The Effect of Magnetic Field on Thermal-Reaction Kinetics of a Paramagnetic Metal Hydride Storage Bed
Abstract
:Featured Application
Abstract
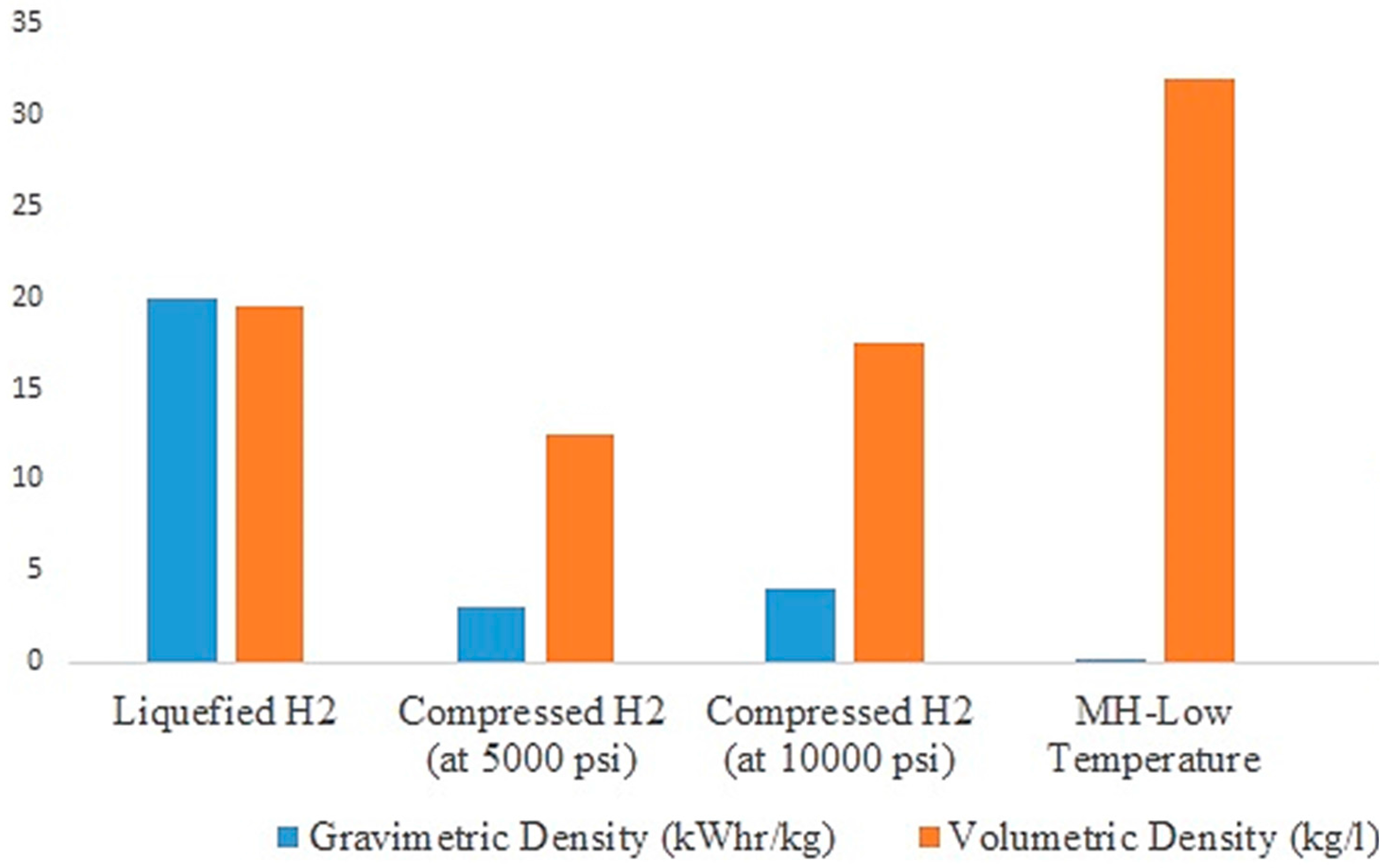
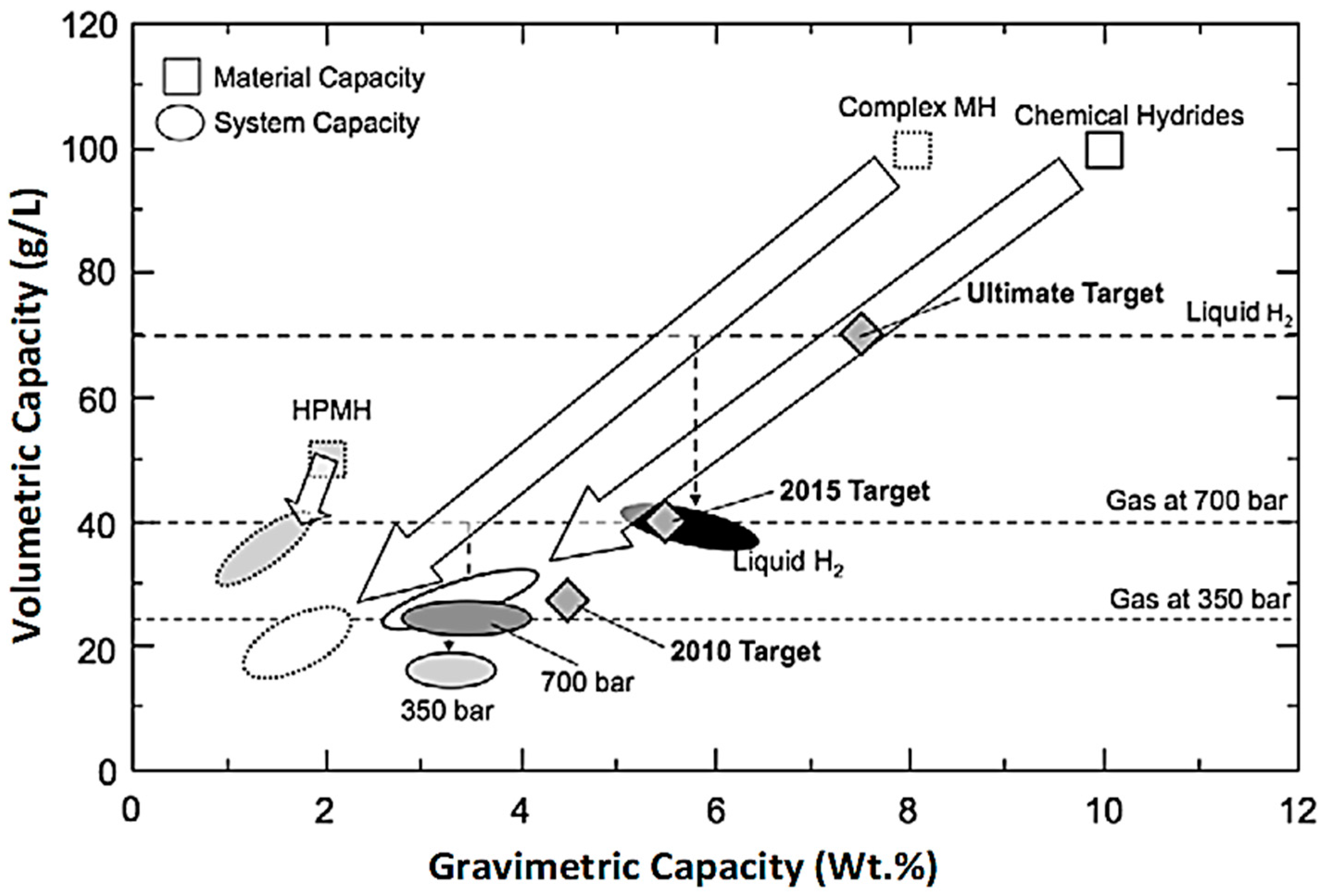
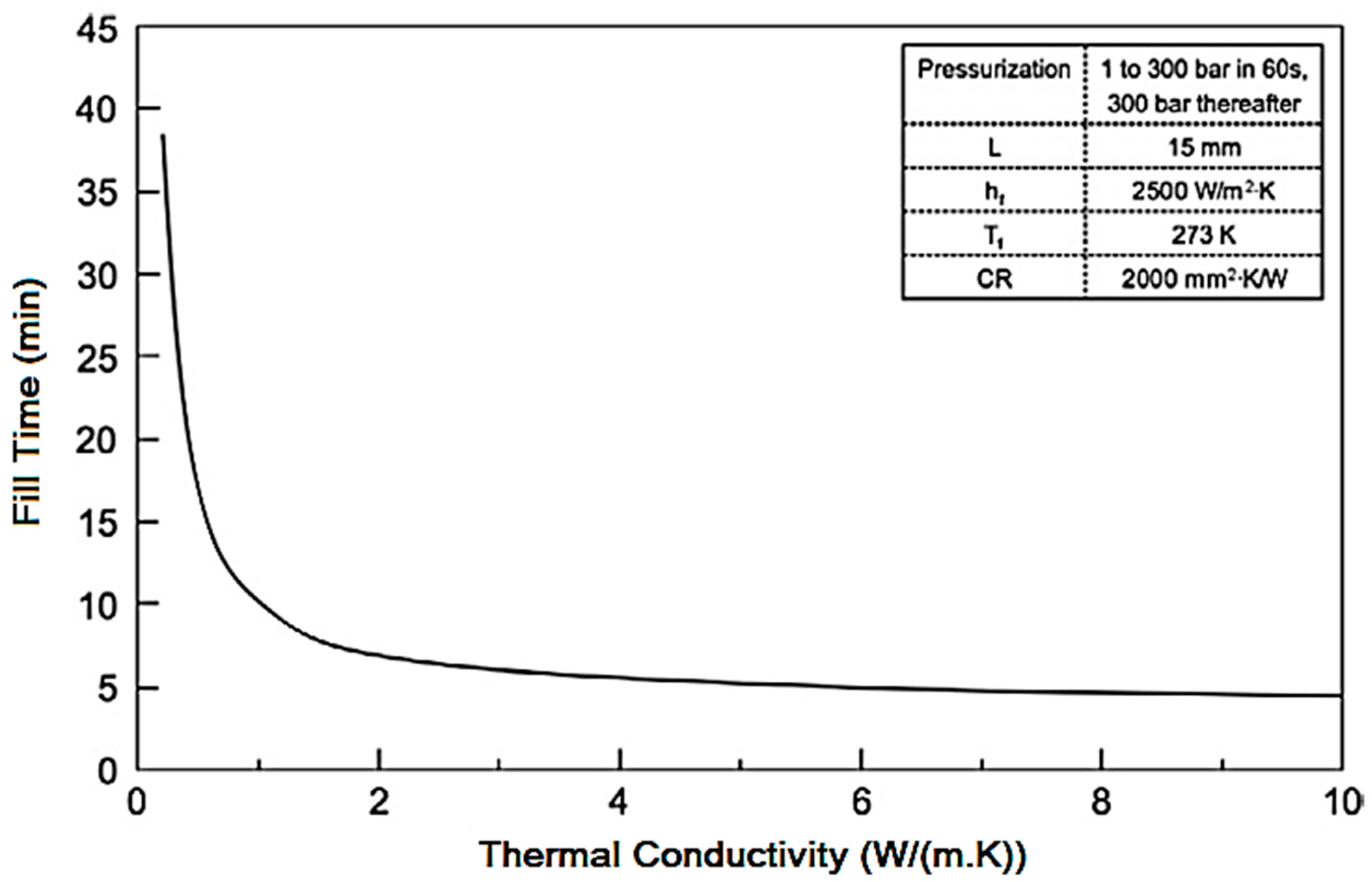
1. Introduction
2. Experimental Setup
2.1. Storage Material and Magnets
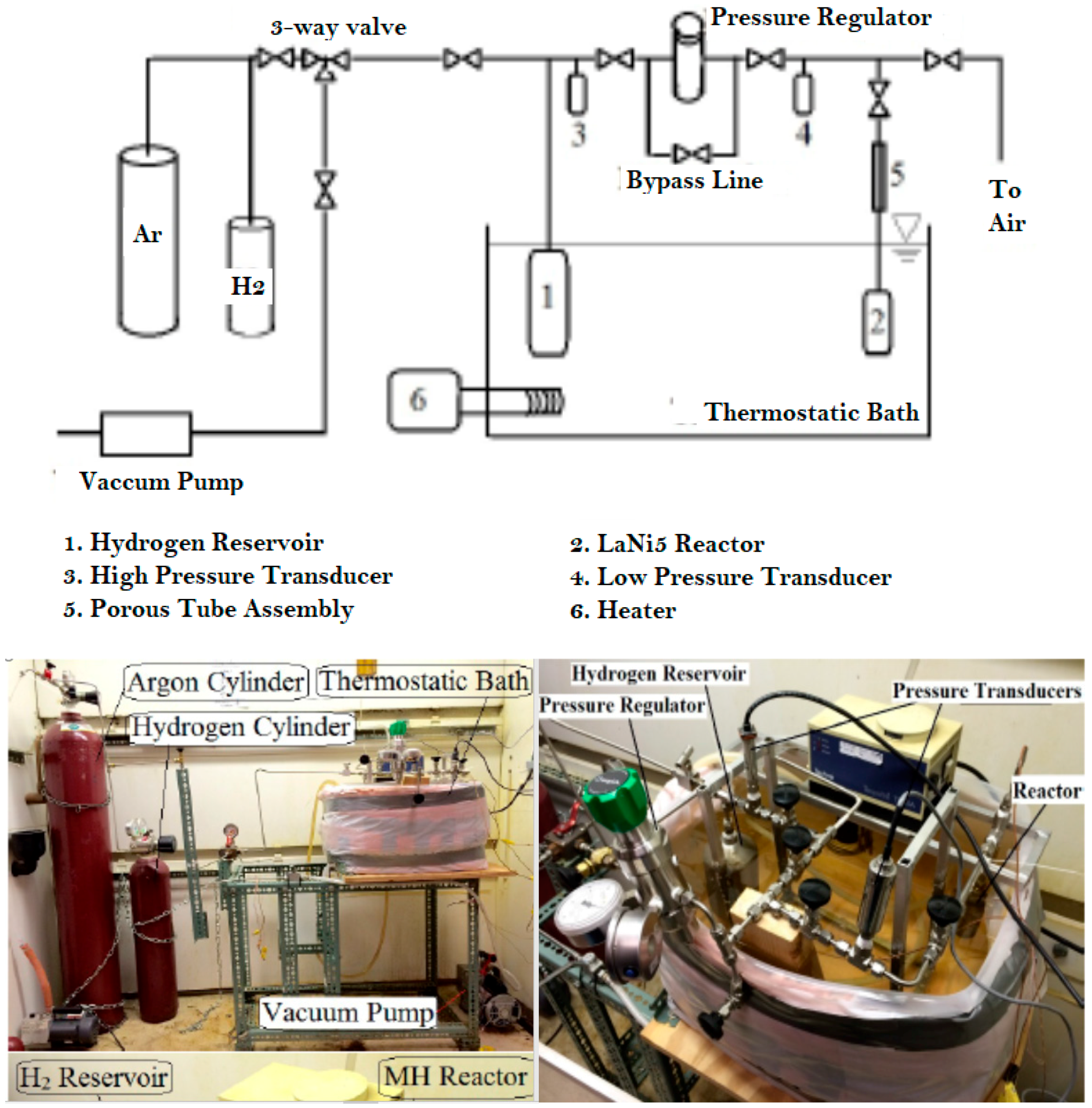
2.2. Metal Hydride Hydrogen Storage System Setup
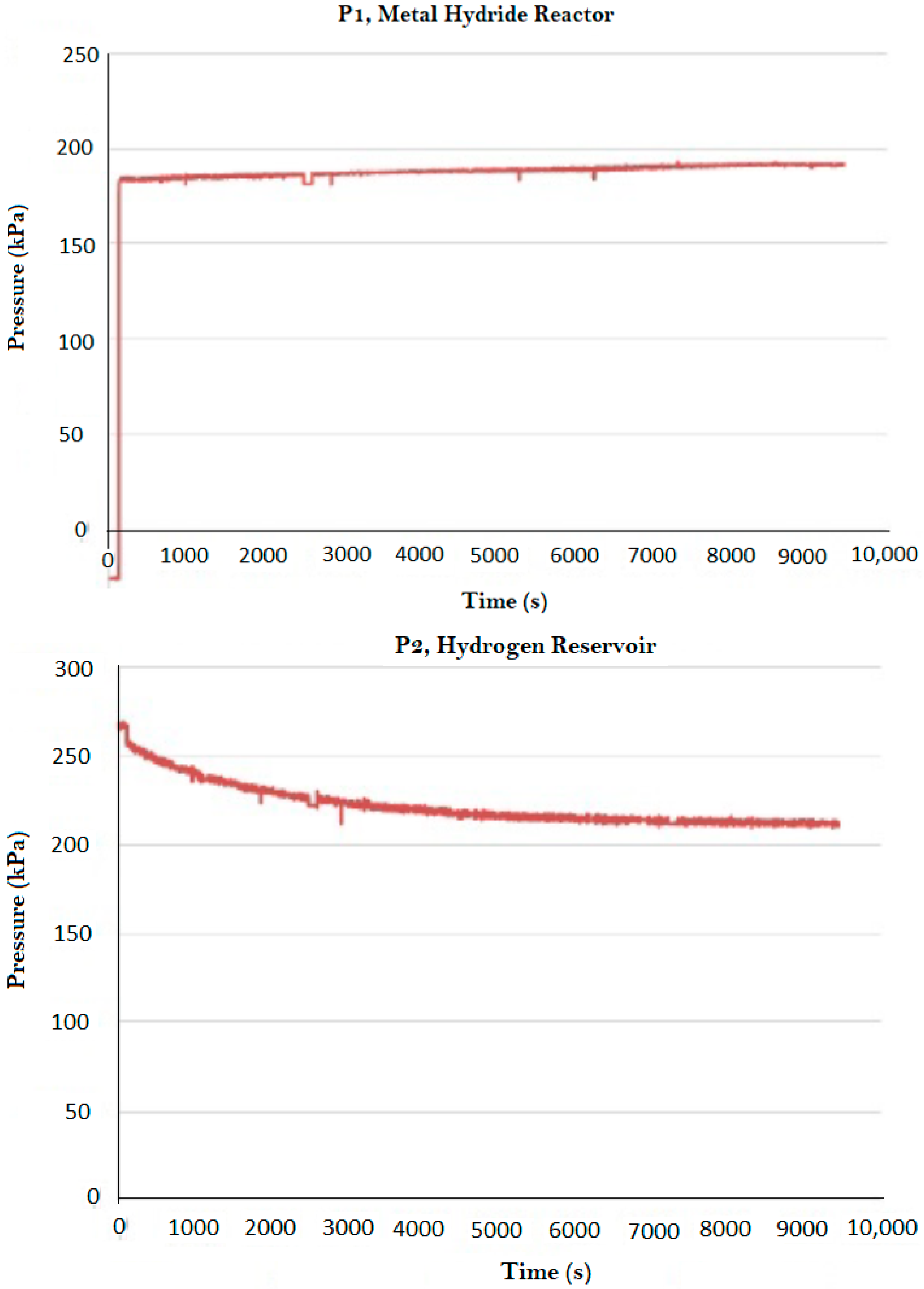
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harries, D.N.; Paskevivius, M.; Sheppard, D.A.; Price, T.E.C.; Buckley, C.E. Concentrating Solar Thermal Heat Storage Using Metal Hydrides. Proc. IEEE 2012, 100, 539–549. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of Hydrogen Storage Techniques for on Board Vehicle Applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Gardiner, M.R.; Burke, A. Comparison of hydrogen storage technologies: A focus on energy required for hydrogen input. ACS Div. Fuel Chem. 2002, 47, 794–795, (Preprints). [Google Scholar]
- Muthukumar, P.; Groll, M. Metal Hydride Based Heating and Cooling Systems: A Review. Int. J. Hydrogen Energy 2010, 35, 3817–3831. [Google Scholar] [CrossRef]
- Werner, R.; Groll, M. Two-stage metal hydride heat transformer laboratory model: Results of reaction bed tests. J. Less Common Met. 1991, 172, 1122–1129. [Google Scholar] [CrossRef]
- Groll, M. Reaction beds for dry sorption machines. Heat Recovery Syst. CHP 1993, 13, 341–346. [Google Scholar] [CrossRef]
- Supper, W.; Groll, M.; Mayer, U. Reaction kinetics in metal hydride reaction beds with improved heat and mass transfer. J. Less Common Met. 1984, 104, 279–286. [Google Scholar] [CrossRef]
- Aardahl, C.L.; Rassat, S.D. Overview of Systems Considerations for On-Board Chemical Hydrogen Storage. Int. J. Hydrogen Energy 2009, 34, 6676–6683. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Marder, T.B. B–N versus C–C: How similar are they? Angew. Chem. Int. Ed. 2008, 47, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Marder, T.B. Will we soon be fueling our automobiles with ammonia–borane? Angew. Chem. Int. Ed. 2007, 46, 8116–8118. [Google Scholar] [CrossRef] [PubMed]
- Kong, V.C.Y.; Kirk, D.W.; Foulkes, F.R.; Hinatsu, J.T. Development of hydrogen storage for fuel cell generators. II: Utilization of calcium hydride and lithium hydride. Int. J. Hydrogen Energy 2003, 28, 205–214. [Google Scholar] [CrossRef]
- Kim, K.J.; Montoya, B.; Razani, A.; Lee, K.H. Metal Hydride Compacts of Improved Thermal Conductivity. Int. J. Hydrogen Energy 2001, 26, 609–613. [Google Scholar] [CrossRef]
- Visaria, M.; Mudawar, I.; Pourpoint, T.; Kumar, S. Study of heat transfer and kinetics parameters influencing the design of heat exchangers for hydrogen storage in high-pressure metal hydrides. Int. J. Heat Mass Transf. 2010, 53, 2229–2239. [Google Scholar] [CrossRef]
- Walker, G. Solid-State Hydrogen Storage: Materials and Chemistry; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar]
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 4569–4575. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.; Dailly, A. Thermodynamic study of the adsorbed hydrogen phase in Cu-based metal-organic frameworks at cryogenic temperatures. J. Phys. Chem. C 2008, 112, 13047–13052. [Google Scholar] [CrossRef]
- Mellouli, S.; Askri, F.; Dhaou, H.; Jemni, A.; Nasrallah, S.B. Parametric Studies on a Metal-Hydride Cooling System. Int. J. Hydrogen Energy 2009, 34, 3945–3952. [Google Scholar] [CrossRef]
- Kim, K.J.; Feldman, K.T.; Lloyd, G.; Razani, A.; Shanahan, K.L. Performance of High Power Metal Hydride Reactors. Int. J. Hydrogen Energy 1998, 23, 355–362. [Google Scholar] [CrossRef]
- Suda, S.; Komazaki, Y.; Kobayashi, N. Effective thermal conductivity of metal hydride beds. J. Less Common Met. 1983, 89, 317–324. [Google Scholar] [CrossRef]
- Yang, F.S.; Wang, G.X.; Zhang, Z.X.; Meng, X.Y.; Rudolph, V. Design of the Metal Hydride Reactors—A Review on the Key Technical Issues. Int. J. Hydrogen Energy 2010, 35, 3832–3840. [Google Scholar] [CrossRef]
- Mellouli, S.; Askri, F.; Dhaou, H.; Jemni, A.; Nasrallah, S.B. Numerical Study of Heat Exchanger Effects on Charge/Discharge Times of Metal-Hydrogen Storage Vessel. Int. J. Hydrogen Energy 2009, 34, 3005–3017. [Google Scholar] [CrossRef]
- Kaplan, Y.; Veziroglu, T.N. Mathematical Modelling of Hydrogen Storage in a LaNi5 Hydride Bed. Int. J. Energy Res. 2003, 27, 1027–1038. [Google Scholar] [CrossRef]
- Cipiti, F.F.; Cacciola, G. Finite Element-Based Simulation of a Metal Hydride-Based Hydrogen Storage Tank. Int. J. Hydrogen Energy 2009, 34, 8574–8582. [Google Scholar]
- Ghafir, M.F.A.; Batcha, M.F.M.; Raghavan, V.R. Prediction of the Thermal Conductivity of Metal Hydrides—The Inverse Problem. Int. J. Hydrogen Energy 2009, 34, 7125–7130. [Google Scholar] [CrossRef] [Green Version]
- Bershadsky, E.; cJosephy, Y.; Ron, M. Permeability and Thermal Conductivity of Porous Metallic Matrix Hydride Compacts. J. Less Common Met. 1989, 153, 65–78. [Google Scholar] [CrossRef]
- Ron, M.; Bershadsky, E.; Josephy, Y. The Thermal Conductivity of Porous Metal Matrix Hydride Compacts. J. Less Common Met. 1991, 172–174, 1138–1146. [Google Scholar] [CrossRef]
- Sanchez, R.; Klein, H.P.; Groll, M. Expanded Graphite as Heat Transfer Matrix in Metal Hydride Beds. Int. J. Hydrogen Energy 2003, 28, 515–527. [Google Scholar]
- Klein, H.P.; Groll, M. Heat Transfer Characteristics of Expanded Graphite Matrices in Metal Hydride Beds. Int. J. Hydrogen Energy 2004, 29, 1503–1511. [Google Scholar] [CrossRef]
- Lee, M.; Kim, K.J.; Hopkins, R.R.; Gawlik, K. Thermal Conductivity Measurements of Copper-Coated Metal Hydrides (LaNi5, Ca0.6Mm0.4Ni5, and LaNi4.75Al0.25) for Use in Metal Hydride Hydrogen Compression Systems. Int. J. Hydrogen Energy 2009, 34, 3185–3190. [Google Scholar] [CrossRef]
- Asano, K.; Yamazaki, Y.; Iijima, Y. Hydrogenation and Dehydrogenation Behavior of LaNi5−xCox (x = 0, 0.25, 2) Alloys Studied by Pressure Differential Scanning Calorimetry. Mater. Trans. 2002, 43, 1095–1099. [Google Scholar] [CrossRef]
- Dhaou, H.; Askri, F.; Salah, M.B.; Jemni, A.; Nasrallah, S.B.; Lamloumi, J. Measurement and Modelling of Kinetics of Hydrogen Sorption by LaNi5 and Two Related Pseudobinary Compounds. Int. J. Hydrogen Energy 2007, 32, 576–587. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Kumar, V.; Singh, S.K. High Capacity Hydrogen Storage: Basic Aspects, New Developments and Milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- Mosher, D.A.; Tang, X.; Brown, R.J.; Arsenault, S.; Saitta, S.; Laube, B.L.; Dold, R.H.; Anton, D.L. High Density Hydrogen Storage System Demonstration Using NaAlH4 Based Complex Compound Hydrides; United Technologies Research Center: East Hartford, CT, USA, 2007. [Google Scholar]
- Bahadori, R.; Gutierrez, H.; Manikonda, S.; Meinke, R. Monte Carlo method simulation for two-dimensional heat transfer in homogenous medium and proposed application to quench propagation simulation. IEEE Trans. Appl. Supercond. 2017, 27, 1–5. [Google Scholar] [CrossRef]
- Shafiee, S.; McCay, M.H. Different Reactor and Heat Exchanger Configurations for Metal Hydride Hydrogen Storage Systems—A Review. Int. J. Hydrogen Energy 2016, 41, 9462–9470. [Google Scholar] [CrossRef]
- Shafiee, S. A Method to Enhance the Heat Transfer Mechanism in Metal Particle Porous Beds with Applicability to Paramagnetic Materials for Hydrogen Storage. Ph.D. Thesis, Florida Institute of Technology, Melbourne, FL, USA, 2016. [Google Scholar]
- Shafiee, S.; McCay, M.H.; Kuravi, S. Effect of Magnetic Fields on Thermal Conductivity in a Ferromagnetic Packed Bed. Exp. Therm. Fluid Sci. 2017, 86, 160–167. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Guo, Q.T.; Yu, F.Y.; Li, J.; Zhang, J.; Li, T.J. Motion Behavior of Non-Metallic Particles under High Frequency Magnetic Field. Trans. Nonferr. Met. Soc. China 2009, 19, 674–680. [Google Scholar] [CrossRef]
- Mei, M.R.; Klausner, J.F.; Rahmatian, N. Interaction Forces Between Soft Magnetic Particles in Uniform and Non-Uniform Magnetic Fields. Acta Mech. Sin. 2010, 26, 921–929. [Google Scholar]
- Stucki, F.; Schlapbach, L. Magnetic Properties of LaNi5, FeTi, Mg2Ni and Their Hydrides. J. Less Common Met. 1980, 74, 143–151. [Google Scholar] [CrossRef]
- Kim, G.H.; Chun, C.H.; Lee, S.G.; Lee, J.Y. A Studey on the Microstructural Change of Surface of the Intermetallic Compound LaNi5 by Hydrogen Absorption. Scr. Metall. Mater. 1993, 29, 485–490. [Google Scholar] [CrossRef]
- Liu, N.; Ma, Q.M.; Xie, Z.; Liu, Y.; Li, Y.C. Structures, Stabilities and Magnetic Moments of Small Lanthanum-Nickel Clusters. Chem. Phys. Lett. 2007, 436, 184–188. [Google Scholar] [CrossRef]
- Alam, F.A.; Matar, S.F.; Nakhl, M.; Ouaini, N. Investigation of Changes in Crystal and Electronic Structures by Hydrogen within LaNi5 from First-Principles. Solid State Sci. 2009, 11, 1098–1106. [Google Scholar] [CrossRef]
- Albertini, F.; Canepa, F.; Cirafici, S.; Franceschi, E.A.; Napoletano, M.; Paoluzi, A.; Pareti, L.; Solzi, M. Composition Dependence of Magnetic and Magnetothermal Properties of Ni-Mn-Ga Shape Memory Alloys. J. Magn. Magn. Mater. 2004, 272–276, 2111–2112. [Google Scholar] [CrossRef]
- Marcos, J.; Manosa, L.; Planes, A.; Casanova, F.; Batlle, X.; Labarta, A.; Martinez, B. Magnetic Field Induced Entropy Change and Magnetoelasticity in Ni-Mn-Ga Alloys. J. Magn. Magn. Mater. 2004, 272–276, 224413. [Google Scholar] [CrossRef]
- Grechnev, G.E.; Logosha, A.V.; Panfilov, A.S.; Kuchin, A.G.; Vasijev, A.N. Effect of Pressure on the Magnetic Properties of YNi5, LaNi5, and CeNi5. Low Temp. Phys. 2011, 37, 138. [Google Scholar] [CrossRef]
- LaNi5; SDS No. 685933; Sigma-Aldrich: Saint Louis, MO, USA, 16 November 2017.
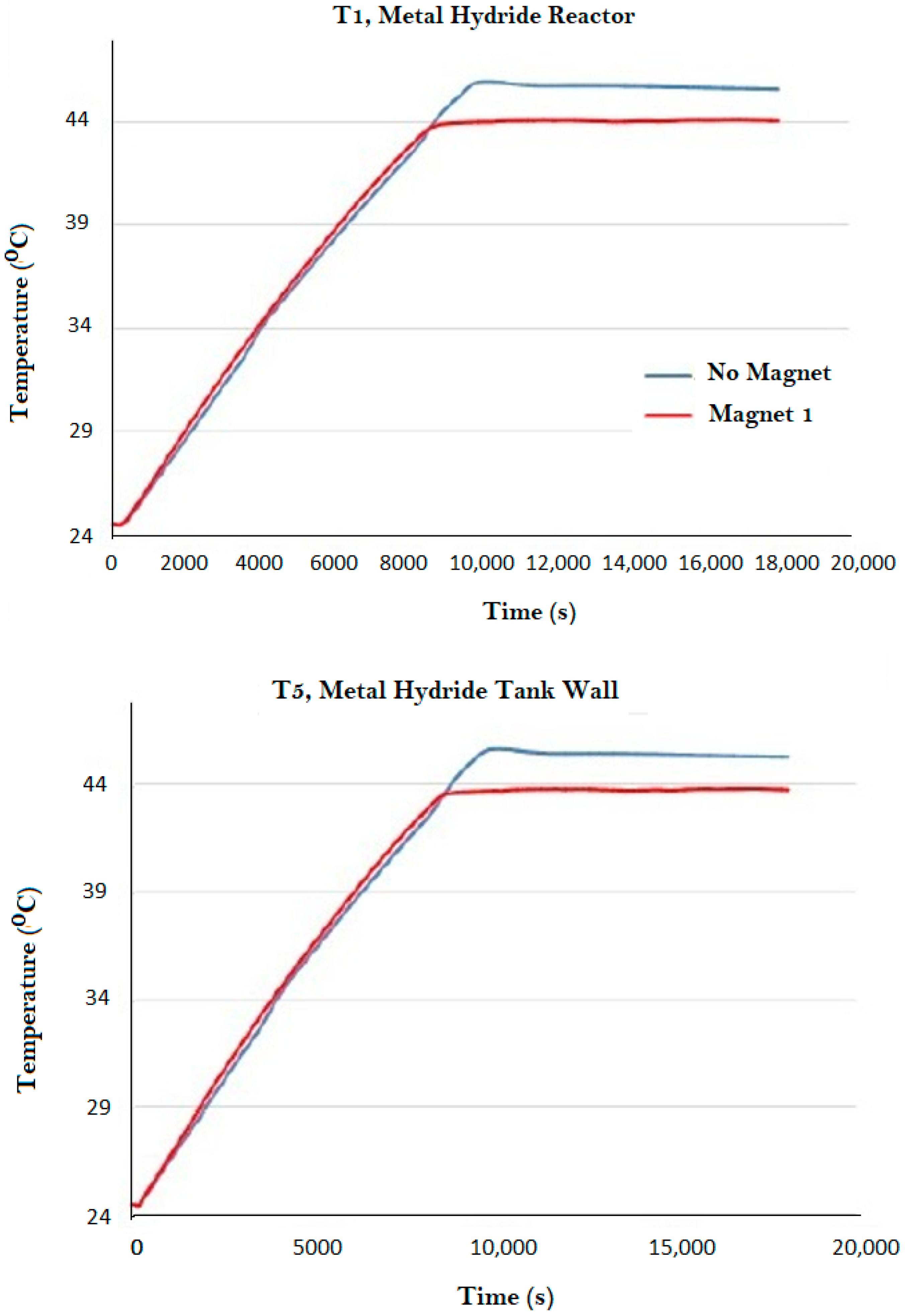
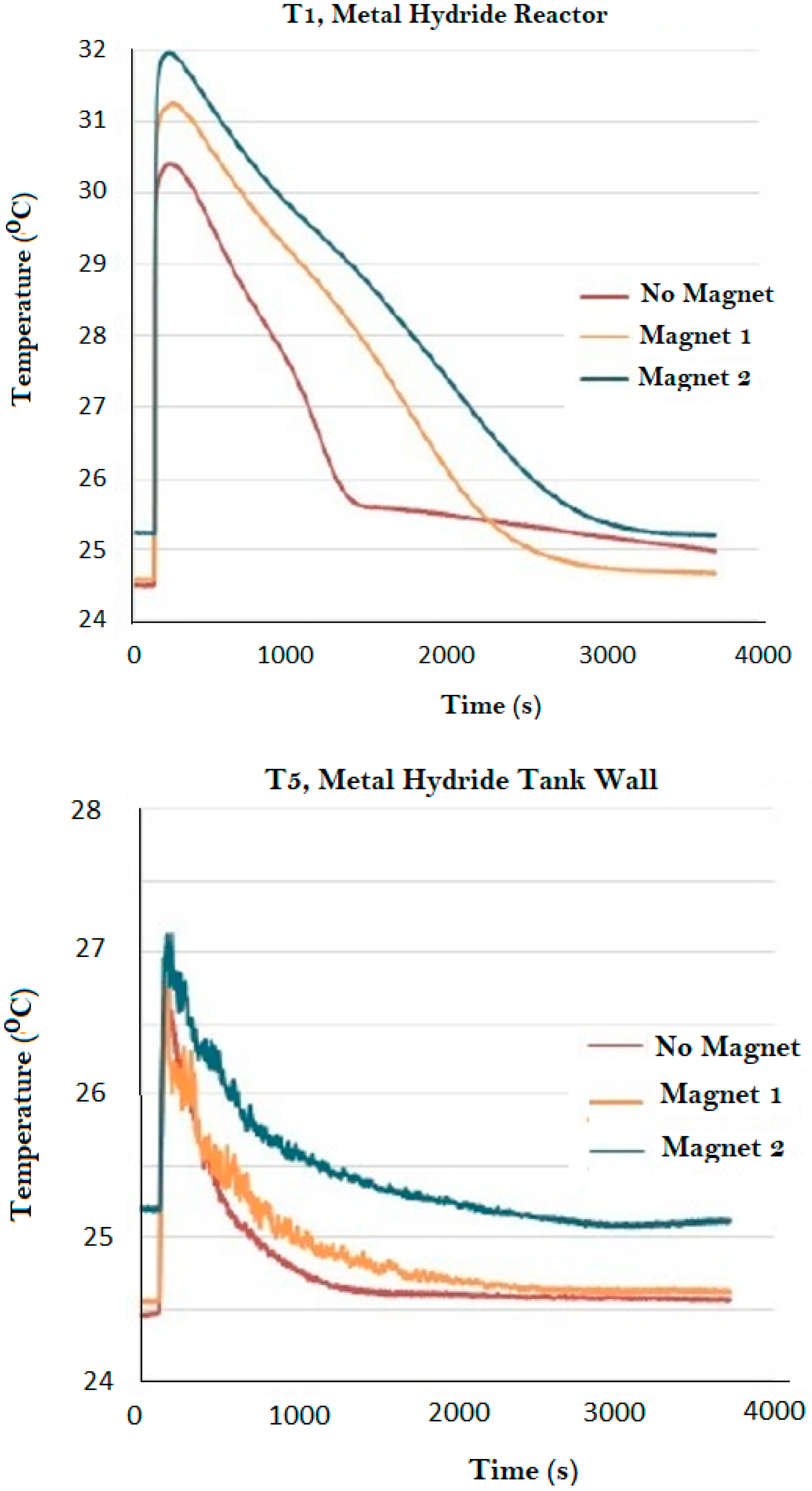
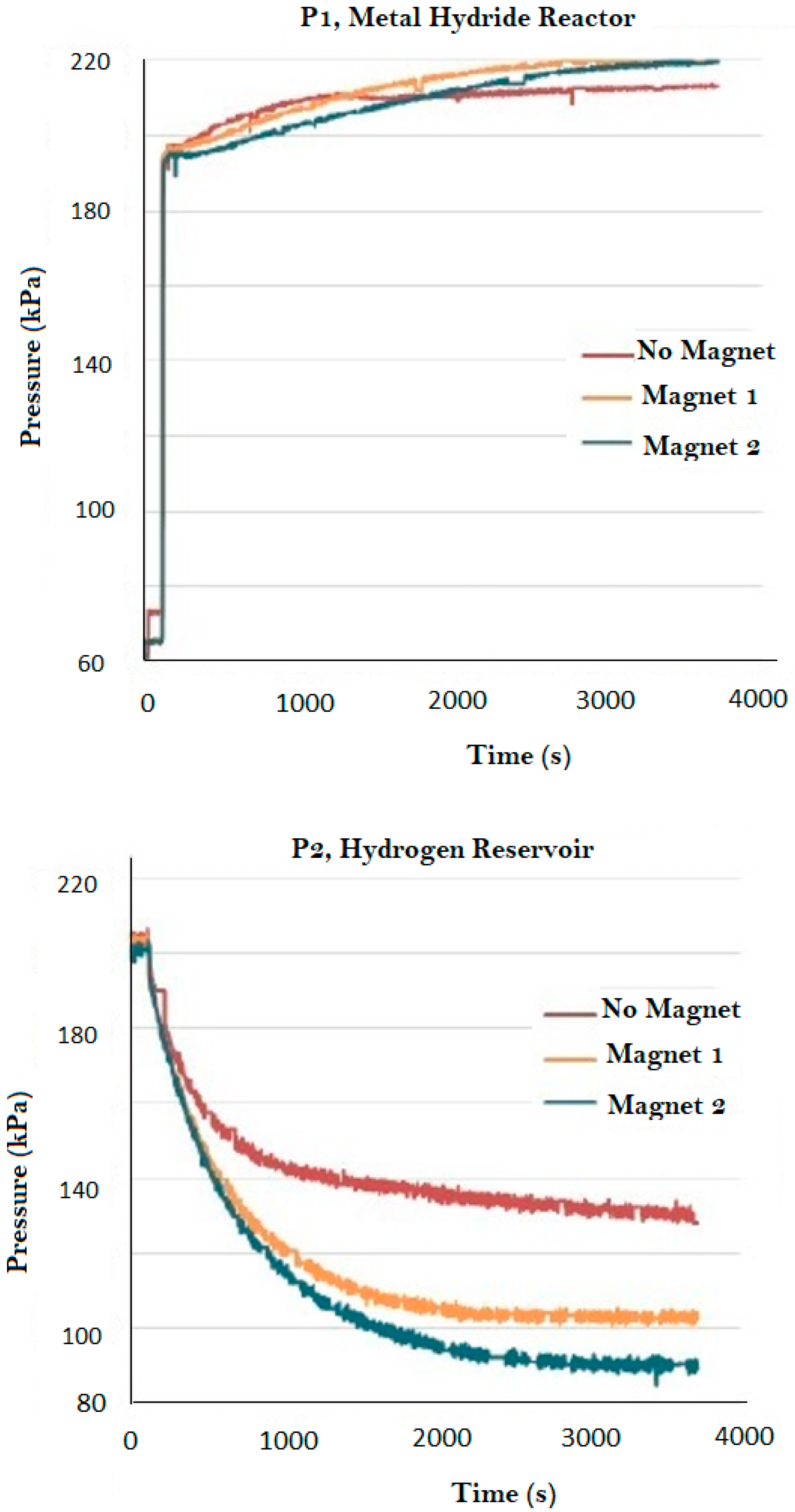
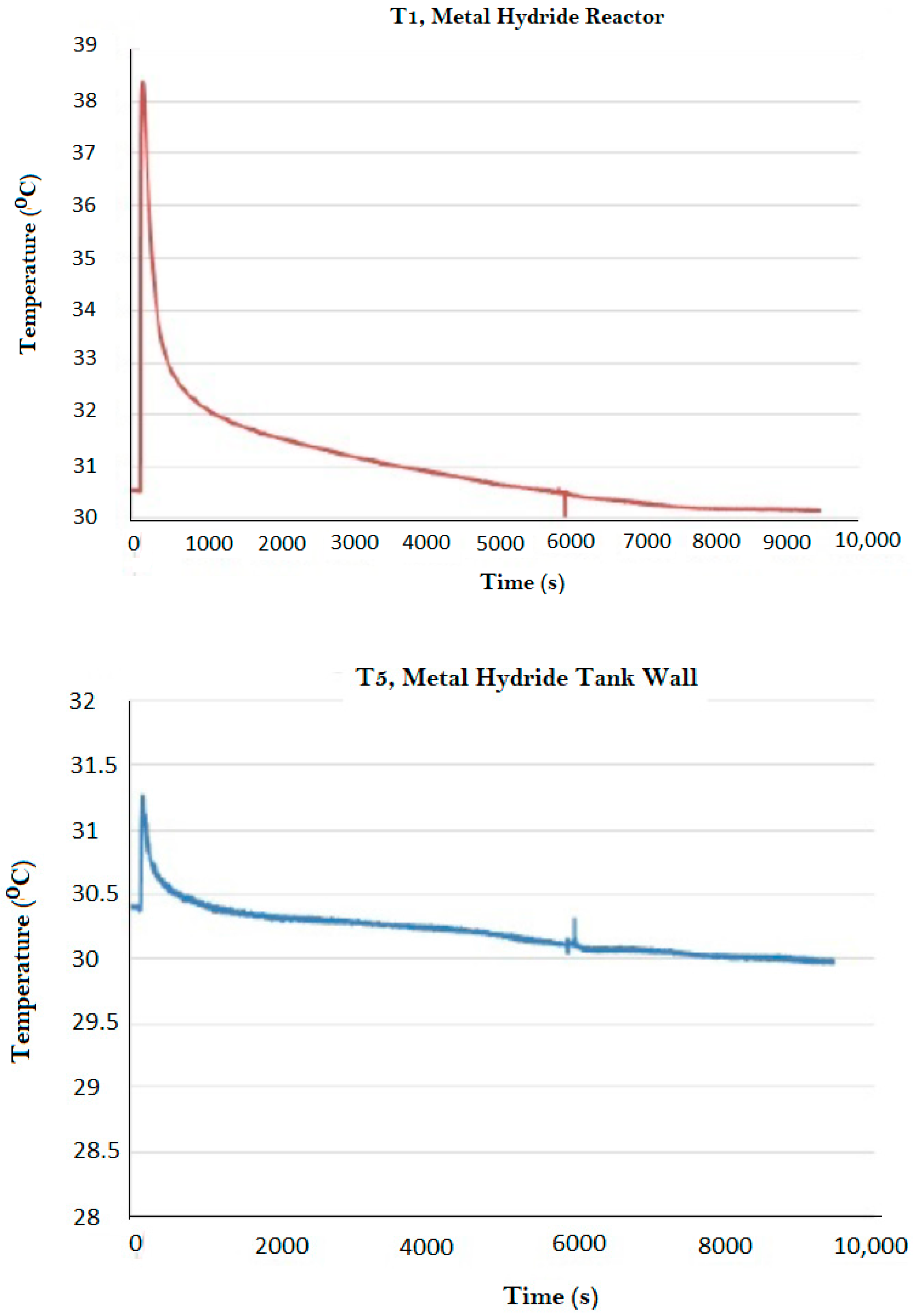
| Magnet Number | Thickness (cm) | Nominal Intensity (Gauss) |
|---|---|---|
| 1 | 1.27 | 2952 |
| 2 | 2.54 | 4667 |
| Thermocouple | Position |
|---|---|
| T1 | Metal hydride reactor |
| T2 | Hydrogen reservoir |
| T3 | Thermostatic bath |
| T4 | Room temperature |
| T5 | Reactor wall |
| Boundary Condition | Δm (gr) |
|---|---|
| No magnet | 0.619 |
| Magnet 1 | 0.818 |
| Magnet 2 | 0.909 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiee, S.; McCay, M.H.; Kuravi, S. The Effect of Magnetic Field on Thermal-Reaction Kinetics of a Paramagnetic Metal Hydride Storage Bed. Appl. Sci. 2017, 7, 1006. https://doi.org/10.3390/app7101006
Shafiee S, McCay MH, Kuravi S. The Effect of Magnetic Field on Thermal-Reaction Kinetics of a Paramagnetic Metal Hydride Storage Bed. Applied Sciences. 2017; 7(10):1006. https://doi.org/10.3390/app7101006
Chicago/Turabian StyleShafiee, Shahin, Mary Helen McCay, and Sarada Kuravi. 2017. "The Effect of Magnetic Field on Thermal-Reaction Kinetics of a Paramagnetic Metal Hydride Storage Bed" Applied Sciences 7, no. 10: 1006. https://doi.org/10.3390/app7101006





