Thermal Stability and Tuning of Thermoelectric Properties of Ag1−xSb1+xTe2+x (0 ≤ x ≤ 0.4) Alloys
Abstract
:1. Introduction
2. Experimental
2.1. Preparation
2.2. Powder X-ray Diffraction, Thermal Analysis and Chemical Analysis
2.3. Metallography and Scanning Electron Microscopy
2.4. High-Resolution Transmission Electron Microscopy
2.5. Spark Plasma Sintering and Thermoelectric Properties
3. Results & Discussion
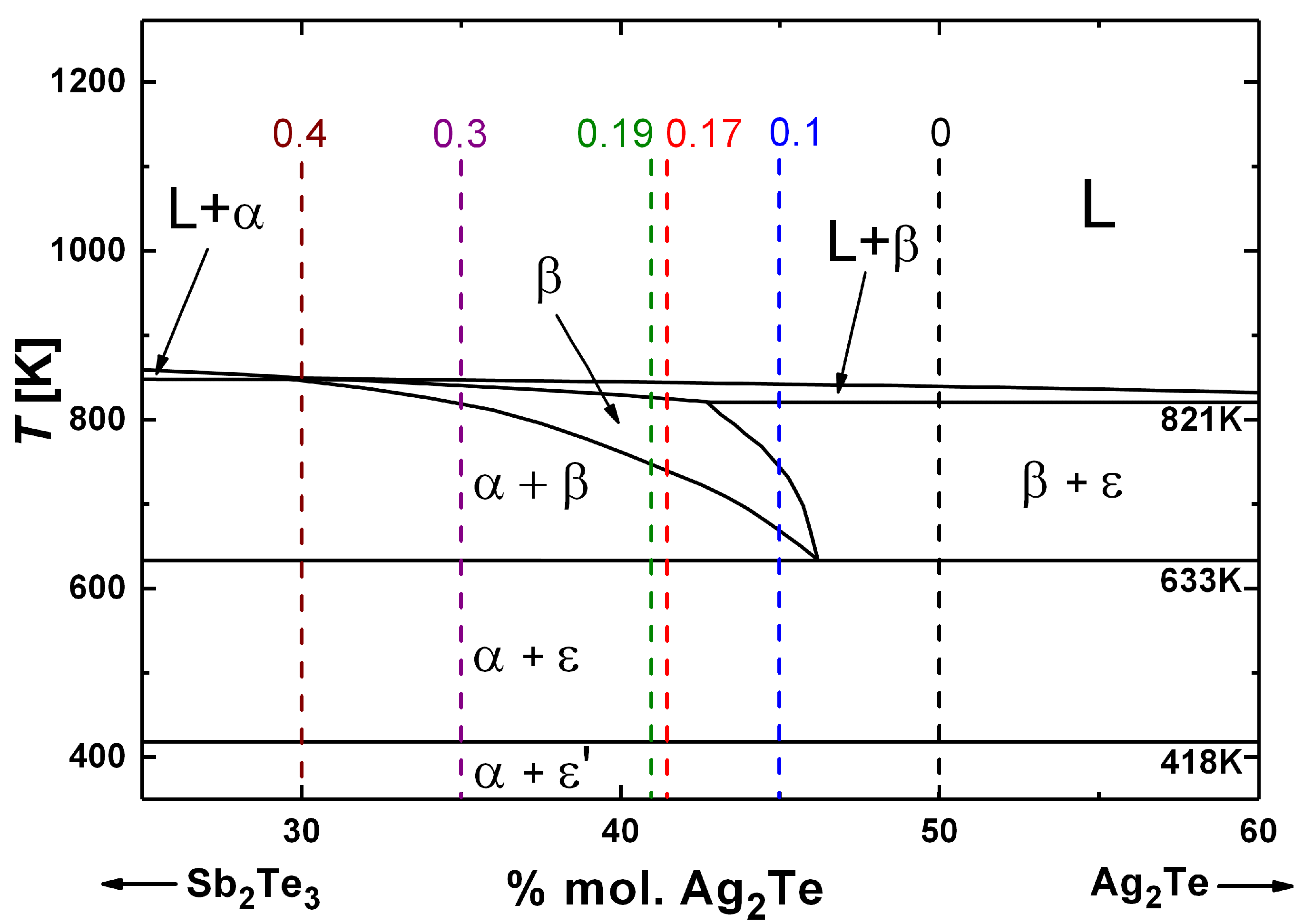
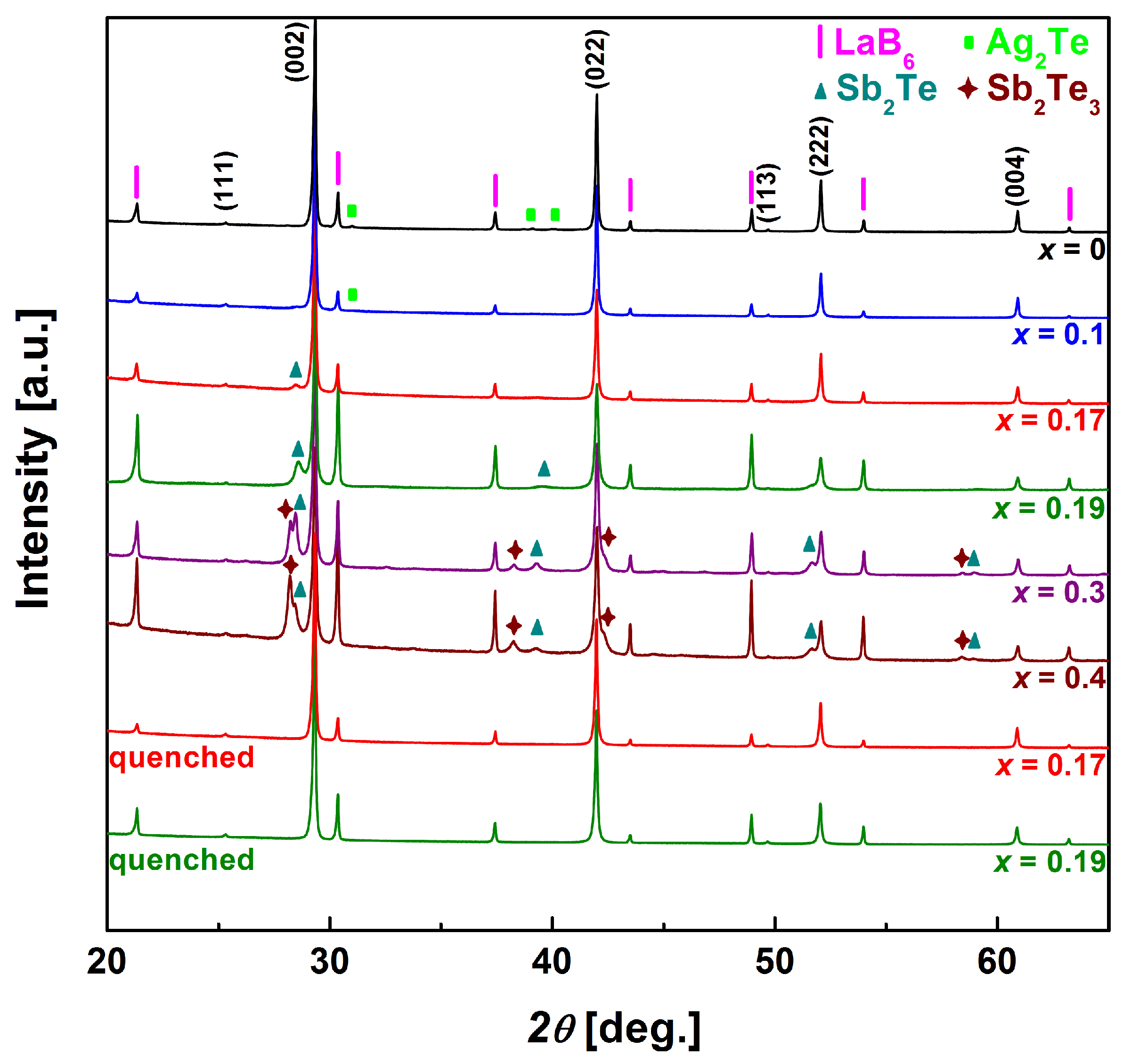
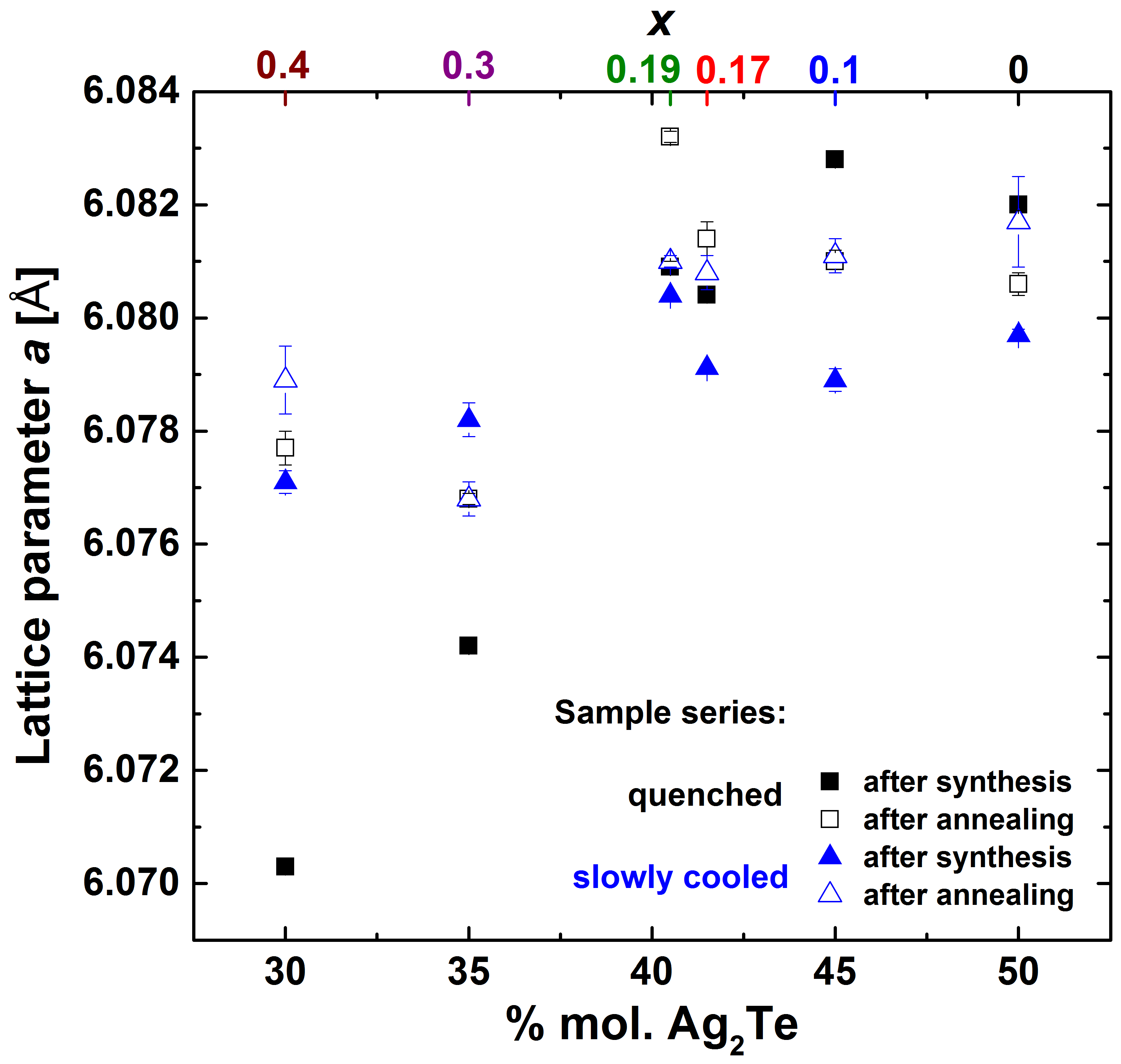
3.1. Structural and Thermal Properties
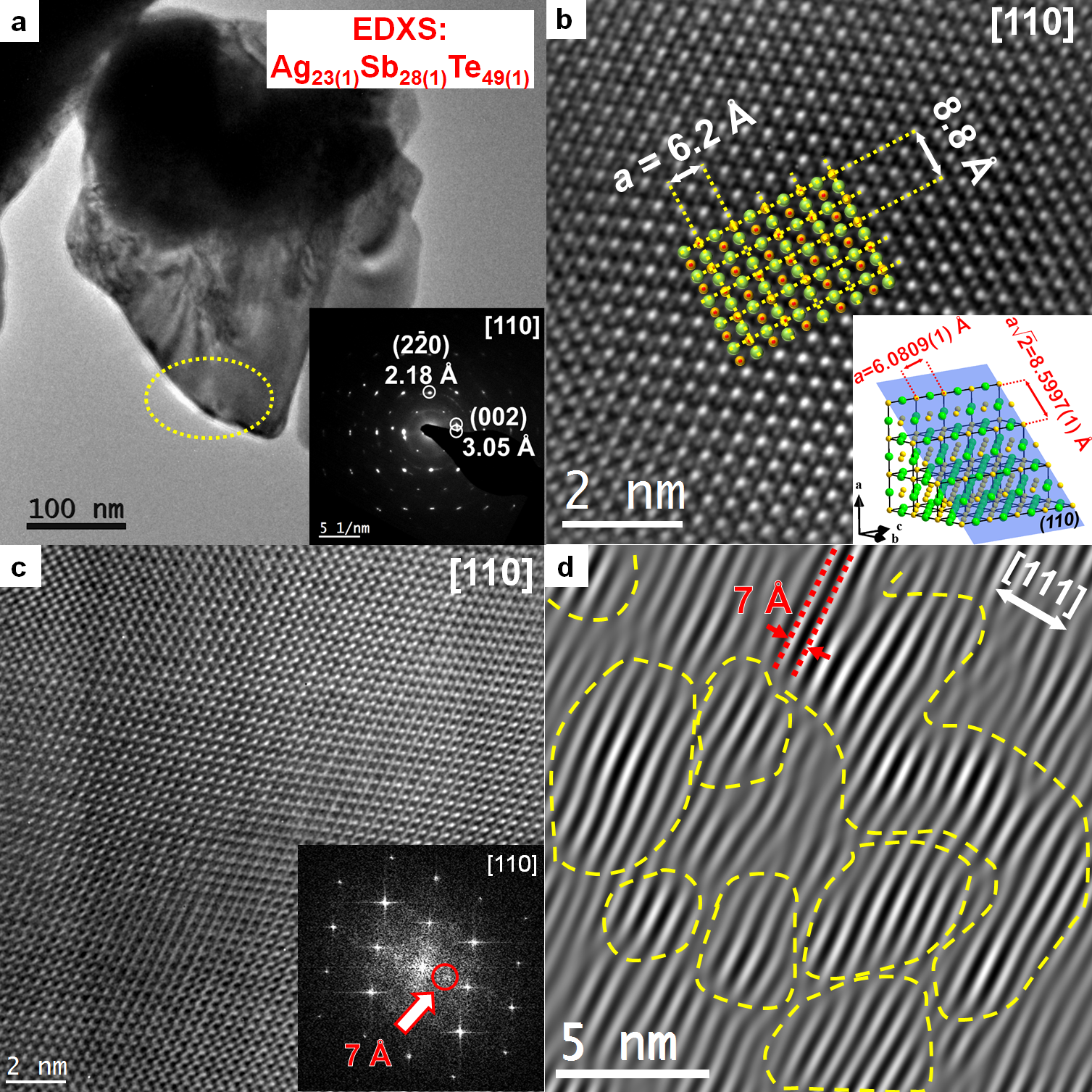
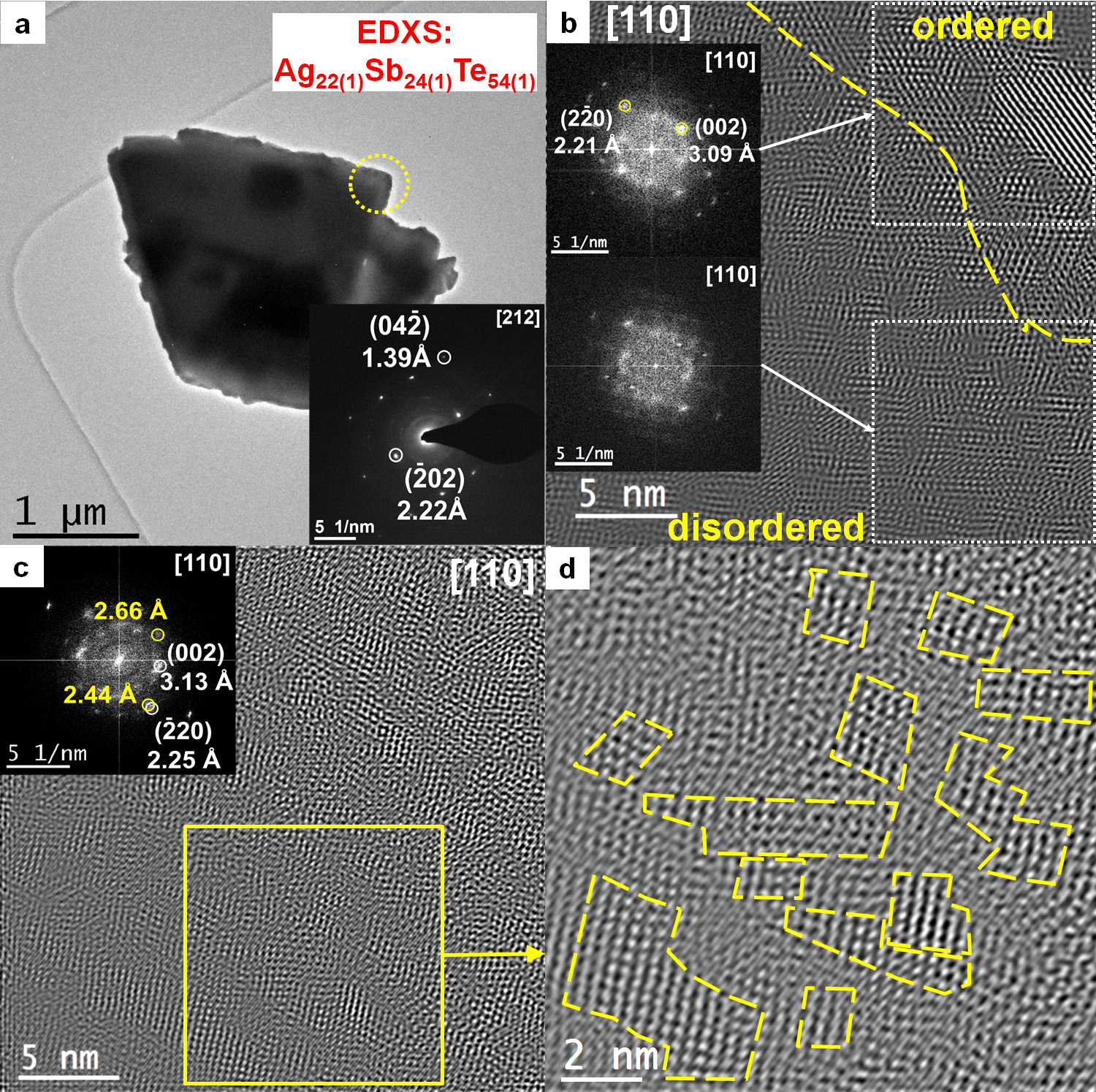
3.2. Microstructures
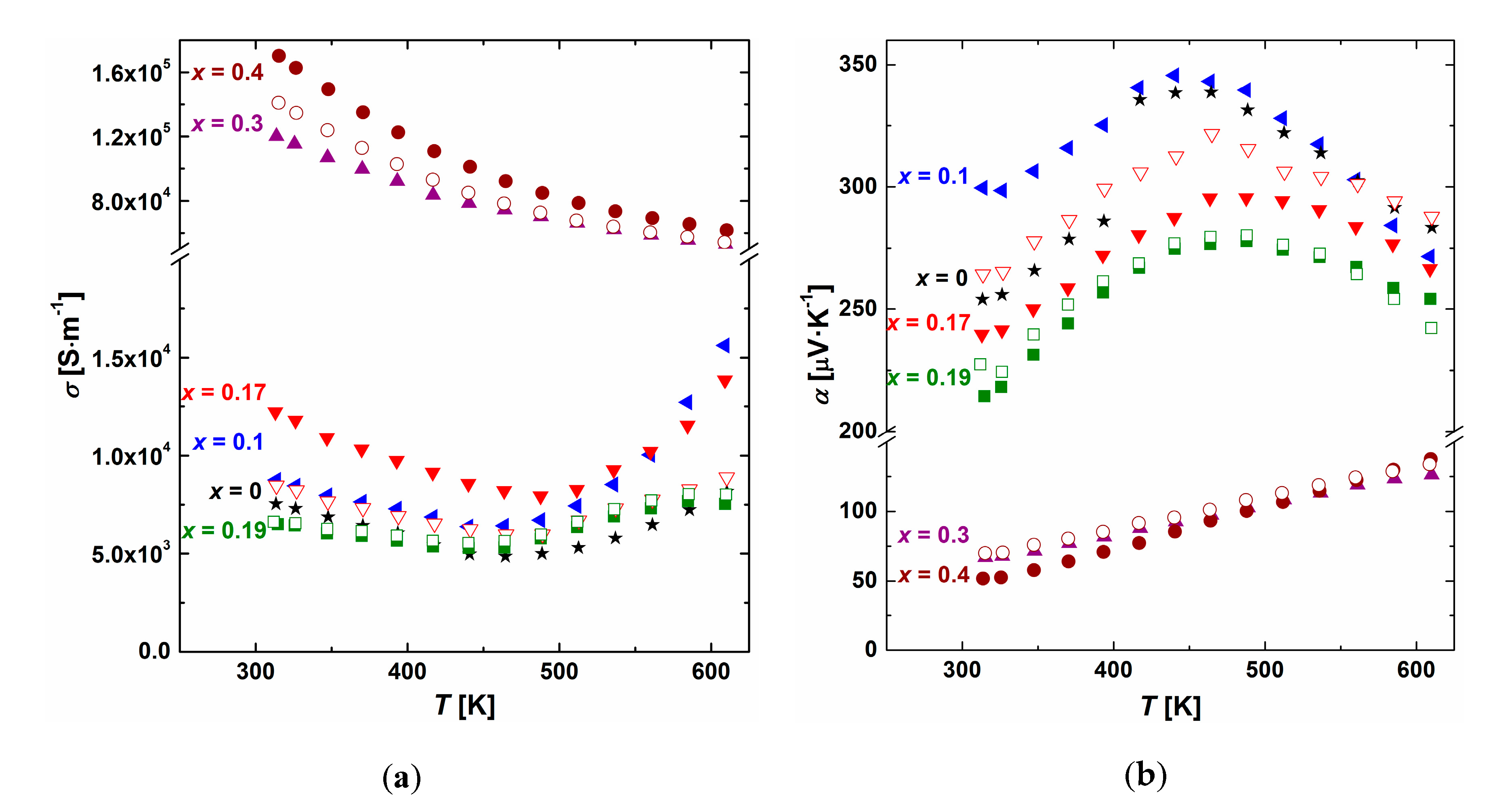
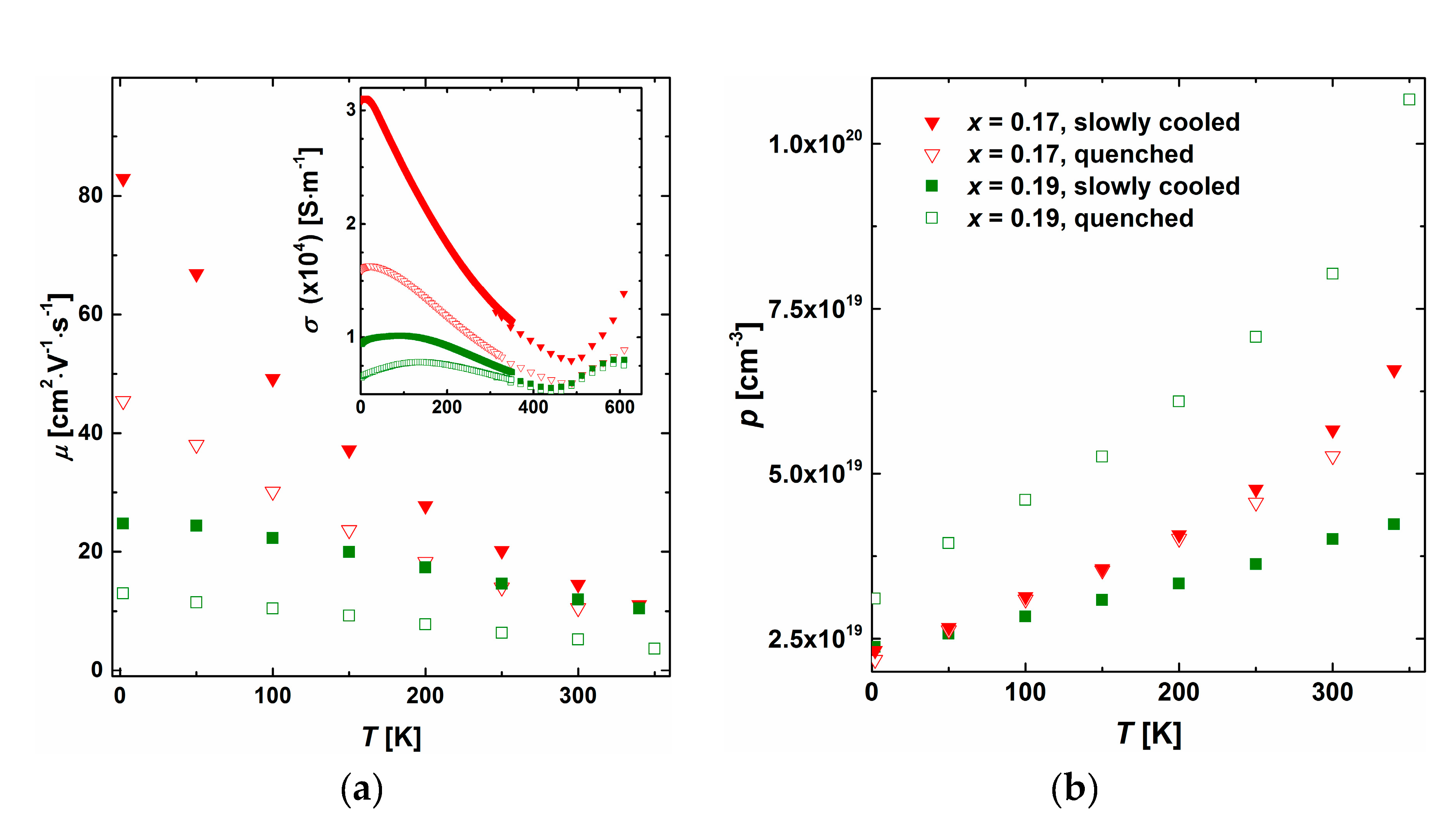
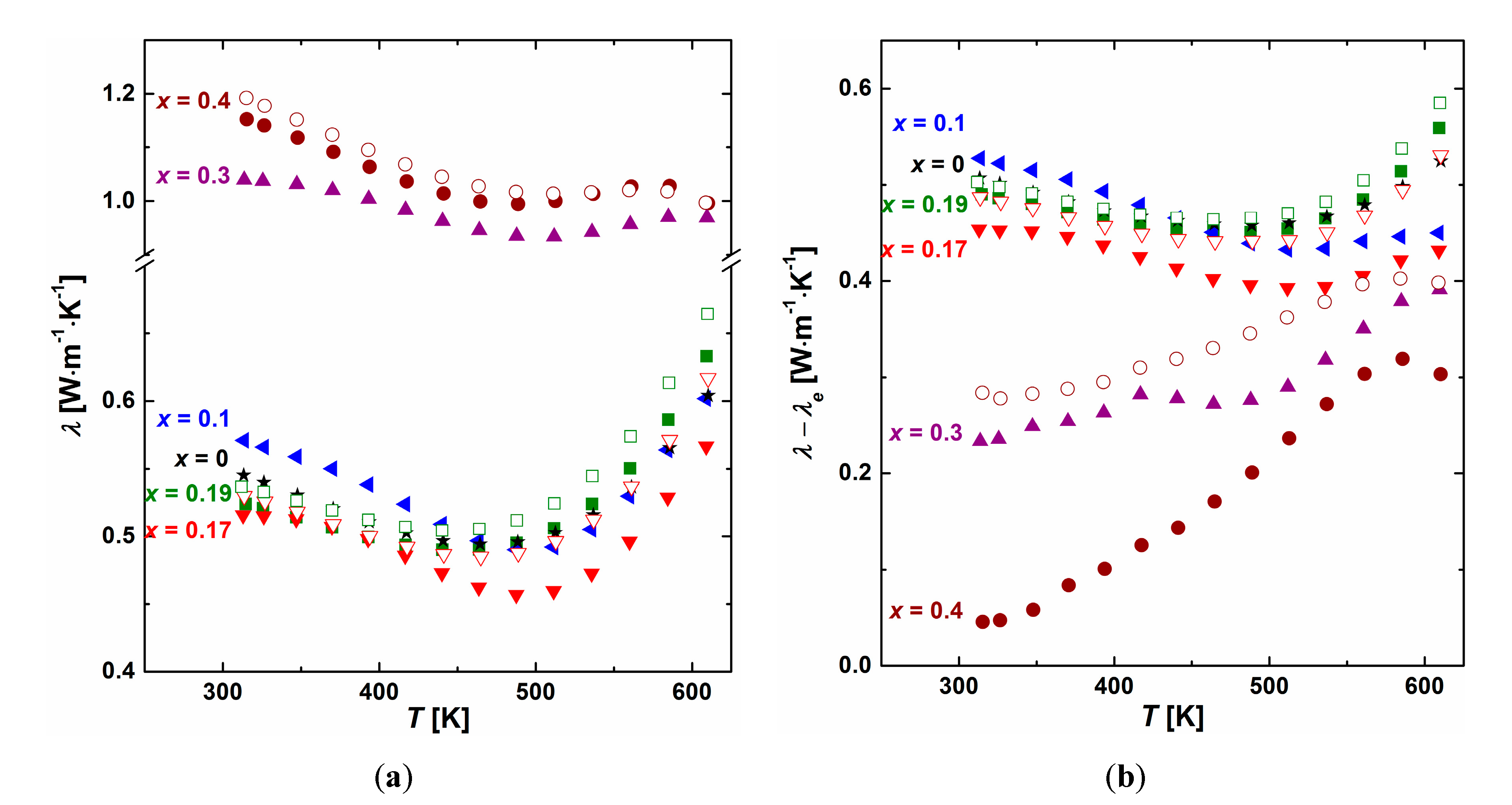
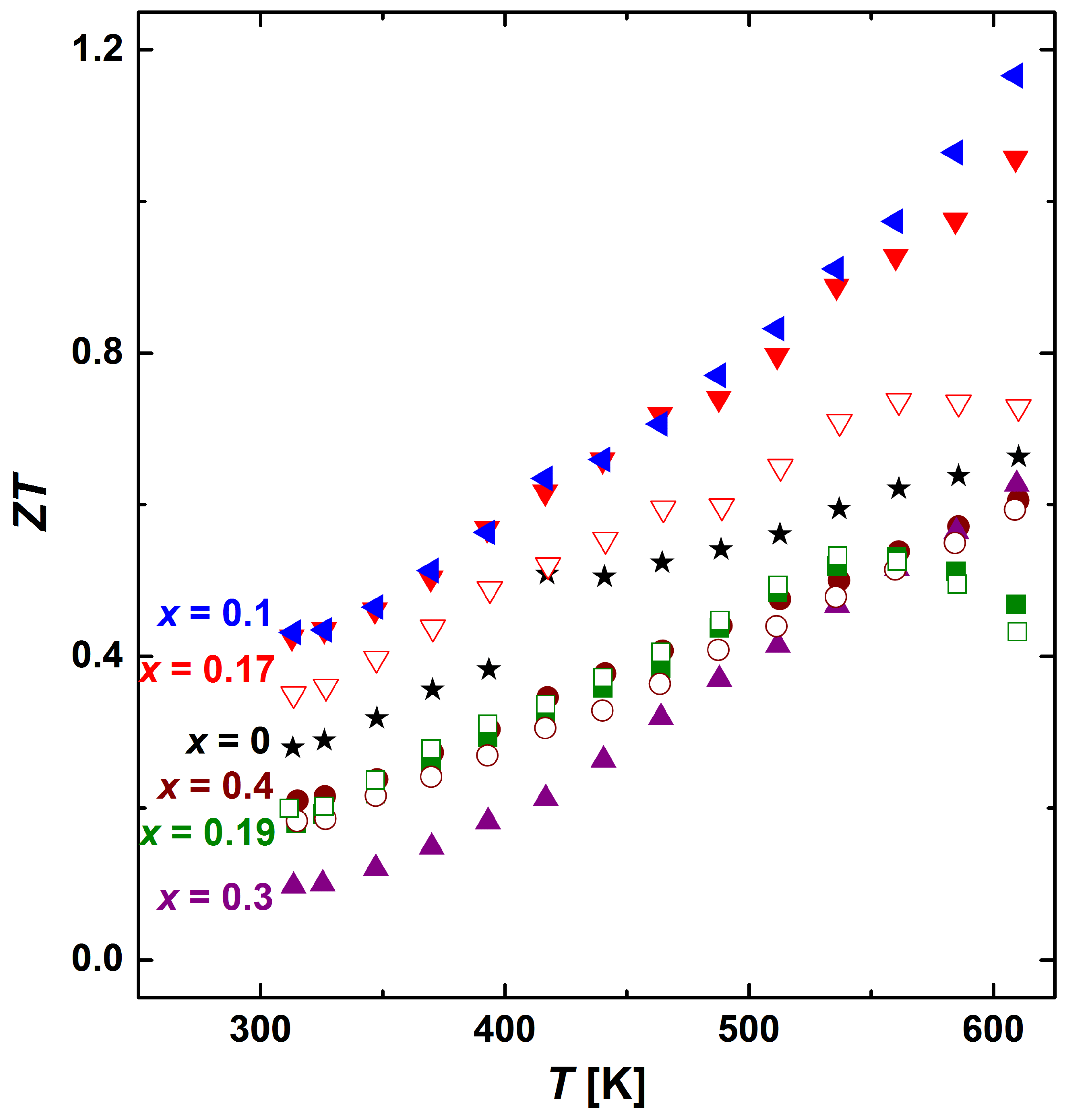
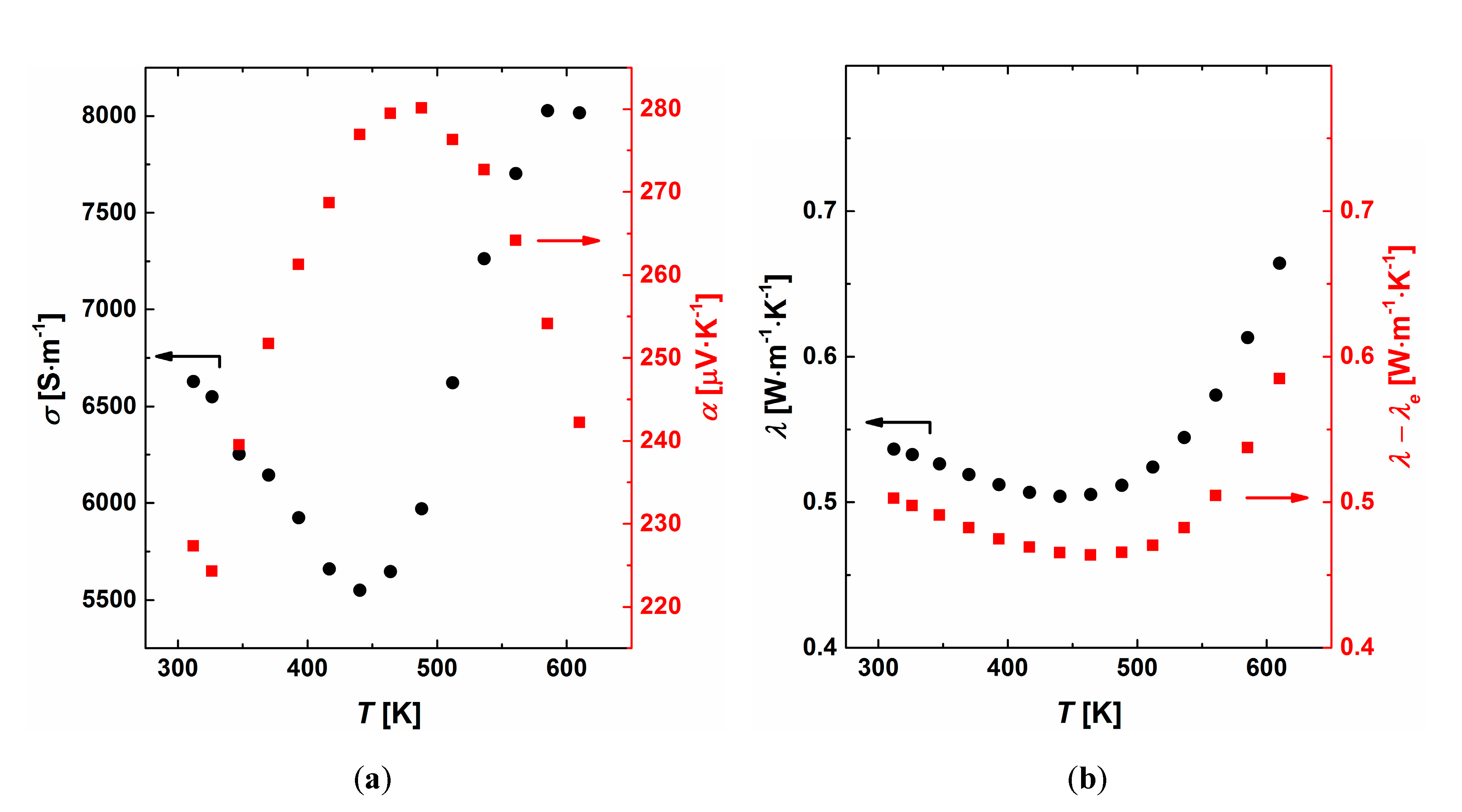
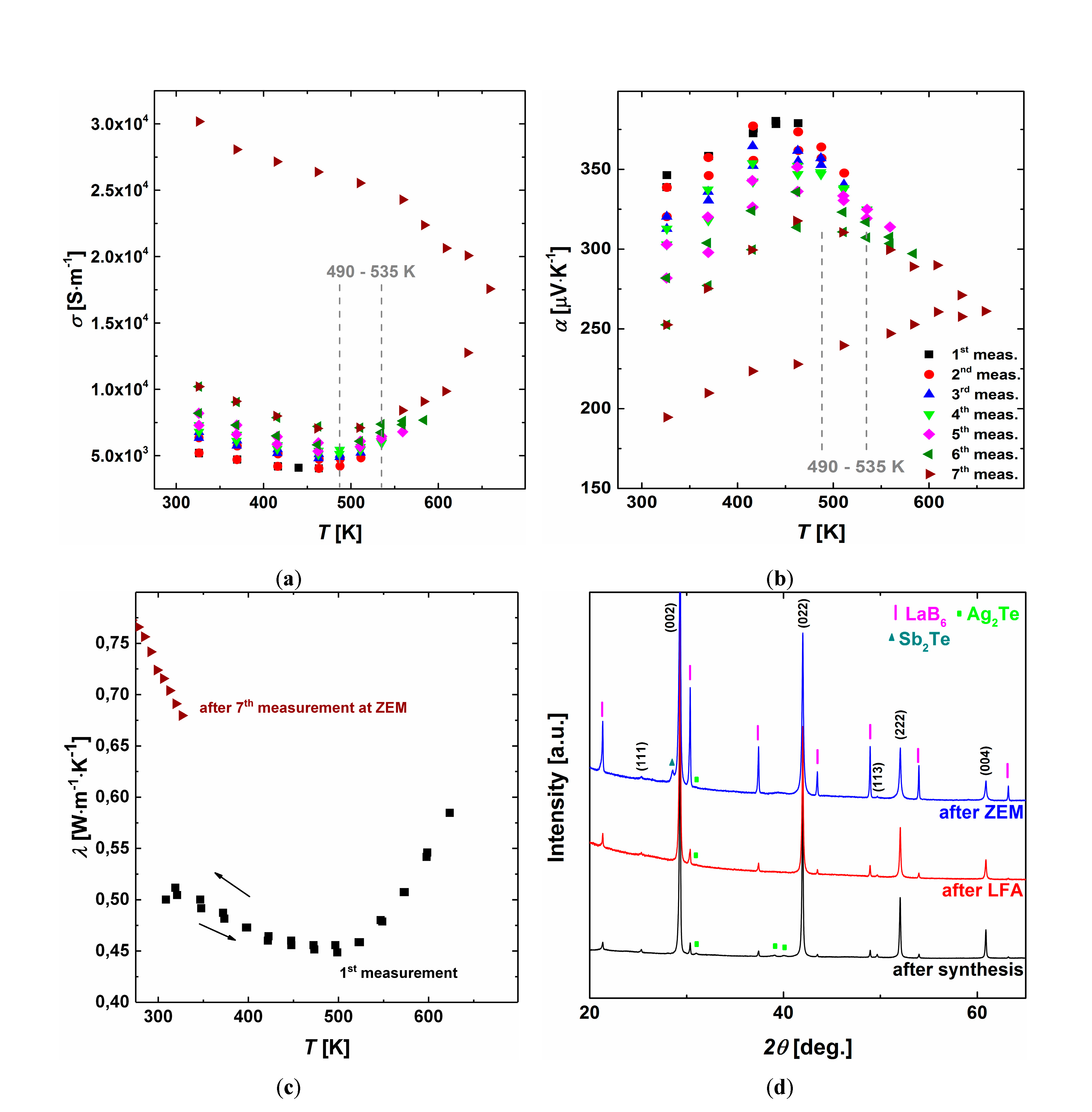
3.3. Thermoelectric Properties
3.4. Intrinsic Properties of the β Phase (Ag0.81Sb2.19Te2.19)
3.5. Nanostructure of the β Phase (Ag0.81Sb1.19Te2.19)
3.6. Thermodynamic Stability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tritt, T.M. Thermoelectric phenomena, materials, and applications. Annu. Rev. Mater. Res. 2011, 41, 433–448. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Panigrahi, R.; Perumal, S.; Biswas, K. Ultrahigh thermoelectric figure of merit and enhanced mechanical stability of p-type AgSb1−xZnxTe2. ACS Energy Lett. 2017, 2, 349–356. [Google Scholar] [CrossRef]
- Schmidt, M.; Wojciechowski, K.T. Reinvestigation of the influence of Se impurity on the structural and thermoelectric properties of AgSbTe2. AIP Conf. Proc. 2012, 1449, 175–178. [Google Scholar] [CrossRef]
- Du, B.; Li, H.; Xu, J.; Tang, X.; Uher, C. Enhanced figure-of-merit in Se-doped p-type AgSbTe2 thermoelectric compound. Chem. Mater. 2010, 22, 5521–5527. [Google Scholar] [CrossRef]
- Wu, H.J.; Lan, T.W.; Chen, S.W.; Chen, Y.Y.; Day, T.; Snyder, G.J. State of the art Ag50−xSbxSe50−yTey alloys: Their high ZT values, microstructures and related phase equilibria. Acta Mater. 2015, 93, 38–45. [Google Scholar] [CrossRef]
- Mohanraman, R.; Sankar, R.; Boopathi, K.M.; Chou, F.C.; Chu, C.W.; Lee, C.H.; Chen, Y.-Y. Influence of In doping on the thermoelectric properties of an AgSbTe2 compound with enhanced figure of merit. J. Mater. Chem. A 2014, 2, 2839–2844. [Google Scholar] [CrossRef]
- Morelli, D.T.; Jovovic, V.; Heremans, J.P. Intrinsically minimal thermal conductivity in cubic I-V-VI2 semiconductors. Phys. Rev. Lett. 2008, 101, 035901. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Chen, K.; Yan, H.; Reece, M.J. Efficacy of lone-pair electrons to engender ultralow thermal conductivity. Scr. Mater. 2016, 111, 49–53. [Google Scholar] [CrossRef]
- Ma, J.; Delaire, O.; May, A.F.; Carlton, C.E.; Mcguire, M.A.; VanBebber, L.H.; Abernathy, D.L.; Ehlers, G.; Hong, T.; Huq, A.; et al. Glass-like phonon scattering from a spontaneous nanostructure in AgSbTe2. Nat. Nanotechnol. 2013, 8, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Petzow, G.; Effenberg, G. Ternary Alloys: A Comprehensive Compendium of Evaluated Constitutional Data and Phase Diagrams; Wileys-VCH Verlag: Weinheim, Germany, 1998; pp. 552–554. ISBN 3-527-26967-3. [Google Scholar]
- Wu, H.J.; Chen, S.W. Phase equilibria of Ag-Sb-Te thermoelec tric materials. Acta Mater. 2011, 59, 6463–6472. [Google Scholar] [CrossRef]
- Wyzga, P.M.; Wojciechowski, K.T. Analysis of the influence of thermal treatment on the stability of Ag1-xSb1+xTe2+x and Se-doped AgSbTe2. J. Electron. Mater. 2016, 45, 1548–1554. [Google Scholar] [CrossRef]
- Wernick, J.H.; Benson, K.E. New semiconducting ternary compounds. J. Phys. Chem. Solids 1957, 3, 157–159. [Google Scholar] [CrossRef]
- Geller, S.; Wernick, J.H. Ternary semiconducting compounds with sodium chloride-like structure: AgSbSe2, AgSbTe2, AgBiS2, AgBiSe2. Acta Crystallogr. 1959, 12, 46–54. [Google Scholar] [CrossRef]
- Quarez, E.; Hsu, K.F.; Pcionek, R.; Frangis, N.; Polychroniadis, E.K.; Kanatzidis, M.G. Nanostructuring, compositional fluctuations, and atomic ordering in the thermoelectric materials AgPbmSbTe2+m. The myth of solid solutions. J. Am. Chem. Soc. 2005, 127, 9177–9190. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.; Toboła, J.; Schmidt, M.; Zybała, R. Structural and thermoelectric properties of AgSbSe2 and AgSbTe2. In Proceedings of the 5th European Conference on Thermoelectrics (ECT2007), Odessa, Ukraine, 10–12 September 2007. [Google Scholar]
- Rezaei, N.; Hashemifar, S.J.; Akbarzadeh, H. Thermoelectric properties of AgSbTe2 from first-principles calculations. J. Appl. Phys. 2014, 116, 103705. [Google Scholar] [CrossRef]
- Hoang, K.; Mahanti, S.D.; Salvador, J.R.; Kanatzidis, M.G. Atomic ordering and gap formation in Ag-Sb-based ternary chalcogenides. Phys. Rev. Lett. 2007, 99, 156403. [Google Scholar] [CrossRef] [PubMed]
- Marin-Ayral, R.M.; Legendre, B.; Brun, G.; Liautard, B.; Tedenac, J.C. The ternary system silver-antimony-tellurium. Study of the subternary Sb2Te3-Ag2Te-Te. Thermochim. Acta 1988, 131, 37–45. [Google Scholar] [CrossRef]
- Wojciechowski, K.T.; Schmidt, M. Structural and thermoelectric properties of AgSbTe2-AgSbSe2 pseudobinary system. Phys. Rev. B 2009, 79, 184202. [Google Scholar] [CrossRef]
- Du, B.; Yan, Y.; Tang, X. Variable-temperature in situ X-ray diffraction study of the thermodynamic evolution of AgSbTe2 thermoelectric compound. J. Electron. Mater. 2015, 44, 2118–2123. [Google Scholar] [CrossRef]
- Aspiala, M.; Taskinen, P. Thermodynamic study of the Ag-Sb-Te system with an advanced EMF method. J. Chem. Thermodyn. 2016, 93, 261–266. [Google Scholar] [CrossRef]
- Armstrong, R.W.; Faust, J.W., Jr.; Tiller, W.A. A structural study of the compound AgSbTe2. J. Appl. Phys. 1960, 31, 1954–1959. [Google Scholar] [CrossRef]
- Matsushita, H.; Hagiwara, E.; Katsui, A. Phase diagram and thermoelectric properties of Ag3−xSb1+xTe4 system. J. Mater. Sci. 2004, 39, 6299–6301. [Google Scholar] [CrossRef]
- Zhang, S.N.; Zhu, T.J.; Yang, S.H.; Yu, C.; Zhao, X.B. Phase compositions, nanoscale microstructures and thermoelectric properties in Ag2−ySbyTe1+y alloys with precipitated Sb2Te3 plates. Acta Mater. 2010, 58, 4160–4169. [Google Scholar] [CrossRef]
- Nielsen, M.D.; Jaworski, C.M.; Heremans, J.P. Off-stoichiometric silver antimony telluride: An experimental study of transport properties with intrinsic and extrinsic doping. AIP Adv. 2015, 5, 053602. [Google Scholar] [CrossRef]
- Jovovic, V.; Heremans, J.P. Measurements of the energy band gap and valence band structure of AgSbTe2. Phys. Rev. B 2008, 77, 245204. [Google Scholar] [CrossRef]
- Jung, H.; Park, H.; Choa, Y.; Myung, N.V. Composition-dependent electrical properties of ternary AgxSb1−xTey thin films synthesized by cationic exchange reaction. Electron. Mater. Lett. 2012, 8, 219–224. [Google Scholar] [CrossRef]
- Akselrud, L.; Grin, Y. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Max-Planck-Institut für Chemische Physik fester Stoffe. Scientific Report 2006–2008; Max-Planck-Institut für Chemische Physik Fester Stoffe: Dresden, Germany, 2009. [Google Scholar]
- Karakaya, I.; Thompson, W.T. The Ag-Te (silver-tellurium) system. J. Phase Equilib. 1991, 12, 56–63. [Google Scholar] [CrossRef]
- Cojocaru-Miredin, O.; Abdellaoui, L.; Nagli, M.; Zhang, S.; Yu, Y.; Scheu, C.; Raabe, D.; Wuttig, M.; Amouyal, Y. Role of nanostructuring and microstructuring in silver antimony telluride compounds for thermoelectric applications. ACS Appl. Matter. Interfaces 2017, 9, 14779–14790. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Chakraborty, B.R.; Bhattacharya, R.; Dutta, A.K. Electrical and magnetic properties of antimony telluride. Solid State Commun. 1978, 25, 617–620. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Introduction to Thermoelectricity, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 203. ISBN 978-3-642-00715-6. [Google Scholar]
- Ragimov, S.S.; Aliev, S.A. α → β phase transition of Ag2Te in the AgSbTe2 alloy of the Ag-Sb-Te system. Inorg. Mater. 2007, 43, 1184–1186. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]

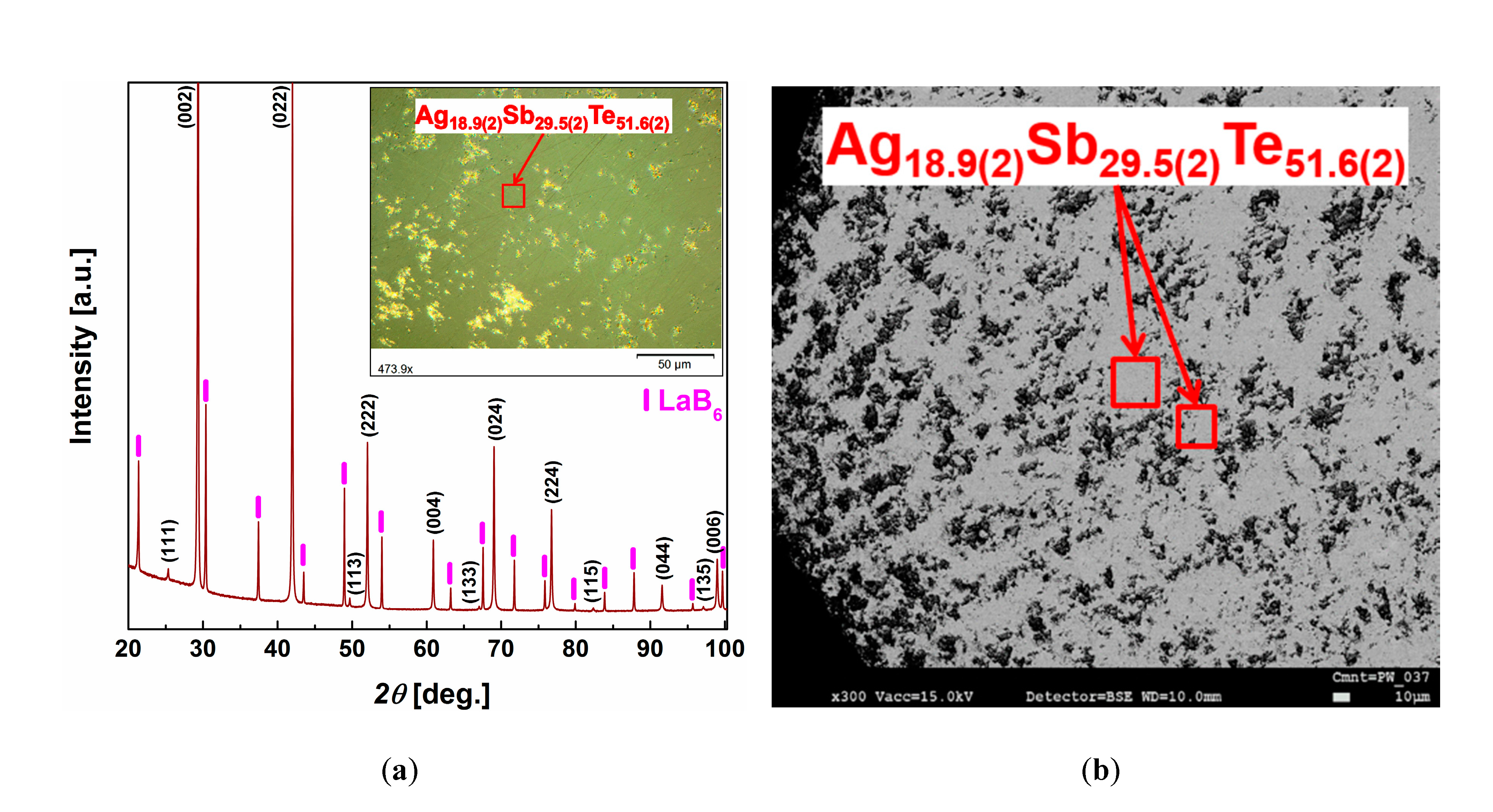
| x | Nominal Composition (at. %) | Composition of the β Phase according to EDXS (at. %) | ||
|---|---|---|---|---|
| After Quenching | After Slow Cooling | After LFA | ||
| 0.4 | Ag13.6Sb31.8Te54.6 | Ag15.6(2)Sb32.0(2)Te52.4(2) | Ag17.9(3)Sb29.3(2)Te52.8(3) | Ag16.6(8)Sb30.8(5)Te52.6(3) |
| 0.3 | Ag16.3Sb30.2Te53.5 | Ag14.9(5)Sb31(1)Te54.1(9) | Ag15.1(8)Sb31.4(5)Te53.5(3) | Ag17.5(2)Sb29.7(1)Te52.8(2) |
| 0.19 | Ag19.0Sb29.0Te52.0 | Ag18.9(2)Sb29.5(2)Te51.6(2) | Ag18.8(3)Sb29.5(2)Te51.7(1) | Ag18.2(6)Sb30.0(8)Te51.8(8) |
| 0.17 | Ag20.0Sb28.0Te52.0 | Ag19.7(3)Sb28.9(3)Te51.4(3) | Ag18.5(7)Sb29.3(3)Te52.2(4) | Ag20.1(6)Sb28.2(4)Te51.8(2) |
| 0.1 | Ag22.0Sb26.8Te51.2 | Ag20.0(1)Sb28.4(1)Te51.6(1) | Ag20.8(2)Sb28.3(2)Te50.9(1) | Ag18.3(4)Sb27.9(4)Te53.8(4) |
| 0 | Ag25.0Sb25.0Te50.0 | Ag21.0(3)Sb28.0(3)Te51.0(2) | Ag20.6(1)Sb28.2(3)Te51.2(2) | Ag21.1(1)Sb27.8(3)Te51.1(3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyżga, P.; Veremchuk, I.; Burkhardt, U.; Simon, P.; Grin, Y.; Wojciechowski, K.T. Thermal Stability and Tuning of Thermoelectric Properties of Ag1−xSb1+xTe2+x (0 ≤ x ≤ 0.4) Alloys. Appl. Sci. 2018, 8, 52. https://doi.org/10.3390/app8010052
Wyżga P, Veremchuk I, Burkhardt U, Simon P, Grin Y, Wojciechowski KT. Thermal Stability and Tuning of Thermoelectric Properties of Ag1−xSb1+xTe2+x (0 ≤ x ≤ 0.4) Alloys. Applied Sciences. 2018; 8(1):52. https://doi.org/10.3390/app8010052
Chicago/Turabian StyleWyżga, Paweł, Igor Veremchuk, Ulrich Burkhardt, Paul Simon, Yuri Grin, and Krzysztof T. Wojciechowski. 2018. "Thermal Stability and Tuning of Thermoelectric Properties of Ag1−xSb1+xTe2+x (0 ≤ x ≤ 0.4) Alloys" Applied Sciences 8, no. 1: 52. https://doi.org/10.3390/app8010052




