A Simplified Method to Estimate Sc-CO2 Extraction of Bioactive Compounds from Different Matrices: Chili Pepper vs. Tomato By-Products
Abstract
:1. Introduction
2. Materials and Methods
3. Results
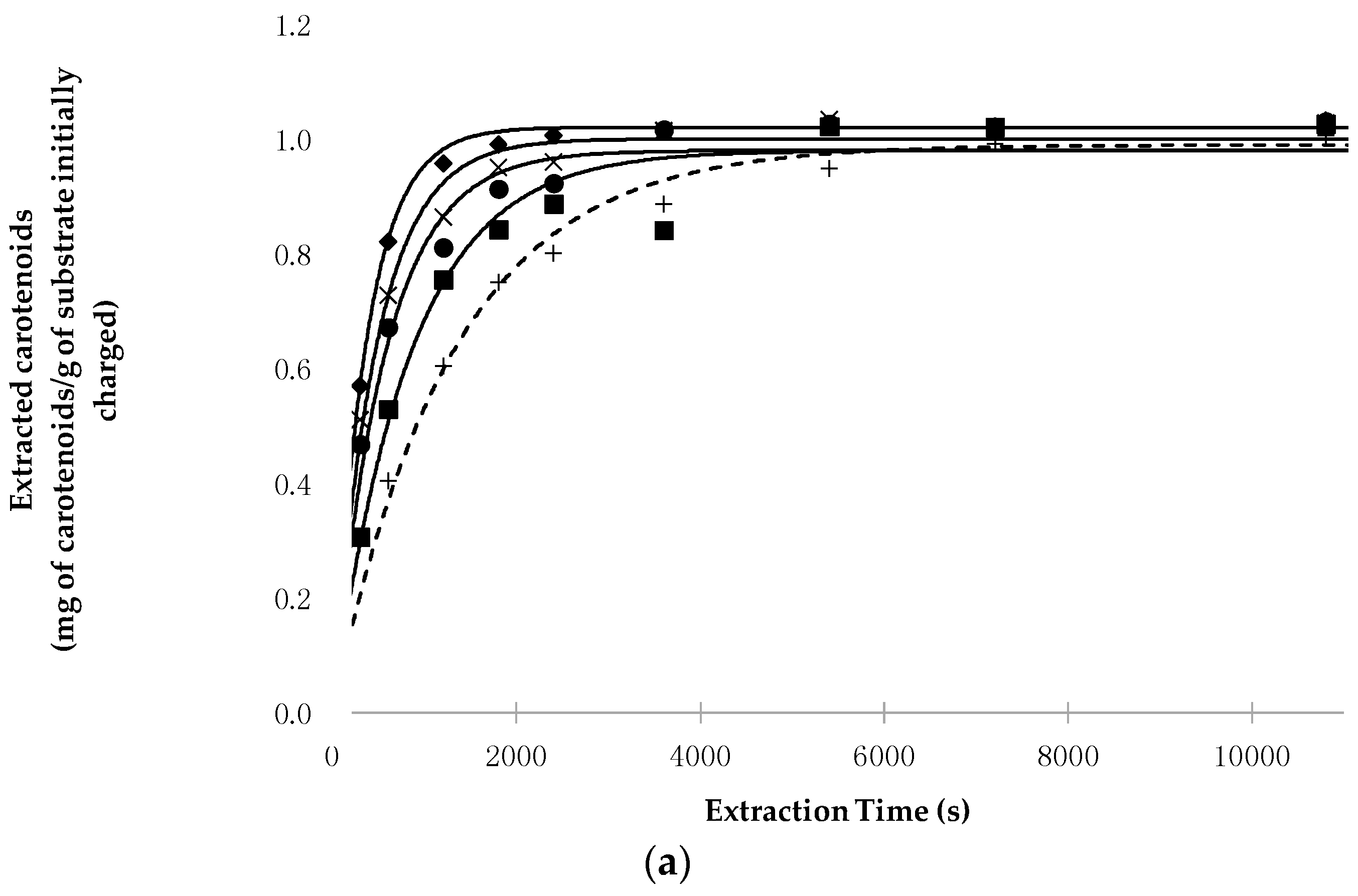
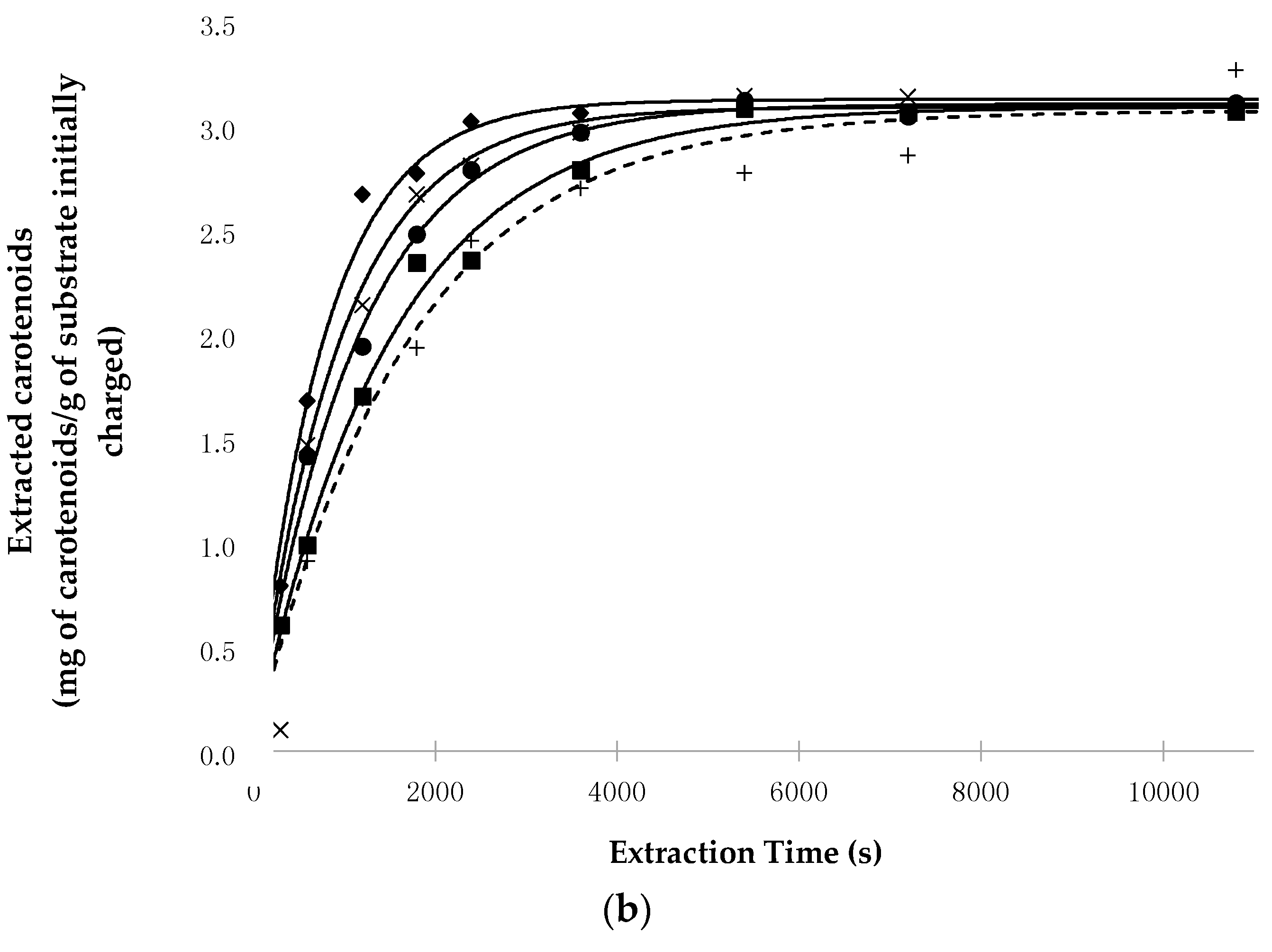
3.1. Kinetic Evaluation of Carotenoid Extraction
- (a)
- The increase in pressure and temperature does not significantly influence the total amount of carotenoids extractable at equilibrium (extraction time = ∞), as shown by the values assumed by the H* × [Cs] parameter. Indeed, this product—which represents the yield in milligrams of carotenoids extracted from one gram of lyophilized material when the equilibrium is reached—assumes that for all the SFE runs, the values used were close to the concentration of carotenoids in the starting material (1016 mg/kg for pepper and 3125 mg/kg for tomato peels) and also to the concentrations obtained with percolation with n-hexane;
- (b)
- P highly affects the extraction kinetics, as confirmed by the values of the k constant when working at the same T;
- (c)
- Secondarily, T also affects the kinetics of the SFE, as shown by the value that k assumes when working at the same P;
- (d)
- The binomial pressure-temperature combination is more crucial than T and P alone in determining the kinetics of the SFE process. In particular, transitioning from the mildest (40 °C and 40 MPa) to the most intense (60 °C and 70 MPa) working conditions, the value of k increases 2.3- and 1.9-fold for chili peppers and tomato peels, respectively.
- (e)
- In all conditions, extraction with Sc-CO2 was completed faster than percolation with n-hexane, as shown by the values assumed by k and/or Rmax. For example, the ratio between the value of k obtained at the most intense SFE conditions and that of the Soxhlet extraction was about 3.5 and 2.2 for chili pepper and tomato peels, respectively.
3.2. Kinetic Evaluation of Polyphenol Extraction
- (a)
- As previously observed with potato by-products [42], pure Sc-CO2 was confirmed to be a poor solvent for polyphenols, even when high density values were used;
- (b)
- EtOH is a suitable co-solvent used to pilot the polarity of the solvent phase, provided that high percentages are utilized (≥50%);
- (c)
- At an equal EtOH/Sc-CO2 ratio (1:1) and P (30 MPa), T greatly affects the extraction process, with particular reference to the total amount of extractable polyphenols. Indeed, while the kinetic constant k′ does not change markedly when T increases from 50 °C to 80 °C, the equation parameter H′* × [Ps] increases almost three-fold for chili peppers and four-fold for tomato by-products, from 50 °C to 80 °C. This means that such temperature increases determine the solubilization of phenolic compounds that are otherwise not easily collectable;
- (d)
- To obtain the extraction of the whole phenolic fraction, pure EtOH at 80 °C, and 30 MPa or 50 MPa conditions were needed. In such conditions, the extraction process was faster than when it was carried out using the Soxhlet apparatus, as shown by the values of the kinetic constant k′. For example, working at 80 °C and 30 MPa, the ratio between the value of Rmax obtained in SFE conditions and of the Soxhlet extraction, was about 2.0 and 1.3 for chili pepper and tomato peels, respectively;
- (e)
- At 80 °C and 30 MPa, if EtOH decreased from 100% to 50%, only a small decrease in the extraction yield and kinetics was observed.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Le Grandois, J.; Guffond, D.; Hamon, E.; Marchioni, E.; Werner, D. Combined microplate-ABTS and HPLC-ABTS analysis of tomato and pepper extracts reveals synergetic and antagonist effect of their lipophilic antioxidative components. Food Chem. 2017, 223, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- FAOSTAT. 2005. Available online: http://faostat.fao.org (accessed on 25 March 2006).
- Al-Wandawi, H.; Abdul-Rahman, M.; Al-Shaikhly, K. Tomato processing wastes as essential raw materials source. J. Agric. Food Chem. 1985, 33, 804–807. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT Food Sci. Technol. 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Curran, T.P.; Ferreira, I.C.F.R. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: A sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 2017. [Google Scholar] [CrossRef]
- Walsh, B.M.; Hoot, S.B. Phylogenetic relashionships of Capsicum (Solanaceae) using DNA sequence from two noncoding regions: The chloroplast atpB-rbcL spacer regions and nuclear waxy introns. Int. J. Plant Sci. 2001, 162, 1409–1418. [Google Scholar] [CrossRef]
- Khan, F.A.; Mahmood, T.; Ali, M.; Saeed, A.; Maalik, A. Pharmacological importance of an ethnobotanical plant: Capsicum annuum L. Nat. Prod. Res. 2014, 28, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, J.F.; Godoy, H.B.; Martínez, J. Supercritical carbon dioxide extraction of Capsicum peppers: Global yield and capsaicinoid content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Topuz, A.; Ozdemir, F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Jin, J.S.; Cho, Y.A.; Lee, J.H.; Park, S.; Jeong, S.W.; Kim, Y.; Lim, C.; Abd El-Aty, A.M.; Kim, G.; et al. Determination of polyphenols in three Capsicum annuum L. (bell pepper) varieties using high-performance liquid chromatography-tandem mass spectrometry: Their contribution to overall antioxidant and anticancer activity. J. Sep. Sci. 2011, 34, 2967–2974. [Google Scholar] [PubMed]
- Meireles, M.A.A. Extracting Bioactive Compounds for Food Products: Theory and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 288–326. [Google Scholar]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical Carbon Dioxide Extraction of Carotenoids from Pumpkin (Cucurbita spp.): A Review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed]
- Maadane, A.; Merghoub, N.; Ainane, T.; ElArroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; García-Cañas, V.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Comparative study of green sub- and supercritical processes to obtain carnosic acid and carnosol-enriched rosemary extracts with in vitro anti-proliferative activity on colon cancer cells. Int. J. Mol. Sci. 2016, 17, 2046. [Google Scholar] [CrossRef] [PubMed]
- Campardelli, R.; Baldino, L.; Reverchon, E. Supercritical fluids applications in nanomedicins. J. Supercrit. Fluids 2015, 101, 193–214. [Google Scholar] [CrossRef]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical fluid extraction using CO2. Main applications and future perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Herrero, M.; Sánchez-Camargo, A.D.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Reverchon, E.; de Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Mattea, F.; Martín, Á.; Cocero, M.J. Carotenoid processing with supercritical fluids. J. Food Eng. 2009, 93, 255–265. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of Solids and Liquids in Supercritical Gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Sovová, H. Steps of Supercritical Fluid Extraction of Natural Products and Their Characteristic Times. J. Supercrit. Fluids 2012, 66, 73–79. [Google Scholar] [CrossRef]
- Andrich, G.; Balzini, S.; Zinnai, A.; de Vitis, V.; Silvestri, S.; Venturi, F.; Fiorentini, R. Supercritical fluid extraction in sunflower seed technology. Eur. J. Lipid Sci. Technol. 2001, 103, 151–157. [Google Scholar] [CrossRef]
- Andrich, G.; Zinnai, A.; Nottoli, S.; Venturi, F.; Fiorentini, R. A Mathematical Model Describing the Supercritical Fluid Extraction (SFE) of Oil from Soybean Seeds. Chem. Eng. Trans. 2002, 2, 397–401. [Google Scholar]
- Andrich, G.; Zinnai, A.; Venturi, F.; Fiorentini, R. A Mathematical Model Describing the Supercritical Fluid Extraction (SFE) of Rapeseed (Brassica Napus) Oil. Chem. Eng. Trans. 2003, 3, 1605–1610. [Google Scholar]
- Andrich, G.; Nesti, U.; Venturi, F.; Zinnai, A.; Fiorentini, R. Supercritical Fluid Extraction of Bioactive Lipids from Microalga Nannochloropsis sp. Eur. J. Lipid Sci. Technol. 2005, 107, 381–386. [Google Scholar] [CrossRef]
- Andrich, G.; Zinnai, A.; Nesti, U.; Venturi, F.; Fiorentini, R. Supercritical Fluid Extraction of Oil from Microalga (Arthrospira platensis). Acta Aliment. 2006, 35, 195–203. [Google Scholar] [CrossRef]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercritical fluid extraction from microalgae with high content of LC-PUFAs. A case of study: Sc-CO2 oil extraction from Schizochytrium spp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Andrich, G. A Simplified Method to Estimate the Time Evolution of Oil Extraction from Different Substrates by Supercritical CO2. Am. J. Anal. Chem. 2012. [Google Scholar] [CrossRef]
- Martinez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicon esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1997, 28, 49–55. [Google Scholar]
- Yu, Z.; Sing, B.; Rizvi, S.H.; Zollweg, J.A. Solubilities of fatty acids, fatty acid esters, triglycerides, and fats and oils in supercritical carbon dioxide. J Supercrit. Fluids. 1994, 7, 51–59. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Xiaoguo, Y.; Andrich, G.; Zinnai, A. The influence of packaging on the sensorial evolution of white wine as a function of the operating conditions adopted during storage. Agrochimica 2016, 60, 150–160. [Google Scholar]
- Andrich, G.; Stevanin, E.; Zinnai, A.; Venturi, F.; Fiorentini, R. Extraction kinetics of natural antioxidants from potato industry by-products. In Proceedings of the VI Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003; Volume 1, pp. 159–163. [Google Scholar]
- Pereira, C.; Meireles, M.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Meireles, M.A.A. Fundamentals of supercritical fluid extraction. In Comprehensive Sampling and Sample Preparation; Janusz, P., Ed.; Academic Press: Oxford, UK, 2012; pp. 117–133. [Google Scholar]


| Matrix | T (°C) | P (MPa) | (k) × 104 (s−1) | (H* × [Cs]) × 103 (Adimensional) | (Rmax) × 106 (s−1) | r2 |
|---|---|---|---|---|---|---|
| (c.i. ≤ 0.01× 10−4) | (c.i. ≤ 0.01 × 10−3) | (c.i. ≤ 0.01 × 10−6) | ||||
| C. annuum L. | 40 | 40 | 11.98 | 0.97 | 1.16 | 0.95 |
| 40 | 70 | 21.32 | 0.99 | 2.11 | 0.97 | |
| 60 | 40 | 17.76 | 0.99 | 1.75 | 0.95 | |
| 60 | 70 | 27.26 | 1.01 | 2.74 | 0.98 | |
| Soxhlet extraction | 7.69 | 0.98 | 0.98 | 0.75 | ||
| L. esculentum L. | 40 | 40 | 6.79 | 3.13 | 2.12 | 0.98 |
| 40 | 70 | 10.65 | 3.13 | 3.33 | 0.98 | |
| 60 | 40 | 8.86 | 3.14 | 2.78 | 0.98 | |
| 60 | 70 | 12.93 | 3.16 | 4.09 | 0.99 | |
| Soxhlet extraction | 6.00 | 3.11 | 1.87 | 0.97 | ||
| Matrix | a ± c.i. | −(b ± c.i.) × 10−3 | −(c ± c.i.) | r2 |
|---|---|---|---|---|
| C. annuum L. | 6.09 ± 0.51 | 2.93 ± 0.23 | 34.47 ± 3.10 | 0.96 |
| L. esculentum L. | 5.21 ± 0.42 | 2.33 ± 0.19 | 29.82 ± 2.72 | 0.98 |
| Matrix | T (°C) | P (MPa) | ρ Sc-CO2 (g/L) | R*max (g/L) | (k × 103) (s−1) |
|---|---|---|---|---|---|
| C. annuum L. | 40 | 40 | 953 | 0.129 | 1.26 |
| 40 | 70 | 1033 | 0.210 | 2.05 | |
| 60 | 40 | 887 | 0.146 | 1.42 | |
| 60 | 70 | 987 | 0.280 | 2.73 | |
| L. esculentum L. | 40 | 40 | 953 | 0.222 | 0.63 |
| 40 | 70 | 1033 | 0.338 | 0.96 | |
| 60 | 40 | 887 | 0.238 | 0.68 | |
| 60 | 70 | 987 | 0.416 | 1.19 |
| Matrix | EtOH (%) | Sc-CO2 (%) | T (°C) | P (MPa) | (k) × 104 (s−1) | (H* × [Cs]) × 103 (Adimensional) | (Rmax) × 106 (s−1) | r2 |
|---|---|---|---|---|---|---|---|---|
| (c.i. ≤ 0.01 × 10−4) | (c.i. ≤ 0.01 × 10−3) | (c.i. ≤ 0.01 × 10−6) | ||||||
| C. annuum L. | 50 | 50 | 50 | 30 | 6.92 | 10.12 | 7.00 | 0.95 |
| 50 | 50 | 80 | 30 | 7.48 | 28.73 | 21.48 | 0.97 | |
| 100 | -- | 80 | 30 | 9.16 | 31.96 | 29.28 | 0.98 | |
| 100 | -- | 80 | 50 | 12.78 | 31.68 | 40.48 | 0.98 | |
| Soxhlet extraction | 5.38 | 30.90 | 16.63 | 0.99 | ||||
| L. esculentum L. | 50 | 50 | 50 | 30 | 5.46 | 0.64 | 0.35 | 0.96 |
| 50 | 50 | 80 | 30 | 9.84 | 2.42 | 2.34 | 0.92 | |
| 100 | -- | 80 | 30 | 10.64 | 2.85 | 3.03 | 0.94 | |
| 100 | -- | 80 | 50 | 14.02 | 2.83 | 3.96 | 0.98 | |
| Soxhlet extraction | 8.33 | 2.86 | 2.38 | 0.91 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Zinnai, A. A Simplified Method to Estimate Sc-CO2 Extraction of Bioactive Compounds from Different Matrices: Chili Pepper vs. Tomato By-Products. Appl. Sci. 2017, 7, 361. https://doi.org/10.3390/app7040361
Venturi F, Sanmartin C, Taglieri I, Andrich G, Zinnai A. A Simplified Method to Estimate Sc-CO2 Extraction of Bioactive Compounds from Different Matrices: Chili Pepper vs. Tomato By-Products. Applied Sciences. 2017; 7(4):361. https://doi.org/10.3390/app7040361
Chicago/Turabian StyleVenturi, Francesca, Chiara Sanmartin, Isabella Taglieri, Gianpaolo Andrich, and Angela Zinnai. 2017. "A Simplified Method to Estimate Sc-CO2 Extraction of Bioactive Compounds from Different Matrices: Chili Pepper vs. Tomato By-Products" Applied Sciences 7, no. 4: 361. https://doi.org/10.3390/app7040361