1. Introduction
Humans need to care for our “mother earth” with intelligence, diligence, and heart. In a society experiencing an energy shortage, the pursuit of sustainable development has become the social mainstream. With rapid economic development, industrial scale expands. However, energy and environmental problems gradually appear at the same time. In the process of industrial production, most energy is released in the form of heat. Thermoelectric (TE) materials can directly convert waste heat into electricity based on the Seebeck effect [
1,
2]. While TE materials show a great prospect environmentally and economically, the lower efficiency of TE materials limits their wide application. The efficiency of TE materials is determined by the dimensionless figure of merit
ZT, which is defined as
ZT = α
2σT/κ, where α is Seebeck coefficient, σ is electrical conductivity, κ is the thermal conductivity, and T is absolute temperature. A good TE material should have high α and σ as well as low κ value. The intrinsic properties of TE materials determine the coupling effect between the conductivity and Seebeck coefficient. Generally, the higher power factor (PF = α
2σ) corresponds to higher power output. PF is proportional to the effect mass (
m*) and mobility of the carriers (
μ). Thermal property is determined by the total thermal conductivity (
κ), which is composed of lattice part (
κL) and carrier part (
κe). Electronic thermal conductivity (
κe) is proportional to the conductivity. Reducing the
κL of a TE material is an effective way to reduce the total thermal conductivity.
Cu
2SnSe
3 with a diamond structure has gained great attention recently due to its low thermal conductivity originating from the complex crystal structure and high specific heat. In addition, Cu
2SnSe
3 has good electrical conductivity and adjustable electrical properties [
3]. To further improve the thermoelectric properties of Cu
2SnSe
3, a great deal of research has been carried out to optimize the material from multiple perspectives. Li et al. obtained a maximum
ZT of 0.45 for Cu
2SnSe
3 material at 773 K by one-step high-pressure combustion synthesis (HPCS) [
4]. Liu et al. prepared the Cu
2SnSe
3 material using combustion synthesis and obtained a maximum
ZT of 0.51 at 773 K, which is the best result for Cu
2SnSe
3 compared to other methods [
5]. Wang et al. optimized the synthesis parameters to form uniform particles of wurtzite, cubic, linear heterostructures, and tetrapods by colloidal synthesis. The facile synthesis strategy opens pathways for extension to other compositions of multielement copper chalcogenides where the cubic and hexagonal phases can be formed [
6]. Ge et al. and Ahmadi et al. synthesized Cu
2SnSe
3 nanocrystals and controlled the nanostructure successfully by solvothermal method [
7,
8]. In addition to optimizing the preparation process, doping of materials is also an important way to enhance the performance of TE materials. Cho et al. synthesized a Ga-doped Cu
2Ga
1−xGe
xSe
3 sample and approached its theoretical minimum value of lattice thermal conductivity, resulting in a
ZT value of 0.5 at 750 K [
9]. Fan et al. reported a lower lattice thermal conductivity of the Cu
2Ga
0.075Sn
0.925Se
3 sample and obtained a maximum
ZT of 0.43 at 700 K [
10]. In addition, Skoug et al. confirmed that substituting Se with S is also an effective method to improve the TE performance of Cu-based ternary selenides [
11]. The combustion synthesis method can prepare high-performance thermoelectric materials, but it is difficult to precisely control. Moreover, many studies report that only Sn location is the best candidate for substituting in the Cu
2Sn(Ge)Se(S)
3 structure, which limits the improvement of TE performance [
3,
8,
10]. Based on those limits mentioned above, the composite was designed on the Cu-based ternary selenides matrix. Some studies confirmed that introducing a nanophase into the Cu-based semiconductor can improve the
ZT. Zhang et al. introduced graphite nanosheets into CuGaTe
2 and obtained the highest
ZT of 0.93 for the CuGaTe
2 composite at 873 K, which is 21% higher than that of CuGaTe
2 matrix [
12]. Li et al. reported that the incorporation of a suitable quantity of nanophase PbTe particles into SnSe matrix could efficiently enhance the TE performance of SnSe material [
13]. TiO
2 has a band gap of 3.0 eV and has good thermal and chemical stability as well as large elastic modulus, which makes it a good candidate as a second phase. TiO
2 has been introduced into a filled-skutterudite matrix in previous studies [
14,
15,
16]. However, introducing nano-TiO
2 into Cu
2SnSe
3 has never been reported. In this study, the technological parameter was optimized to synthesize pure Cu
2SnSe
3 material. The nano-TiO
2 was incorporated into the Cu
2SnSe
3 matrix by ball milling method, and the TiO
2/Cu
2SnSe
3 thermoelectric composites were prepared by spark plasma sintering (SPS). The thermoelectric properties of TiO
2/Cu
2SnSe
3 composites were investigated in detail.
3. Results and Discussion
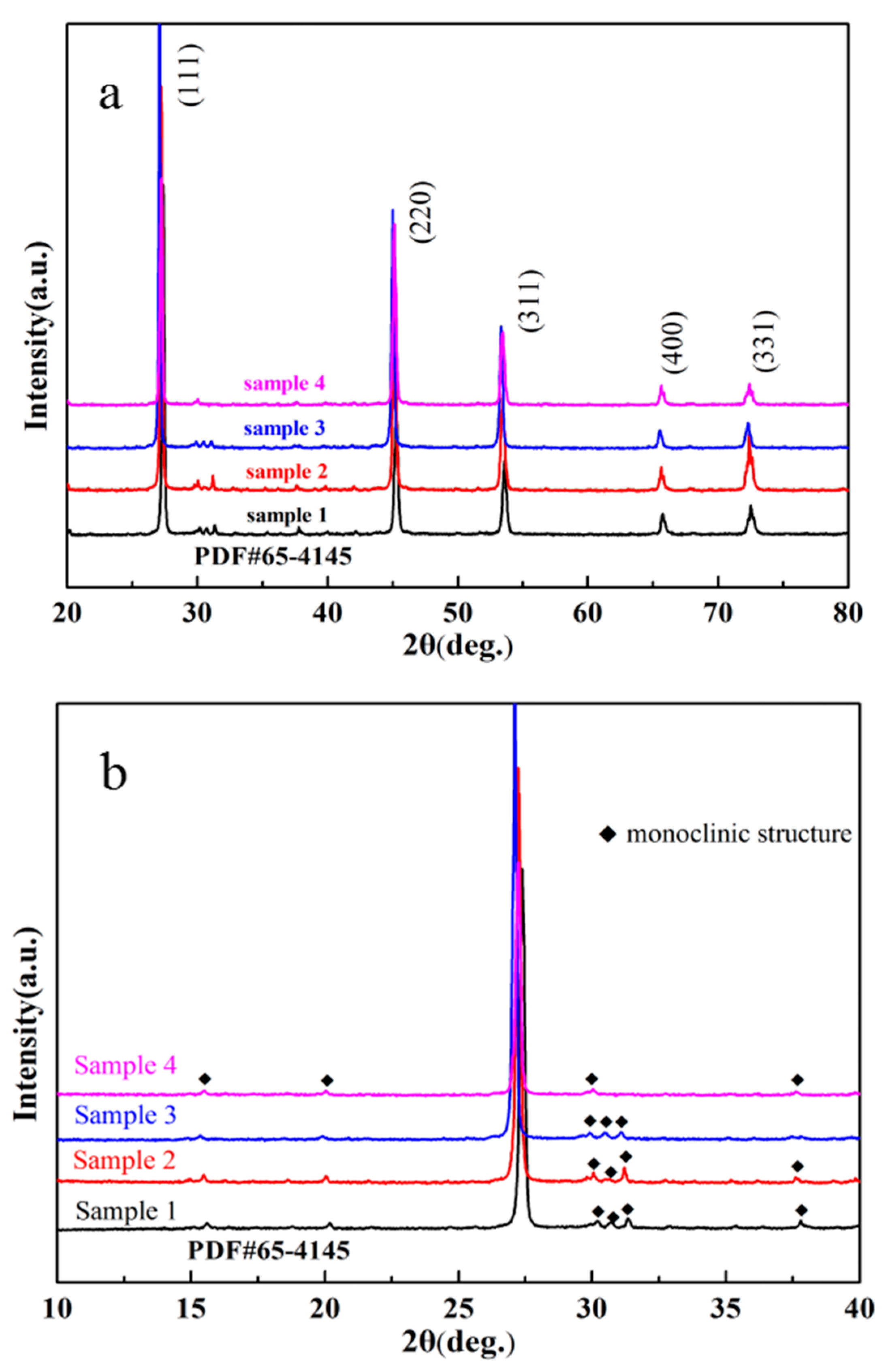
Figure 1a shows the XRD patterns of the resultant Cu
2SnSe
3 samples after melting and annealing with different cooling rates. Samples 1 and 2 were cooled from 1273 K to room temperature in 24 h and 48 h, respectively. Sample 3 was directly quenched in salt water. Sample 4 was annealed at 923 K for 24 h after quenching in salt water. The XRD patterns indicate that Cu
2SnSe
3 can be synthesized by melting at 1273 K for 12 h and annealing. The Cu
2SnSe
3 matrix is a disordered zincblende structure with a lattice parameter of 5.684 Å.
Figure 1b shows the XRD patterns of different samples from 10° to 40°. There was no significant difference between sample 1 and sample 2, while the sample 4 had fewer peaks, which may be indexed to the monoclinic structure [
3]. The structure transformation in Cu
2SnSe
3 from monoclinic to cubic can be attributed to the different cooling rates. Skoug reported that slow cooling could result in a monoclinic structure while water quenching led to a disordered cubic zincblende-like structure [
17]. Delgado et al. reported that an order–disorder transformation in Cu
2SnSe
3 can occur at 723 K and the modified phase was found to be sphalerite [
18]. In order to comprehensively and thoroughly study these phases, Marcano et al. found that the phase in low diffraction position was likely to be SnSe
2 on the basis of differential thermal analysis (DTA) [
19]. Therefore, the structure of Cu
2SnSe
3 can be described as an adamantane compound which is derivative of the sphalerite structure. In this study, the Cu
2SnSe
3 material prepared by the melting, quenching, and annealing process was selected as the matrix phase (sample 4) to carry out the following experiment.
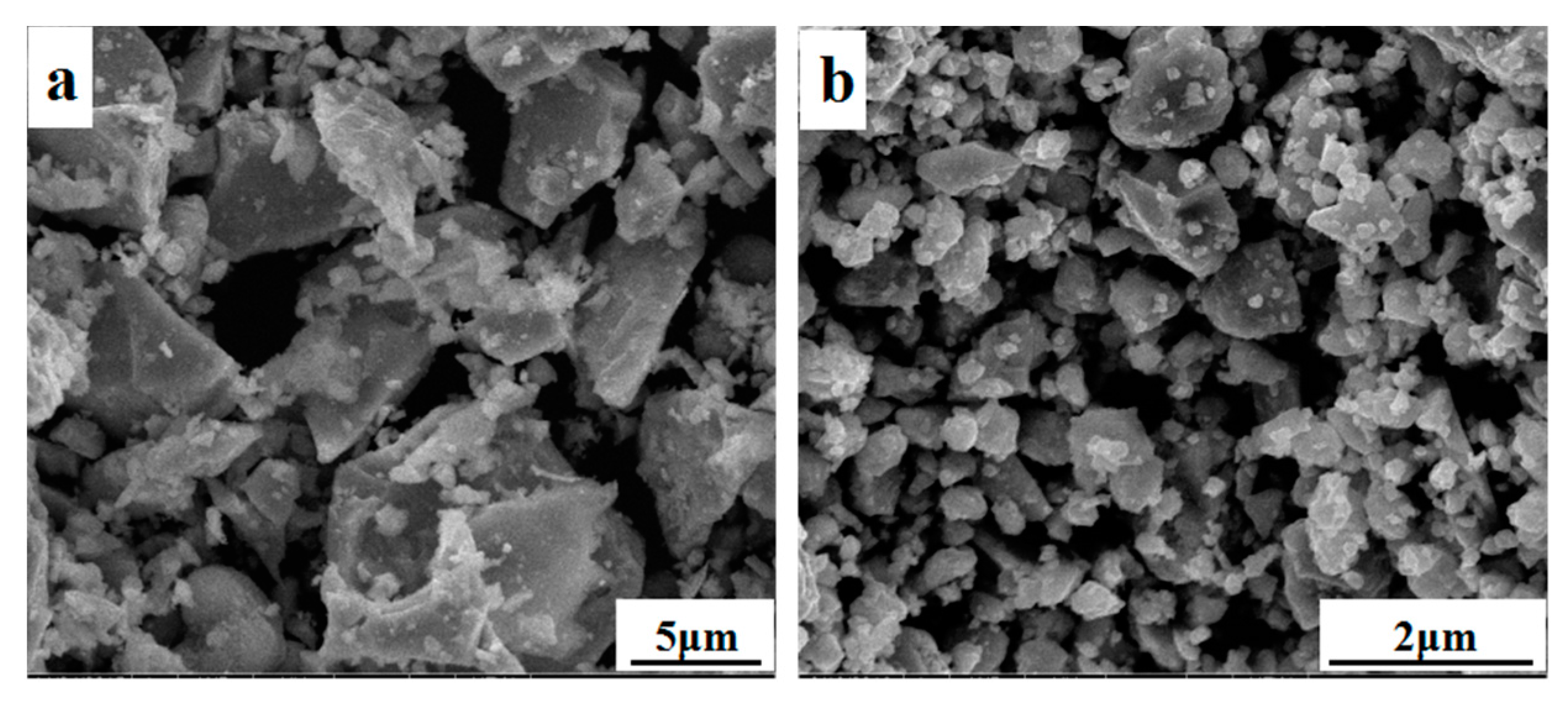
The Cu
2SnSe
3 bulk ingot was artificially ground into powder, as shown in
Figure 2a. It can be seen that the size distribution of Cu
2SnSe
3 particles is not uniform. In order to obtain smaller uniform particles, the powder was subjected to mechanical ball milling, and the SEM image of Cu
2SnSe
3 powder after ball milling is shown in
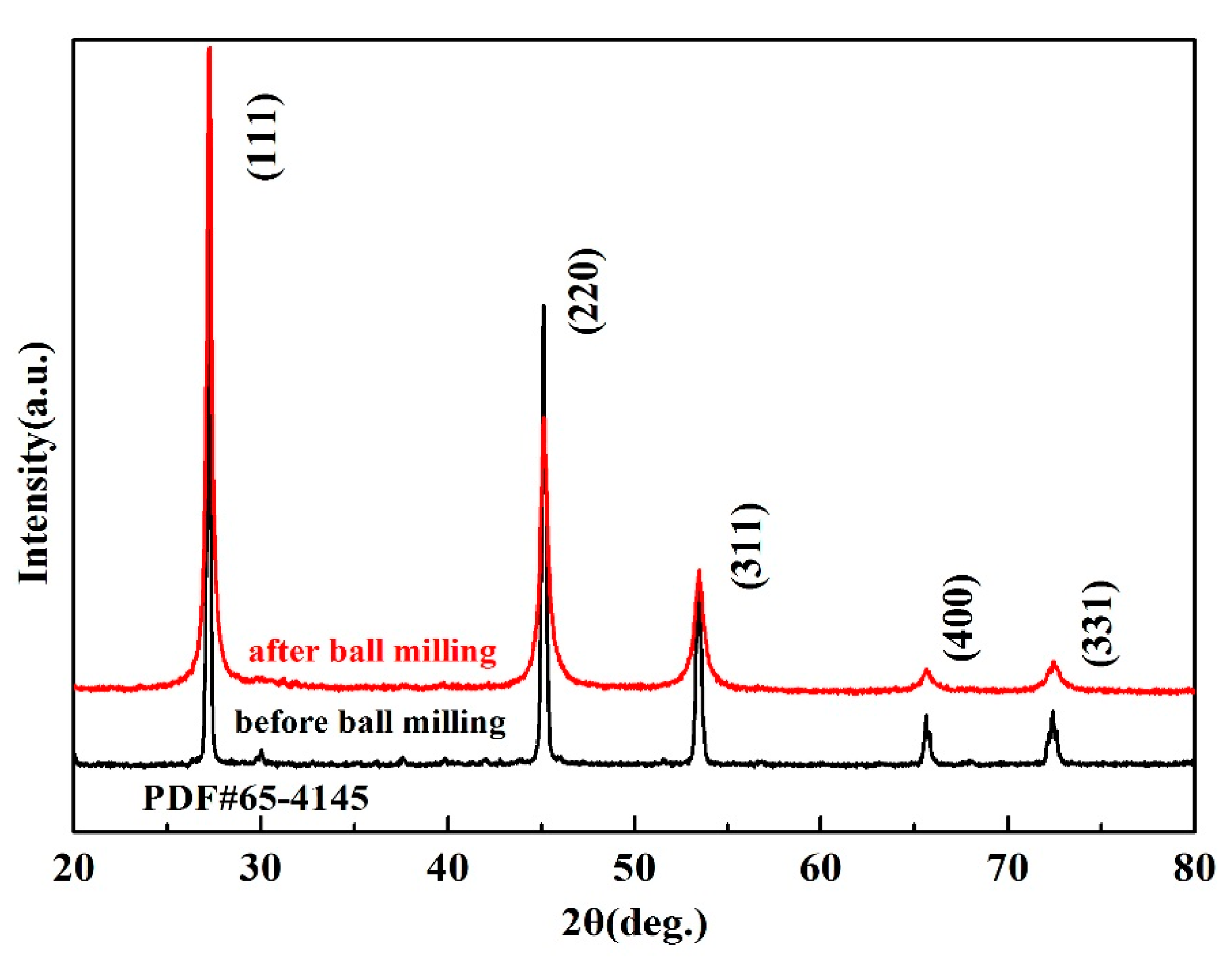
Figure 2b. The XRD of Cu
2SnSe
3 matrix was analyzed before and after ball milling, as shown in
Figure 3. There was no phase change reaction in the process of ball milling of Cu
2SnSe
3 matrix.
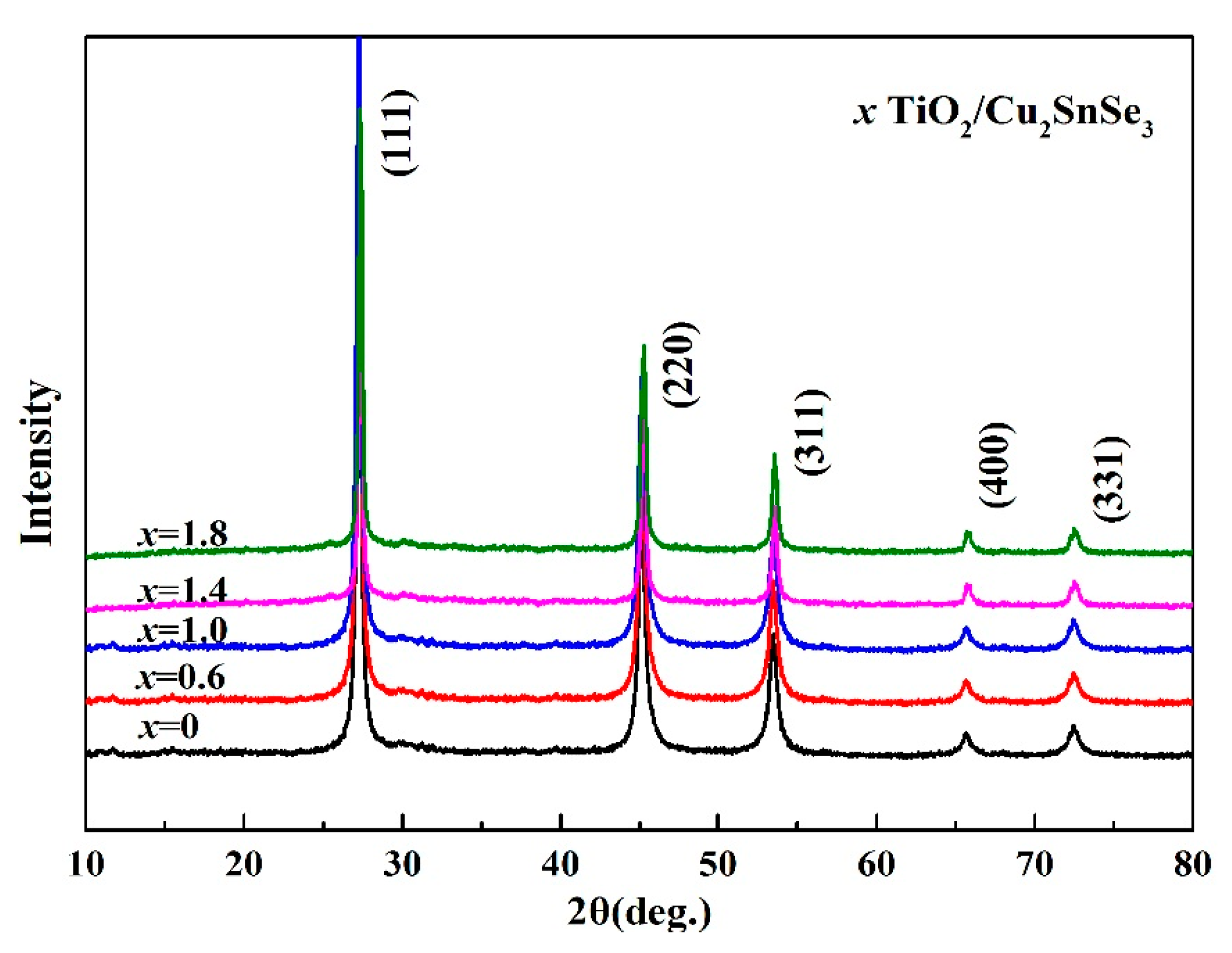
Figure 4 shows the XRD patterns of
x vol %TiO
2/Cu
2SnSe
3 (
x = 0, 0.6, 1.0, 1.4, 1.8) composites after ball-milling. The XRD patterns of composites samples are similar to that of Cu
2SnSe
3 matrix samples. The diffraction peaks of all the samples correspond to the Cu
2SnSe
3 phase (PDF#65-4145). The peak of TiO
2 phase is hardly found in the XRD patterns, which may be attributed to its low content. In addition, no diffraction peaks corresponding to other phase was found, which indicates that there was no reaction between the introduced nano-TiO
2 particles and the Cu
2SnSe
3 matrix in the process of ball-milling.
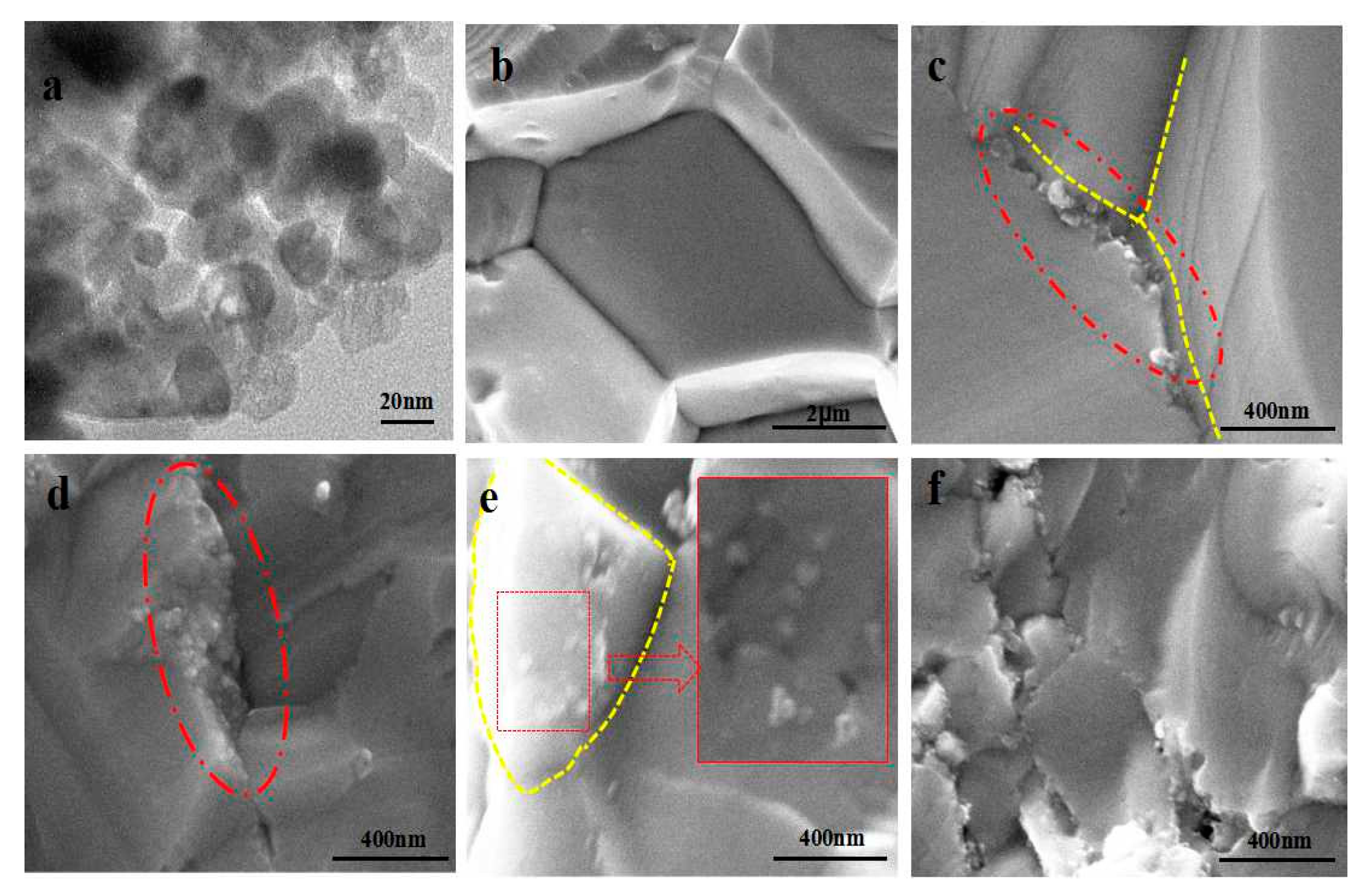
Figure 5a shows the TEM image of the starting powders of TiO
2 nanoparticles. It is shown that the size of spherical TiO
2 particles is 10–20 nm.
Figure 5b–f shows the SEM images of the fracture surface of TiO
2/Cu
2SnSe
3 composite samples. It can be observed in
Figure 5b that the grain size of Cu
2SnSe
3 matrix is about 3 μm. Rapid sintering process with SPS technology can effectively inhibit the growth of grains, which would result in more grain boundaries and scatter more phonons. It also can be found in
Figure 5c,d, and f that the nano-TiO
2 particles are mainly distributed on the grain boundary. With the content of nano-TiO
2 increasing from 0.6 vol % to 1.8 vol %, the grain size of Cu
2SnSe
3 decreases from 2.2 μm to 0.9 μm. The nano-TiO
2 particles in the sintering process effectively inhibited the growth of grains, and the inhibitory effect is more obvious with the increase of nanoparticles [
20,
21,
22]. In contrast, it can be found in
Figure 5e that most TiO
2 particles in the 1.4%TiO
2/Cu
2SnSe
3 composites were distributed in the internal grain. This is possibly related to the surfactivity of powder, SPS process, and so on. This issue will be investigated in future work. In addition, when the amount of nano-TiO
2 reached 1.8 vol %, the TiO
2/Cu
2SnSe
3 composite kept more holes at the grain boundary. The microstructure of TiO
2/Cu
2SnSe
3 composites could influence the electrical and thermal transport properties of the materials. The relative density of TiO
2/Cu
2SnSe
3 composites is shown in
Table 1. It can be seen that the addition of nano-TiO
2 reduced the relative density of TiO
2/Cu
2SnSe
3 composites.
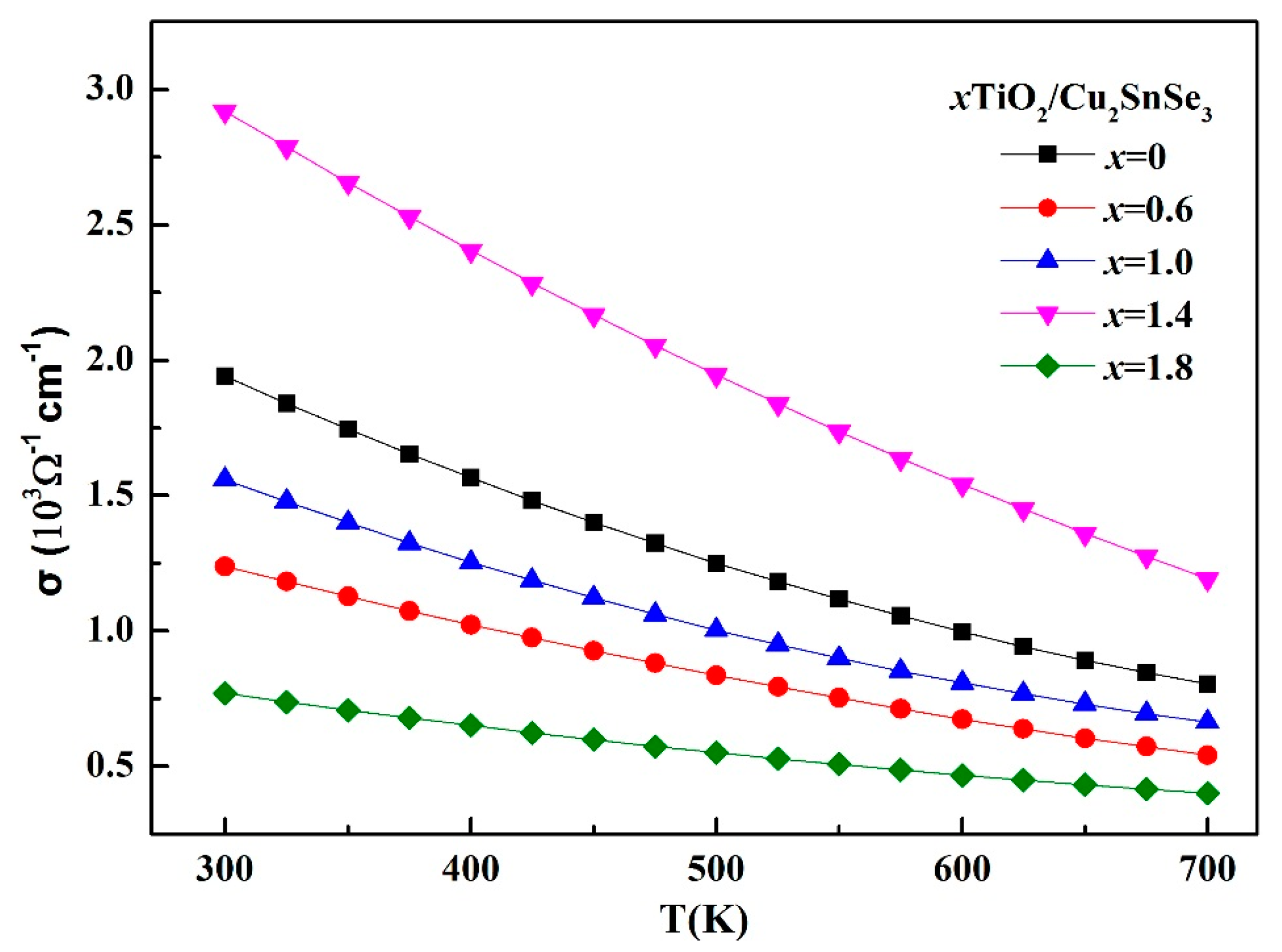
Figure 6 shows the temperature dependence of electrical conductivity (σ) for the TiO
2/Cu
2SnSe
3 composites in the range of 300–700 K. The electrical conductivity of all samples decreased with increasing temperature in all of the investigated temperatures, which indicates that all samples demonstrated degenerate semiconductor behavior. The composites samples showed lower σ than the Cu
2SnSe
3 matrix except for the 1.4%TiO
2/Cu
2SnSe
3 sample, which suggests that the addition of nano-TiO
2 into the Cu
2SnSe
3 matrix can reduce the electrical conductivity. Nano-TiO
2 particles affect the carrier transport, leading to a reduction in the conductivity of the material. On the other hand, the nano-TiO
2 particles which were distributed at the grain boundary effectively suppressed the grain growth, resulting in a large number of grain boundaries during the sintering process. These grain boundaries have great effects on the transport of the carriers, which reduce the conductivity of the material. However, the conductivity of the 1.4%TiO
2/Cu
2SnSe
3 sample was effectively improved. As shown in
Figure 5e, the nano-TiO
2 particles in the 1.4%TiO
2/Cu
2SnSe
3 composites were mainly distributed in the internal grain. Therefore the effect of suppressing the grain growth was not as obvious as that of other composites. Meanwhile, the carrier concentration of 1.4%TiO
2/Cu
2SnSe
3 composite at room temperature is listed in
Table 2. The increase in the conductivity of the 1.4%TiO
2/Cu
2SnSe
3 composite is attributed to the high carrier mobility. These nanoparticles distributed in the grain provide a special channel and contribute to the transport for the carrier. The electrical conductivity of 1.4%TiO
2/Cu
2SnSe
3 sample was 2.91 × 10
3 Ω
−1/cm at room temperature. Compared with the electrical conductivity of doped-Cu
2SnSe
3 sample in the previous studies [
8,
9,
10,
11], the electrical conductivity of TiO
2/Cu
2SnSe
3 sample was higher, which is possibly due to high carrier concentration (about 10
21 cm
−3), as shown in
Table 2.
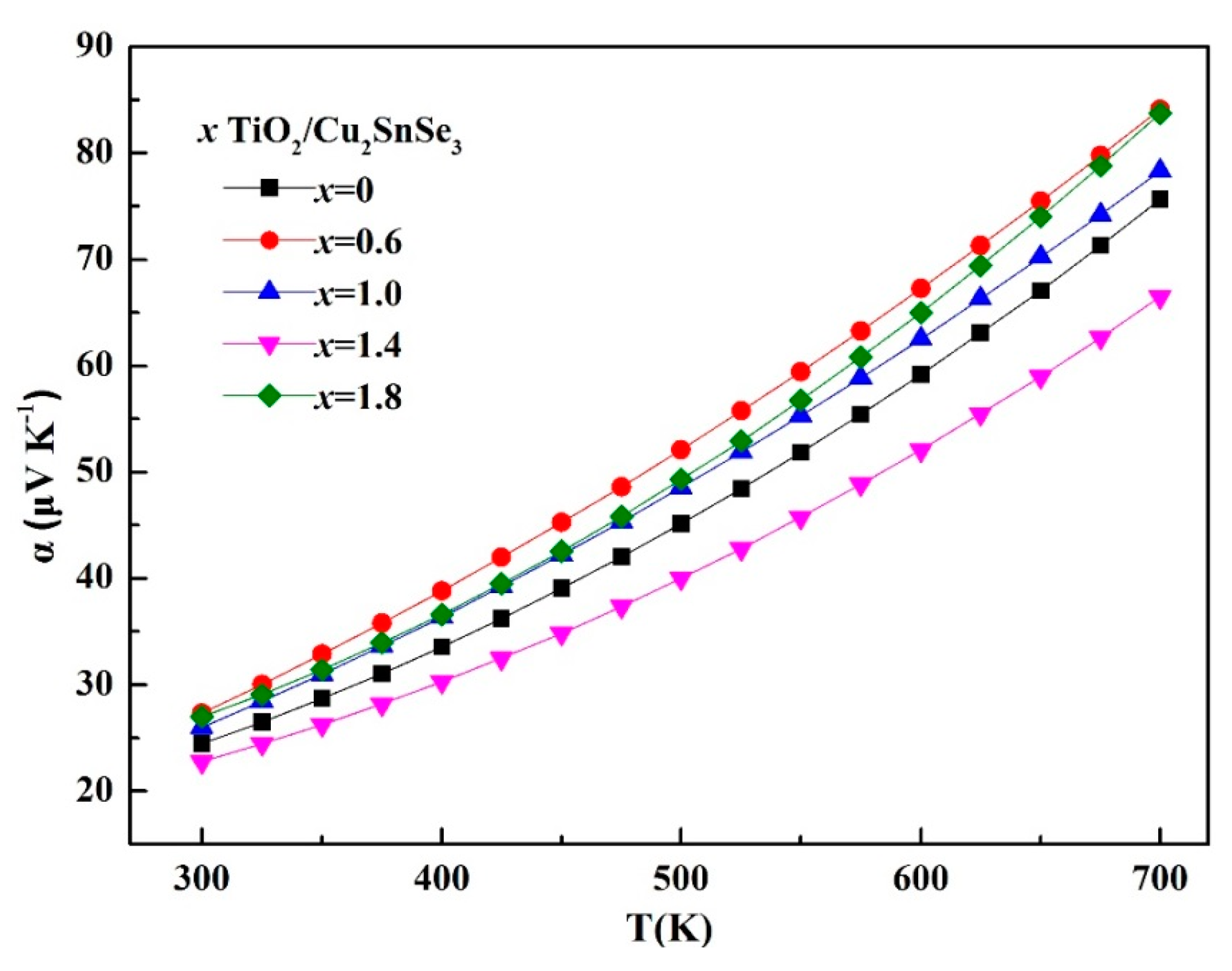
Figure 7 shows the temperature dependence of Seebeck coefficient (α) of the TiO
2/Cu
2SnSe
3 composite samples. All samples kept positive values of α in the whole temperature range, indicating that they are
p-type semiconductors. The Seebeck coefficient of all samples increased with increasing temperature. The Seebeck coefficient of TiO
2/Cu
2SnSe
3 composites was higher than that of the matrix material, except for the 1.4%TiO
2/Cu
2SnSe
3 sample. The introduction of nano-TiO
2 brings a large number of phase interfaces, which can filter out the low-energy carriers so as to increase the density of states of the material. As a result, the Seebeck coefficient of composites was improved. As for the 1.4%TiO
2/Cu
2SnSe
3 sample, the lower Seebeck coefficient should be related with the different distribution of nano-TiO
2 particles in the TiO
2/Cu
2SnSe
3 composite. Combined with the above analysis of the microstructure and conductivity, there is an effective channel in the composite which allows a large number of low-energy carriers to pass through, so the composite maintains a lower Seebeck coefficient. The detailed reason will be further investigated.
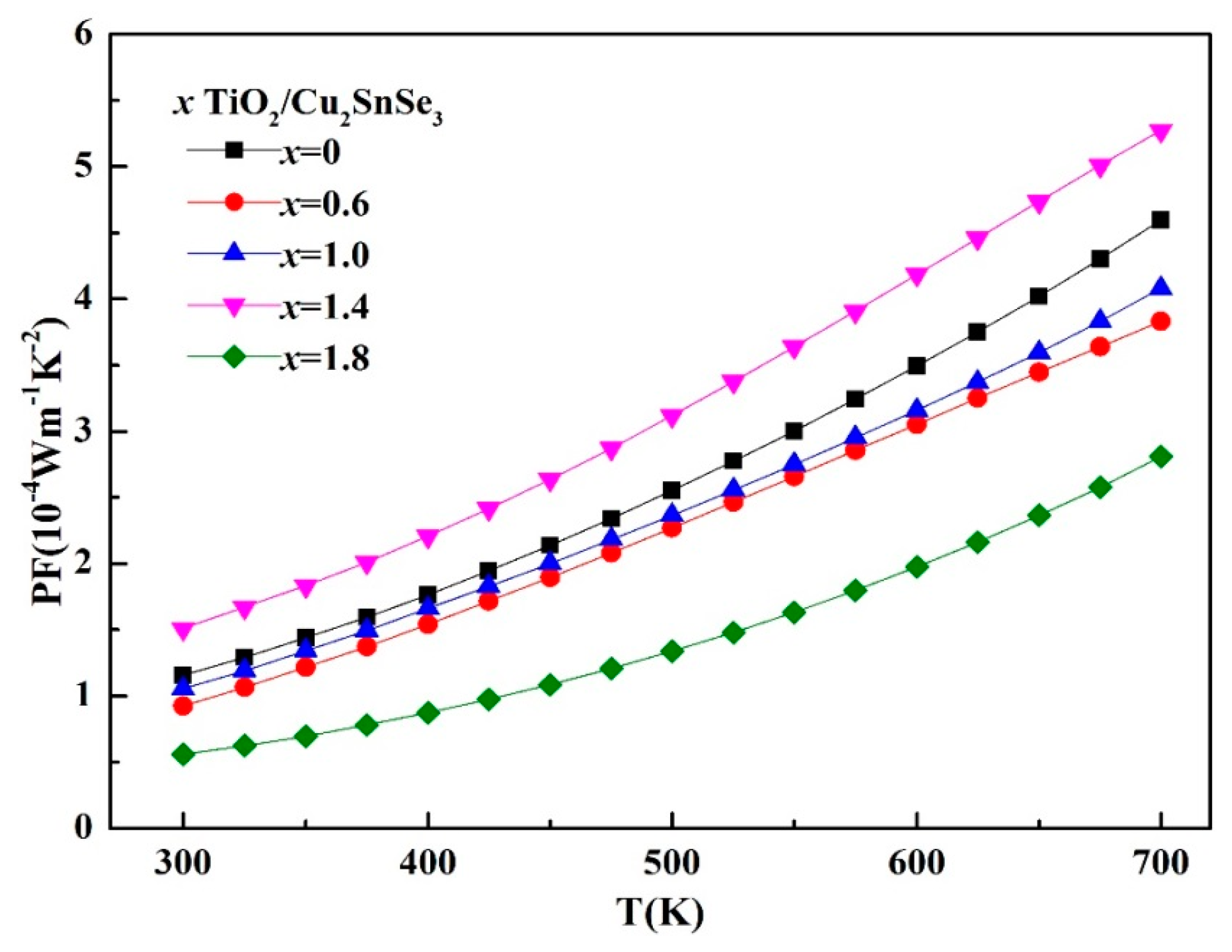
Figure 8 shows the temperature dependence of the power factor (PF) of the TiO
2/Cu
2SnSe
3 composites. The PF of 1.4%TiO
2/Cu
2SnSe
3 reached 5.27 × 10
−4 Wm
−1 K
−2 at 700 K, which is 20% higher than that of Cu
2SnSe
3 matrix material (4.59 × 10
−4 Wm
−1 K
−2). For the other TiO
2/Cu
2SnSe
3 composites, since the increase in the Seebeck coefficient is not sufficient to compensate for the decrease in conductivity, the PF of the composite samples is maintained at a lower level than that of the Cu
2SnSe
3 matrix.
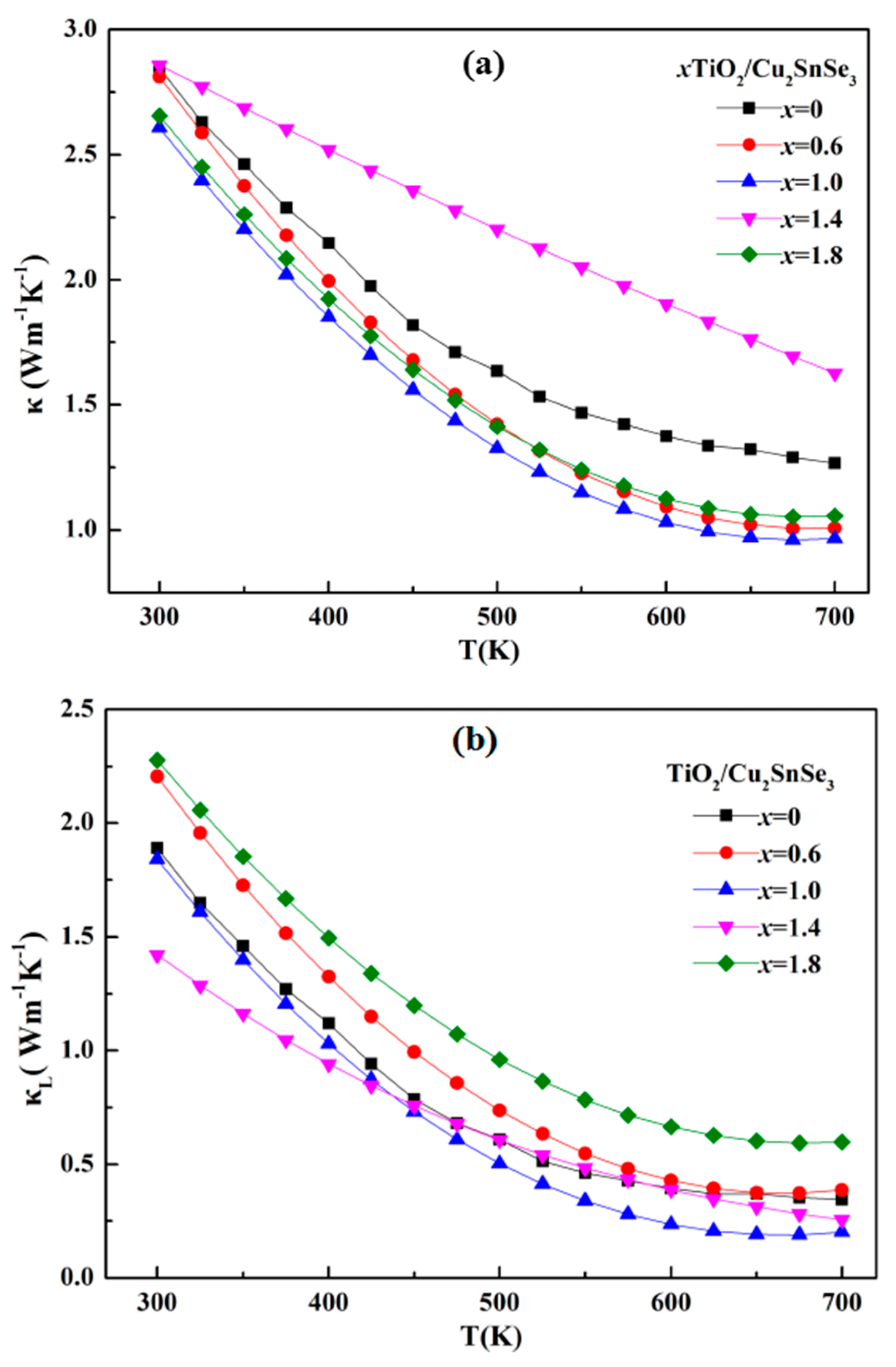
Figure 9 shows the temperature dependence of thermal conductivity (
κ) of the TiO
2/Cu
2SnSe
3 composite. Compared with the
κ of Cu
2SnSe
3 matrix, it can be seen in
Figure 9a that the TiO
2/Cu
2SnSe
3 composites maintained the lower
κ, except for the 1.4%TiO
2/Cu
2SnSe
3 sample. The 1.4%TiO
2/Cu
2SnSe
3 composite kept higher
κ than that of the Cu
2SnSe
3 matrix. The lattice thermal conductivity (
κL) of the TiO
2/Cu
2SnSe
3 composite can be calculated from the total thermal conductivity (
κ) minus the carrier thermal conductivity
κe (
κe =
Lσ
T), as shown in
Figure 9b. It can be observed that the
κL of 1.4%TiO
2/Cu
2SnSe
3 sample was significantly reduced before 450 K. The higher
κ of 1.4%TiO
2/Cu
2SnSe
3 in the temperature of 450–700 K is mainly due to the high
κe, which is closely related with the distribution of nano-TiO
2 phase in the internal grain. The expected decrease of
κL due to the addition of nano-TiO
2 is not evident in other TiO
2/Cu
2SnSe
3 composites, which is possibly attributed to the distribution of nano-TiO
2 in the grain boundaries. However, the
κL of 1.4%TiO
2/Cu
2SnSe
3 sample is 1.43 W/mK at room temperature, which is now the lowest value of lattice conductivity for Cu
2SnSe
3 sample and obviously lower than the previous studies. Fan et al. reported a lattice thermal conductivity of about 2.25 W/mK at room temperature for Ga-doped Cu
2SnSe
3 sample fabricated by hot pressing sintering [
10]. Compared with the Ga- or In-doped Cu
2SnSe
3 sample, the lower
κL of TiO
2/Cu
2SnSe
3 sample in this study should be related with the distribution of TiO
2.
Figure 10 shows the temperature dependence of the dimensionless figure of merit
ZT of TiO
2/Cu
2SnSe
3 composites. It can be seen that the
ZT value of 1.4%TiO
2/Cu
2SnSe
3 composite was not improved due to its higher
κ. The maximum
ZT value for 1.0%TiO
2/Cu
2SnSe
3 composite is 0.30 at 700 K, which is 17% higher than that of Cu
2SnSe
3 matrix.