Macroporous Activated Carbon Derived from Rapeseed Shell for Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Rapeseed Shell-Derived AC
2.2. Preparation of AC/S Composite
2.3. Material Characterization
2.4. Electrochemical Characterization
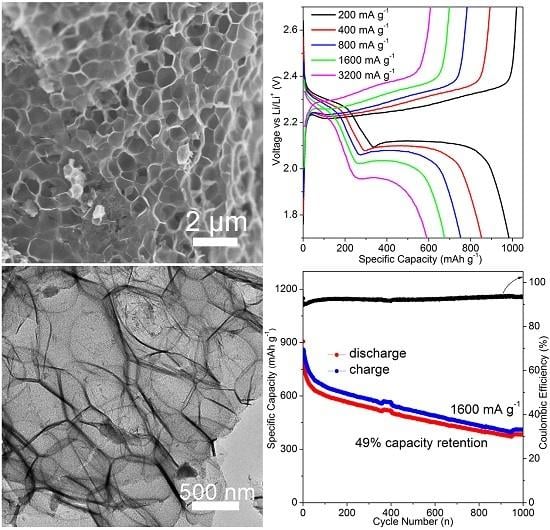
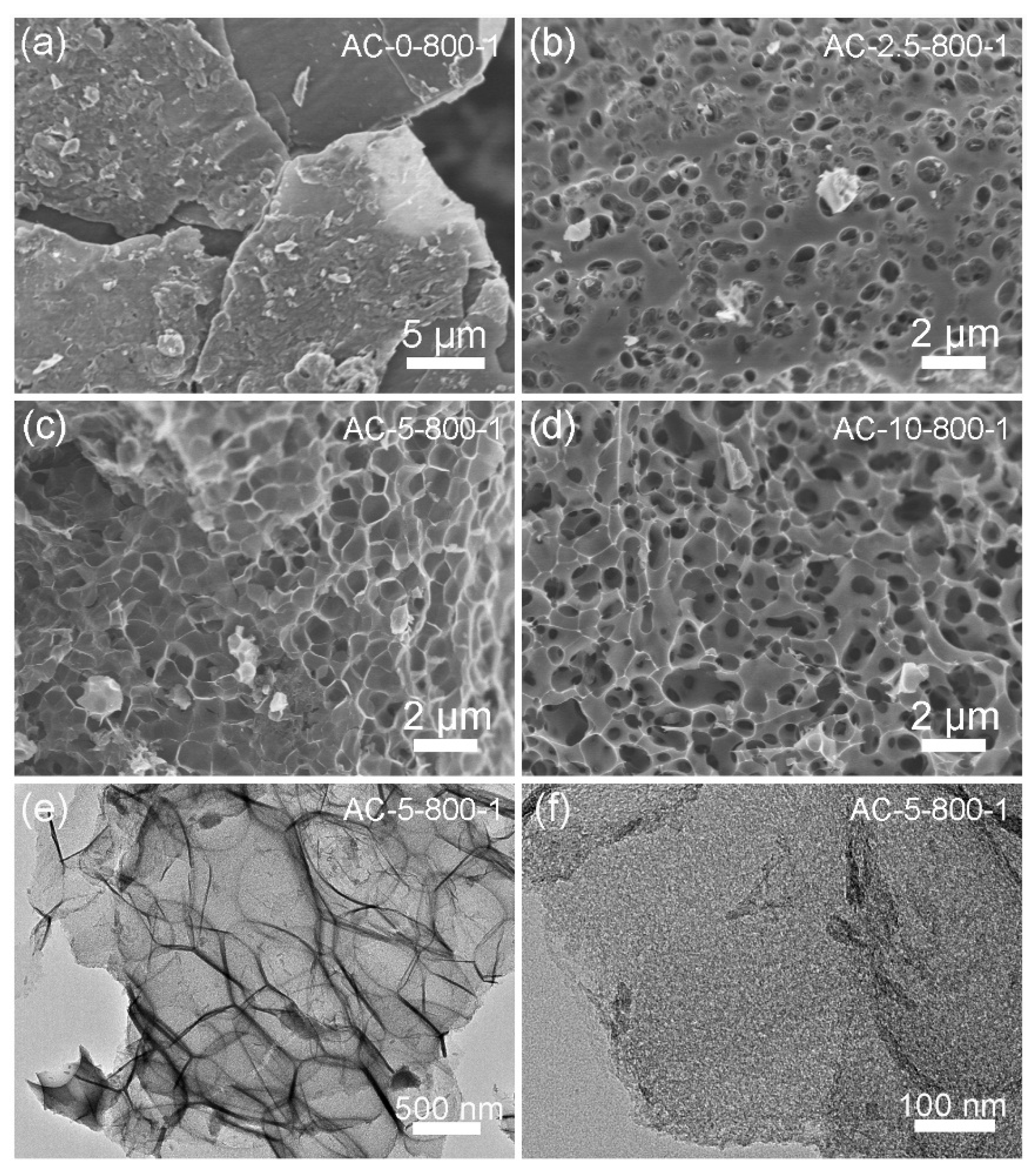
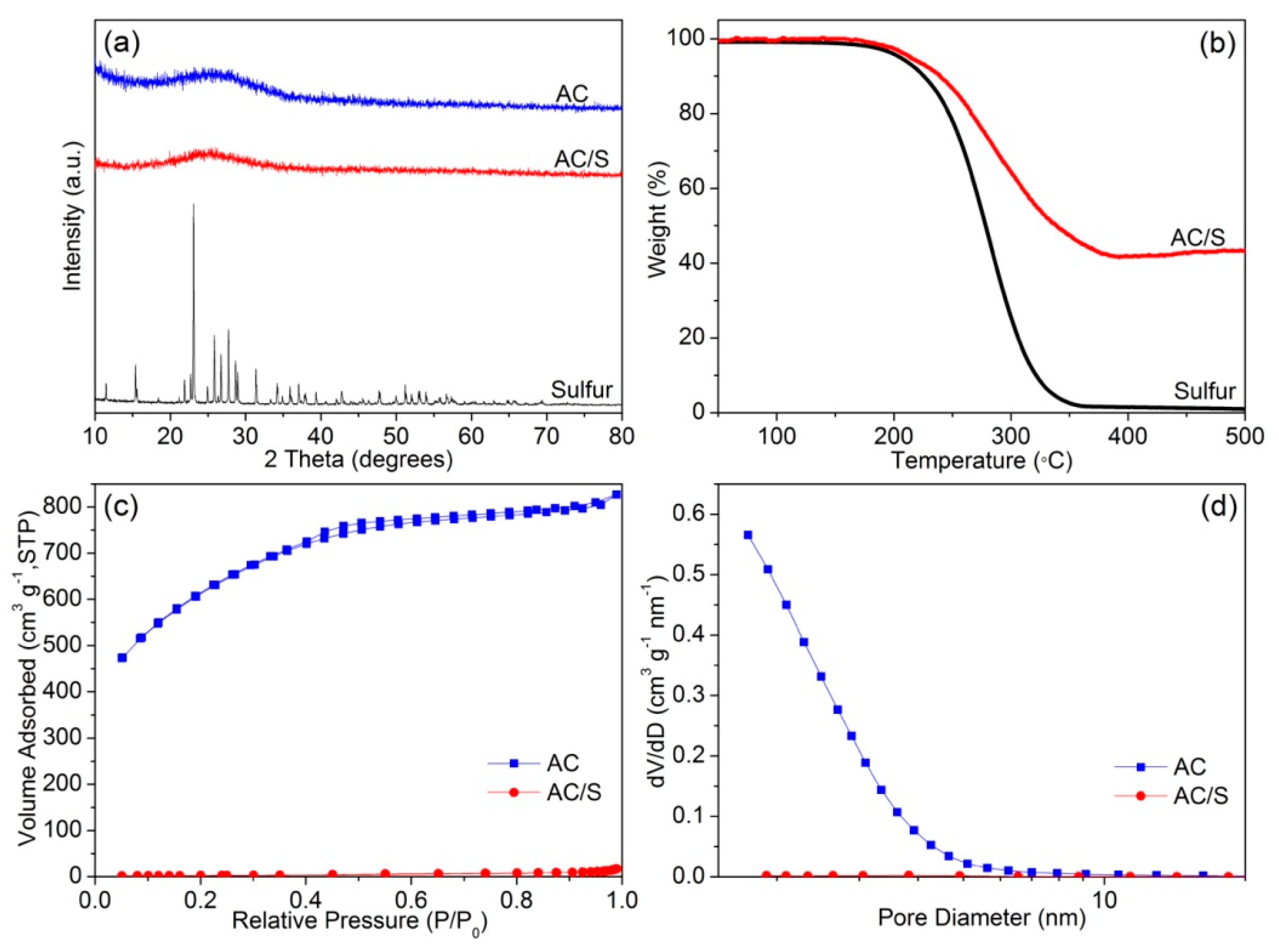
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, B.; Lai, M.O.; Liu, Z.; Liu, H.; Lu, L. Graphene-based surface modification on layered Li-rich cathode for high-performance Li-ion batteries. J. Mater. Chem. A 2013, 1, 9954–9965. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Bian, X.; Liu, J.; Xu, H.; An, Y. A controlled red phosphorus@Ni–P core@shell nanostructure as an ultralong cycle-life and superior high-rate anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1222–1233. [Google Scholar] [CrossRef]
- Demirocak, D.; Srinivasan, S.; Stefanakos, E. A review on nanocomposite materials for rechargeable Li-ion batteries. Appl. Sci. 2017, 7, 731. [Google Scholar] [CrossRef]
- Lee, D.; Ahn, S. Investigation of laser cutting width of LiCoO2 coated aluminum for lithium-ion batteries. Appl. Sci. 2017, 7, 914. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, Z.; Li, T.; Sun, X.; Liu, J. Hierarchical construction of core-shell metal oxide nanoarrays with ultrahigh areal capacitance. Nano Energy 2014, 7, 170–178. [Google Scholar] [CrossRef]
- Wu, X.; Lu, Z.; Zhu, W.; Yang, Q.; Zhang, G.; Liu, J.; Sun, X. High-performance aqueous battery with double hierarchical nanoarrays. Nano Energy 2014, 10, 229–234. [Google Scholar] [CrossRef]
- Li, B.; Gu, P.; Feng, Y.; Zhang, G.; Huang, K.; Xue, H.; Pang, H. Ultrathin nickel-cobalt phosphate 2D nanosheets for electrochemical energy storage under aqueous/solid-state electrolyte. Adv. Funct. Mater. 2017, 27, 1605784. [Google Scholar] [CrossRef]
- Zheng, S.; Li, X.; Yan, B.; Hu, Q.; Xu, Y.; Xiao, X.; Xue, H.; Pang, H. Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage. Adv. Energy Mater. 2017, 1602733. [Google Scholar] [CrossRef]
- Jiang, J.; He, P.; Tong, S.; Zheng, M.; Lin, Z.; Zhang, X.; Shi, Y.; Zhou, H. Ruthenium functionalized graphene aerogels with hierarchical and three-dimensional porosity as a free-standing cathode for rechargeable lithium-oxygen batteries. NPG Asia Mater. 2016, 8, e239. [Google Scholar] [CrossRef]
- Chen, M.; Li, W.; Shen, X.; Diao, G. Fabrication of core-shell α-Fe2O3@Li4 Ti5O12 composite and its application in the lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 4514–4523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lu, F.; Shen, X.; Xia, W.; He, H.; Zeng, X. One-pot construction of three dimensional CoMoO4/Co3O4 hybrid nanostructures and their application in supercapacitors. J. Mater. Chem. A 2015, 3, 21201–21210. [Google Scholar] [CrossRef]
- Hu, G.; Sun, Z.; Shi, C.; Fang, R.; Chen, J.; Hou, P.; Liu, C.; Cheng, H.-M.; Li, F. A sulfur-rich copolymer@CNT hybrid cathode with dual-confinement of polysulfides for high-performance lithium-sulfur batteries. Adv. Mater. 2017, 29, 1603835. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, J.; Yin, L.; Hu, G.; Fang, R.; Cheng, H.-M.; Li, F. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium-sulfur batteries. Nat. Commun. 2017, 8, 14627. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal-organic framework-based separator for lithium-sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Liu, X.-Y.; Cheng, X.-B.; Xu, W.-T.; Zhao, C.-Z.; Wei, F.; Zhang, Q. Healing high-loading sulfur electrodes with unprecedented long cycling life: Spatial heterogeneity control. J. Am. Chem. Soc. 2017, 139, 8458–8466. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kretschmer, K.; Choi, S.; Pang, H.; Xue, H.; Wang, G. Fabrication methods of porous carbon materials and separator membranes for lithium-sulfur batteries: Development and future perspectives. Small Methods 2017, 1, 1700089. [Google Scholar] [CrossRef]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium-sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, T.; Li, D.; Gao, H.; Gao, G.; Du, A.; Liu, H.; Guo, Z. Ultrathin cobaltosic oxide nanosheets as an effective sulfur encapsulation matrix with strong affinity toward polysulfides. ACS Appl. Mater. Interfaces 2017, 9, 4320–4325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Liu, H.; Guo, Z. A strategy for configuration of an integrated flexible sulfur cathode for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 3992–3996. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wu, Z.; Zhao, G.; Sun, C.; Zhou, C.; Gong, X.; Diao, G. Core-shell structure and interaction mechanism of γ-MnO2 coated sulfur for improved lithium-sulfur batteries. Small 2017, 13, 1603466. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, J.; Yang, K.R.; Mi, Y.; Kumaravadivel, P.; Zhong, Y.; Fan, Q.; Weng, Z.; Wu, Z.; Cha, J.J.; et al. Ultrathin dendrimer-graphene oxide composite film for stable cycling lithium-sulfur batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 3578–3583. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Yuan, S.; Wang, Y.; Xia, Y. To mitigate self-discharge of lithium-sulfur batteries by optimizing ionic liquid electrolytes. Energy Environ. Sci. 2016, 9, 224–231. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, X.; Jiang, Q.; Huo, J.; Wang, S. Enhanced cycling stability of lithium–sulfur batteries by electrostatic-interaction. Electrochim. Acta 2015, 182, 884–890. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Q.-H. Packing sulfur into carbon framework for high volumetric performance lithium-sulfur batteries. Sci. China Mater. 2015, 58, 349–354. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.; Sun, H.; Lai, C.; Zhao, X.; Peng, H.; Liu, T. A hybrid carbon aerogel with both aligned and interconnected pores as interlayer for high-performance lithium-sulfur batteries. Nano Res. 2016, 9, 3735–3746. [Google Scholar] [CrossRef]
- Ding, Y.-L.; Kopold, P.; Hahn, K.; van Aken, P.A.; Maier, J.; Yu, Y. Facile solid-state growth of 3D well-interconnected nitrogen-rich carbon nanotube-graphene hybrid architectures for lithium-sulfur batteries. Adv. Funct. Mater. 2016, 26, 1112–1119. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.-P.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Sulfur encapsulated in graphitic carbon nanocages for high-rate and long-cycle lithium-sulfur batteries. Adv. Mater. 2016, 28, 9539–9544. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, B.-Q.; Zhang, Q.; Zhu, L.; Wang, H.-F.; Shi, J.-L.; Wei, F. CaO-templated growth of hierarchical porous graphene for high-power lithium-sulfur battery applications. Adv. Funct. Mater. 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Ma, Z.; Tao, L.; Liu, D.; Li, Z.; Zhang, Y.; Liu, Z.; Liu, H.; Chen, R.; Huo, J.; Wang, S. Ultrafine nano-sulfur particles anchored on in situ exfoliated graphene for lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 9412–9417. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Yuan, L.; Hao, Z.; Huang, Y. Status and prospects in sulfur-carbon composites as cathode materials for rechargeable lithium-sulfur batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Yuan, S.; Yang, Y.; Zheng, J.; Hu, J.; Yang, D.; Wang, Y.; Dong, A. Three-dimensionally ordered, ultrathin graphitic-carbon frameworks with cage-like mesoporosity for highly stable Li-S batteries. Nano Res. 2017, 10, 2495–2507. [Google Scholar] [CrossRef]
- Chen, S.; Sun, B.; Xie, X.; Mondal, A.K.; Huang, X.; Wang, G. Multi-chambered micro/mesoporous carbon nanocubes as new polysulfides reserviors for lithium-sulfur batteries with long cycle life. Nano Energy 2015, 16, 268–280. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Qi, W.; Saraf, L.V.; Xiao, J.; Nie, Z.; Mietek, J.; Zhang, J.-G.; Schwenzer, B.; Liu, J. Optimization of mesoporous carbon structures for lithium-sulfur battery applications. J. Mater. Chem. 2011, 21, 16603–16610. [Google Scholar] [CrossRef]
- Li, N.; Zheng, M.; Lu, H.; Hu, Z.; Shen, C.; Chang, X.; Ji, G.; Cao, J.; Shi, Y. High-rate lithium-sulfur batteries promoted by reduced graphene oxide coating. Chem. Commun. 2012, 48, 4106–4108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Guo, Z.; Wang, L.; Hu, S.; Wang, Y.; Xia, Y. Leaf-like graphene-oxide-wrapped sulfur for high-performance lithium-sulfur battery. Adv. Sci. 2015, 2, 1500071. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Pan, F.; Li, W.; Jiang, Y.; Zhong, X.; Yu, Y. Free-standing porous carbon nanofibers-sulfur composite for flexible Li-S battery cathode. Nanoscale 2014, 6, 9579–9587. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, X.; Liu, H.; Sun, B.; Yeoh, W.; Li, K.; Zhang, J.; Wang, G. 3D hyperbranched hollow carbon nanorod architectures for high-performance lithium-sulfur batteries. Adv. Energy Mater. 2014, 4, 1301761. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Sun, B.; Zhang, J.; Liu, H.; Wang, G. Multi-shelled hollow carbon nanospheres for lithium-sulfur batteries with superior performances. J. Mater. Chem. A 2014, 2, 16199–16207. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, S.; Chen, S.; Lin, Z.; Pang, H.; Yu, Y. Activated Graphene with Tailored Pore Structure Parameters for Long Cycle-Life Lithium-Sulfur Batteries. Available online: https://link.springer.com/article/10.1007%2Fs12274-017-1659-3 (accessed on 7 June 2017).
- Yao, Y.; Wu, F. Naturally derived nanostructured materials from biomass for rechargeable lithium/sodium batteries. Nano Energy 2015, 17, 91–103. [Google Scholar] [CrossRef]
- Imtiaz, S.; Zhang, J.; Zafar, Z.A.; Ji, S.; Huang, T.; Anderson, J.A.; Zhang, Z.; Huang, Y. Biomass-derived nanostructured porous carbons for lithium-sulfur batteries. Sci. China Mater. 2016, 59, 389–407. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, M.; Lin, Z.; Zhao, B.; Zhang, S.; Yang, J.; Zhu, C.; Zhang, H.; Sun, D.; Shi, Y. Flexible cathodes and multifunctional interlayers based on carbonized bacterial cellulose for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 10910–10918. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.; Wang, W.; Zhang, H.; Gao, M.; Xiong, X.; Wang, A.; Yuan, K.; Huang, Y.; Wang, F. A novel porous nanocomposite of sulfur/carbon obtained from fish scales for lithium-sulfur batteries. J. Mater. Chem. A 2013, 1, 3334–3339. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, C.; Cao, Z.; Sun, Z.; Jia, Y.; Yang, S. A novel sulfur/carbon composite for low cost lithium-sulfur batteries with high cycling stability. RSC Adv. 2014, 4, 28871–28874. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, M.; Lin, Z.; Li, N.; Liu, Y.; Zhao, B.; Pang, H.; Cao, J.; He, P.; Shi, Y. Activated carbon with ultrahigh specific surface area synthesized from natural plant material for lithium-sulfur batteries. J. Mater. Chem. A 2014, 2, 15889–15896. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, M.; Lin, Z.; Zang, R.; Huang, Q.; Xue, H.; Cao, J.; Pang, H. Mango stone-derived activated carbon with high sulfur loading as a cathode material for lithium-sulfur batteries. RSC Adv. 2016, 6, 39918–39925. [Google Scholar] [CrossRef]
- Helen, M.; Reddy, M.A.; Diemant, T.; Golla-Schindler, U.; Behm, R.J.; Kaiser, U.; Fichtner, M. Single step transformation of sulphur to Li2S2/Li2S in Li-S batteries. Sci. Rep. 2015, 5, 12146. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, H.; Huang, Y.; Wang, W.; Xia, Y.; Yu, Z. Pig bone derived hierarchical porous carbon and its enhanced cycling performance of lithium-sulfur batteries. Energy Environ. Sci. 2011, 4, 736–740. [Google Scholar] [CrossRef]
- Wang, D.; Fu, A.; Li, H.; Wang, Y.; Guo, P.; Liu, J.; Zhao, X.S. Mesoporous carbon spheres with controlled porosity for high-performance lithium-sulfur batteries. J. Power Sources 2015, 285, 469–477. [Google Scholar] [CrossRef]
- You, Y.; Zeng, W.; Yin, Y.-X.; Zhang, J.; Yang, C.-P.; Zhu, Y.; Guo, Y.-G. Hierarchically micro/mesoporous activated graphene with a large surface area for high sulfur loading in Li-S batteries. J. Mater. Chem. A 2015, 3, 4799–4802. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zhang, F.; Huang, Y.; Chen, Y. Sulfur-infiltrated graphene-based layered porous carbon cathodes for high-performance lithium-sulfur batteries. ACS Nano 2014, 8, 5208–5215. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, Z.; Zhang, X.; Ren, G.; Wang, X.; Lai, Y.; Liu, Y.; Li, J. Synthesis of hierarchical porous honeycomb carbon for lithium-sulfur battery cathode with high rate capability and long cycling stability. Electrochim. Acta 2014, 137, 439–446. [Google Scholar] [CrossRef]
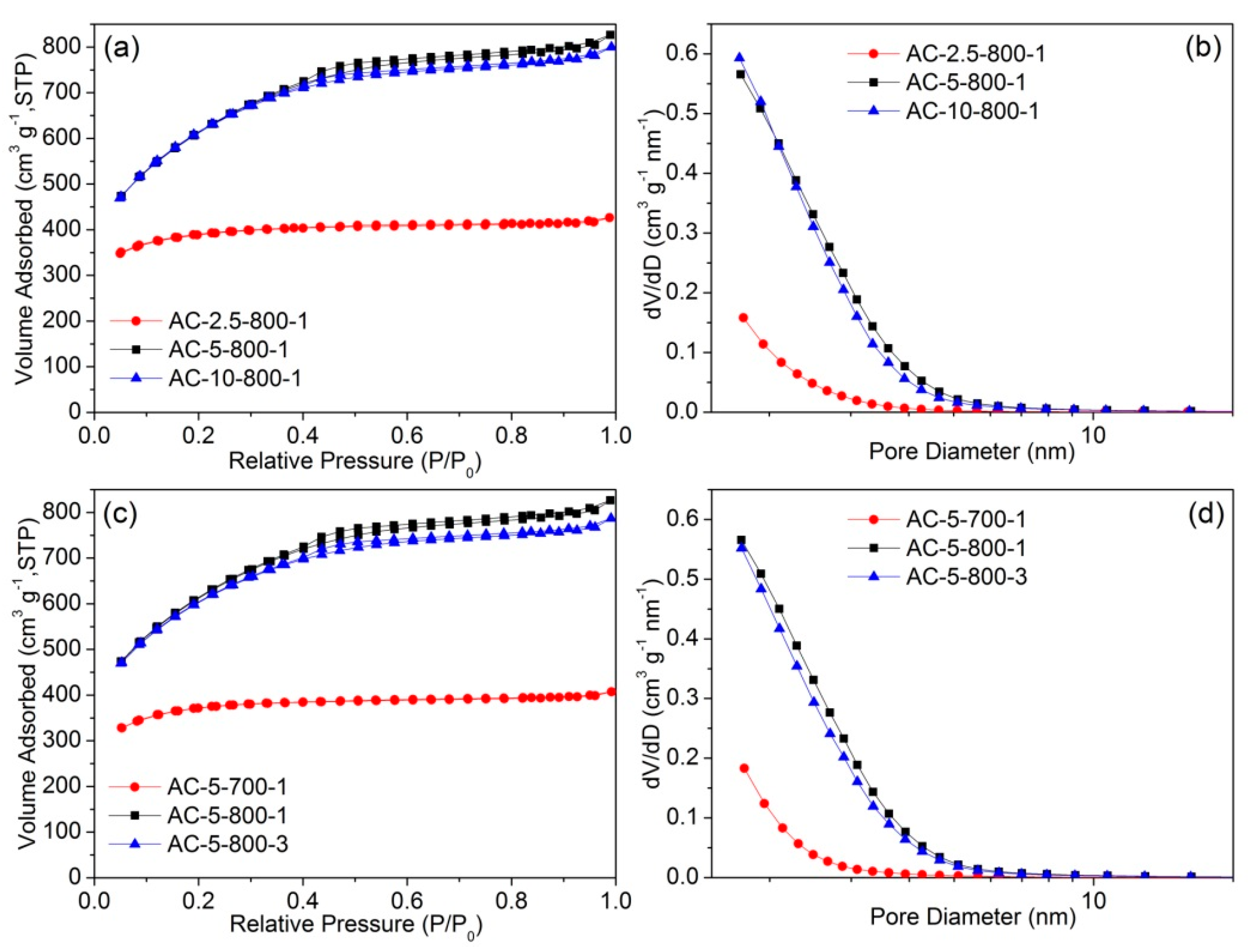
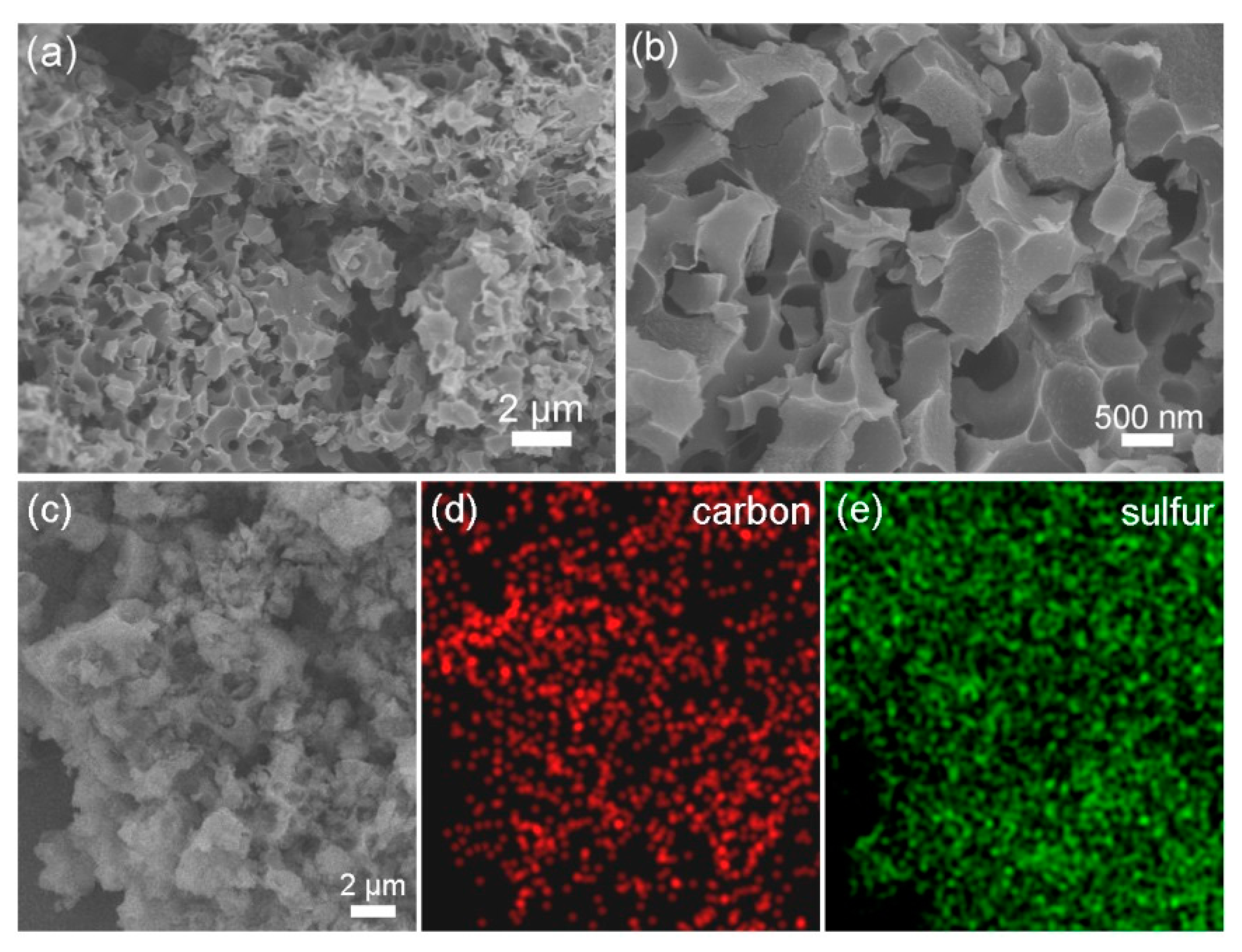

| Samples | Brunauer–Emmett–Teller (BET) Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | BJH Average Pore Size (nm) |
|---|---|---|---|
| AC-2.5-800-1 | 1211 | 0.66 | 2.8 |
| AC-5-800-1 | 2090 | 1.28 | 2.7 |
| AC-10-800-1 | 2082 | 1.24 | 2.6 |
| AC-5-700-1 | 1157 | 0.63 | 2.7 |
| AC-5-800-3 | 2035 | 1.22 | 2.7 |
| Carbon Host Materials | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | S (wt %) | Initial Capacity (mA h g−1) | Capacity Retention (mA h g−1) | Cycle Number | Current Density (mA g−1) | Refs. |
|---|---|---|---|---|---|---|---|---|
| AC from coconut shell | 677 | 0.29 | 60 | 878 | 774 | 50 | 167.5 | [44] |
| Microporous carbon from coconut shell | 1600 | 0.66 | 45.8 | 1458 | 411 | 400 | 335 | [47] |
| Meso/micro/macroporous carbon | 2157 | 2.26 | 63 | 1265 | 643 | 50 | 400 | [48] |
| Mesoporous carbon sphere | 1270 | 4.1 | 60 | 1388 | 857 | 100 | 335 | [49] |
| Micro/mesoporous activated graphene | 2995 | 2.14 | 75 | 620 | 471 | 200 | 1675 | [50] |
| Graphene-based layered porous carbon | 2500 | 1.94 | 68 | 886 | 620 | 100 | 837.5 | [51] |
| Honeycomb carbon | 614 | 1.34 | 66 | 923 | 564 | 100 | 3350 | [52] |
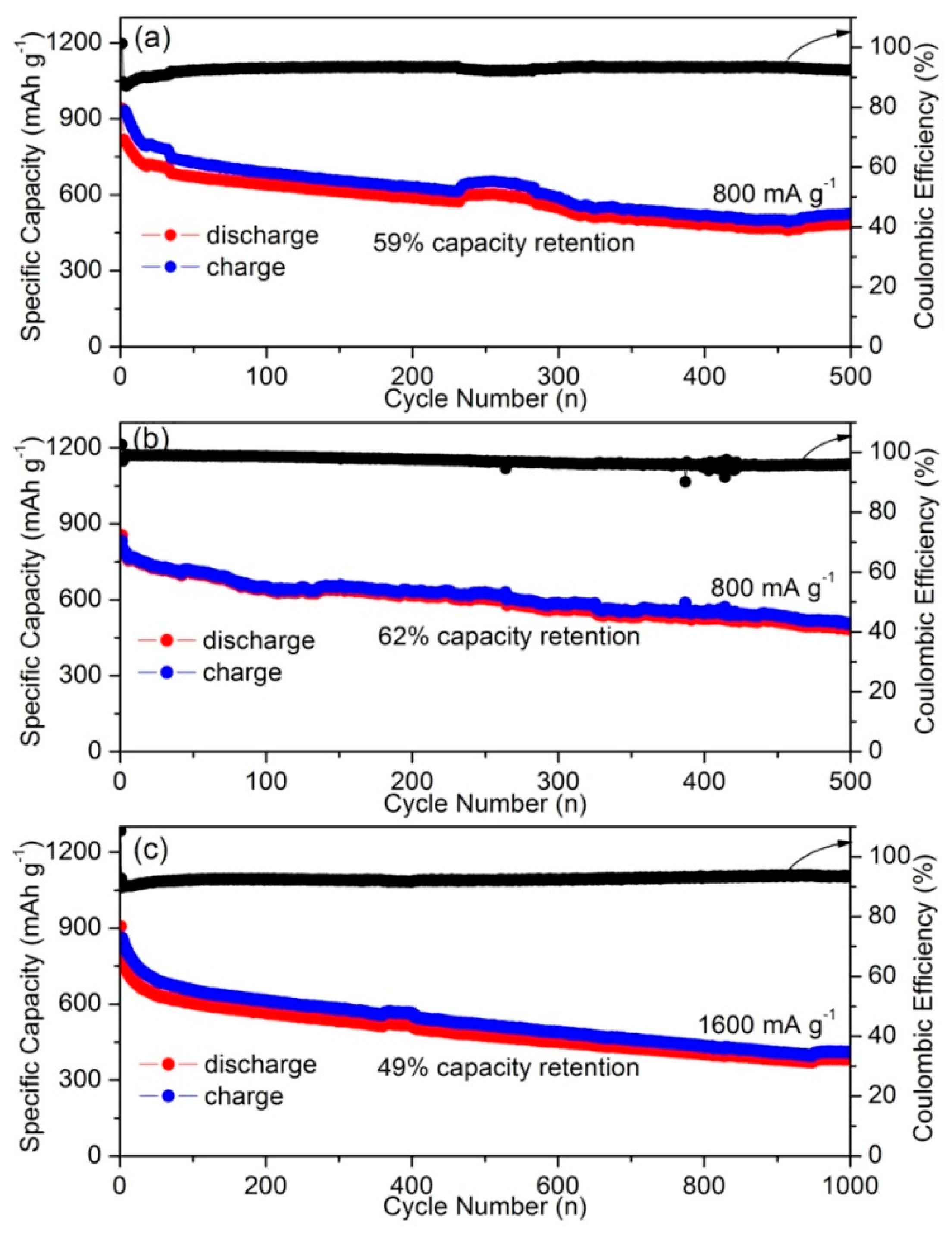
| AC from rapeseed shell | 2090 | 1.28 | 60 | 942 | 486 | 500 | 800 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Hu, Q.; Zhang, S.; Tang, H.; Li, L.; Pang, H. Macroporous Activated Carbon Derived from Rapeseed Shell for Lithium–Sulfur Batteries. Appl. Sci. 2017, 7, 1036. https://doi.org/10.3390/app7101036
Zheng M, Hu Q, Zhang S, Tang H, Li L, Pang H. Macroporous Activated Carbon Derived from Rapeseed Shell for Lithium–Sulfur Batteries. Applied Sciences. 2017; 7(10):1036. https://doi.org/10.3390/app7101036
Chicago/Turabian StyleZheng, Mingbo, Qin Hu, Songtao Zhang, Hao Tang, Lulu Li, and Huan Pang. 2017. "Macroporous Activated Carbon Derived from Rapeseed Shell for Lithium–Sulfur Batteries" Applied Sciences 7, no. 10: 1036. https://doi.org/10.3390/app7101036