kim, M.S.; Cho, H.; O’Donnell, C.P. Hyperspectral fluorescence imaging using violet LEDs as excitation sources for fecal matter contaminate identification on spinach leaves. J. Food Meas. Charact. 2016, 10, 56–63. [Google Scholar] [CrossRef]
- Brandl, M.T. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl. Environ. Microbiol. 2008, 74, 5285–5289. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Analysis and Evaluation of Preventive Control Measures for the Control and Reduction/Elimination of Microbial Hazards on Fresh and Fresh-Cut Produce; Center for Food Safety and Applied Nutrition, US FDA: Washington, DC, USA, 2001.
- Xicohtencatl-Cortes, J.; Sánchez Chacón, E.; Saldaña, Z.; Freer, E.; Girón, J.A. Interaction of Escherichia coli O157:H7 with leafy green produce. J. Food Prot. 2009, 72, 1531–1537. [Google Scholar] [PubMed]
- Armstrong, G.L.; Hollingsworth, J.; Morris, J.G., Jr. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiologic Rev. 1996, 18, 29–51. [Google Scholar] [CrossRef]
- Wiederoder, M.S.; Liu, N.; Lefcourt, A.M.; Kim, M.S.; Lo, Y.M. Use of a portable hyperspectral imaging system for monitoring the efficacy of sanitation procedures in produce processing plants. J. Food Eng. 2013, 117, 217–226. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). Preliminary incidence and trends of infection with pathogens transmitted commonly through food—Foodborne diseases active surveillance network. Morb. Mortal. Wkly. Rep. 2015, 64, 495–499. [Google Scholar]
- Kim, M.S.; Chappelle, E.W.; Corp, L.A.; McMurtrey, J.E. The contribution of chlorophyll fluorescence to the reflectance spectra of green vegetation. In Proceedings of the IGARSS '93–IEEE International Geoscience and Remote Sensing Symposium, Tokyo, Japan, 18–21 August 1993; pp. 1325–1328.
- Lichtenthaler, H.K.; Buschmann, C.; Rinderle, U.; Schmuck, G. Application of chlorophyll fluorescence in ecophysiology. Radiat. Environ. Biophys. 1986, 25, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Chappelle, E.W.; Kim, M.S.; Mulchi, C.L.; Daughtry, C.S.T.; McMurtrey, J.E.; Corp, L.A. Laser Induced Fluorescence (LIF) as a Remote Sensing Tool: A Review; Technical Report for NASA Goddard Space Flight Center: Greenbelt, MD, USA, January 1999. [Google Scholar]
- Campbell, P.K.E.; Middleton, E.M.; Corp, L.A.; Kim, M.S. Contribution of chlorophyll fluorescence to the apparent vegetation reflectance. Sci. Total Environ. 2008, 404, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Guanter, L.; Rossini, M.; Colombo, R.; Meroni, M.; Frankenberg, C.; Lee, J.E.; Joiner, J. Using field spectroscopy to assess the potential of statistical approaches for the retrieval of sun-induced chlorophyll fluorescence from ground and space. Remote Sens. Environ. 2013, 133, 52–61. [Google Scholar] [CrossRef]
- Milton, E.J.; Schaepman, M.E.; Anderson, K.; Kneubühler, M.; Fox, N. Progress in field spectroscopy. Remote Sens. Environ. 2009, 113, S92–S109. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Kriebel, K.T. Average variability of the radiation reflected by vegetated surfaces due to differing irradiations. Remote Sens. Environ. 1978, 7, 81–83. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Meroni, M.; Colombo, R. 3S: A novel program for field spectroscopy. Comput. Geosci. 2009, 35, 1491–1496. [Google Scholar] [CrossRef]
- Everard, C.D.; Kim, M.S.; Lee, H. A comparison of hyperspectral reflectance and fluorescence imaging techniques for detection of contaminants on spinach leaves. J. Food Eng. 2014, 143, 139–145. [Google Scholar] [CrossRef]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]




| Sample | MC, g 100 g−1 | OM, g 100 g−1 (db) |
|---|---|---|
| Fecal Matter | 88.0 ± 0.3 | 88.6 ± 0.1 |
| Soil | 19.2 ± 1.1 | 2.1 ± 0.3 |
| Date | Sun’s Elevation | Sun’s Azimuth | Temperature (°C) | Humidity (%) | Conditions |
|---|---|---|---|---|---|
| 9 July 2015 | 73.07° | 169.72° | 31 | 55 | Scattered Clouds |
| 10 July 2015 | 72.95° | 169.67° | 27 | 51 | Scattered Clouds |
| 15 July 2015 | 72.22° | 169.56° | 25 | 78 | Clear |
| 17 July 2015 | 71.88° | 169.59° | 26 | 61 | Overcast |
| 21 July 2015 | 71.15° | 169.74° | 29 | 66 | Overcast |
| Sample Type | Minimum Value Observed | Maximum Value Observed |
|---|---|---|
| Fecal Matter on Leaf | 1.05 | 1.67 |
| Soil on Leaf | 0.85 | 0.95 |
| Uncontaminated Leaf | 2.03 | 3.75 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Everard, C.D.; Kim, M.S.; O’Donnell, C.P. Distinguishing Bovine Fecal Matter on Spinach Leaves Using Field Spectroscopy. Appl. Sci. 2016, 6, 246. https://doi.org/10.3390/app6090246
Everard CD, Kim MS, O’Donnell CP. Distinguishing Bovine Fecal Matter on Spinach Leaves Using Field Spectroscopy. Applied Sciences. 2016; 6(9):246. https://doi.org/10.3390/app6090246
Chicago/Turabian StyleEverard, Colm D., Moon S. Kim, and Colm P. O’Donnell. 2016. "Distinguishing Bovine Fecal Matter on Spinach Leaves Using Field Spectroscopy" Applied Sciences 6, no. 9: 246. https://doi.org/10.3390/app6090246





