Dense Pure Tungsten Fabricated by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Methods
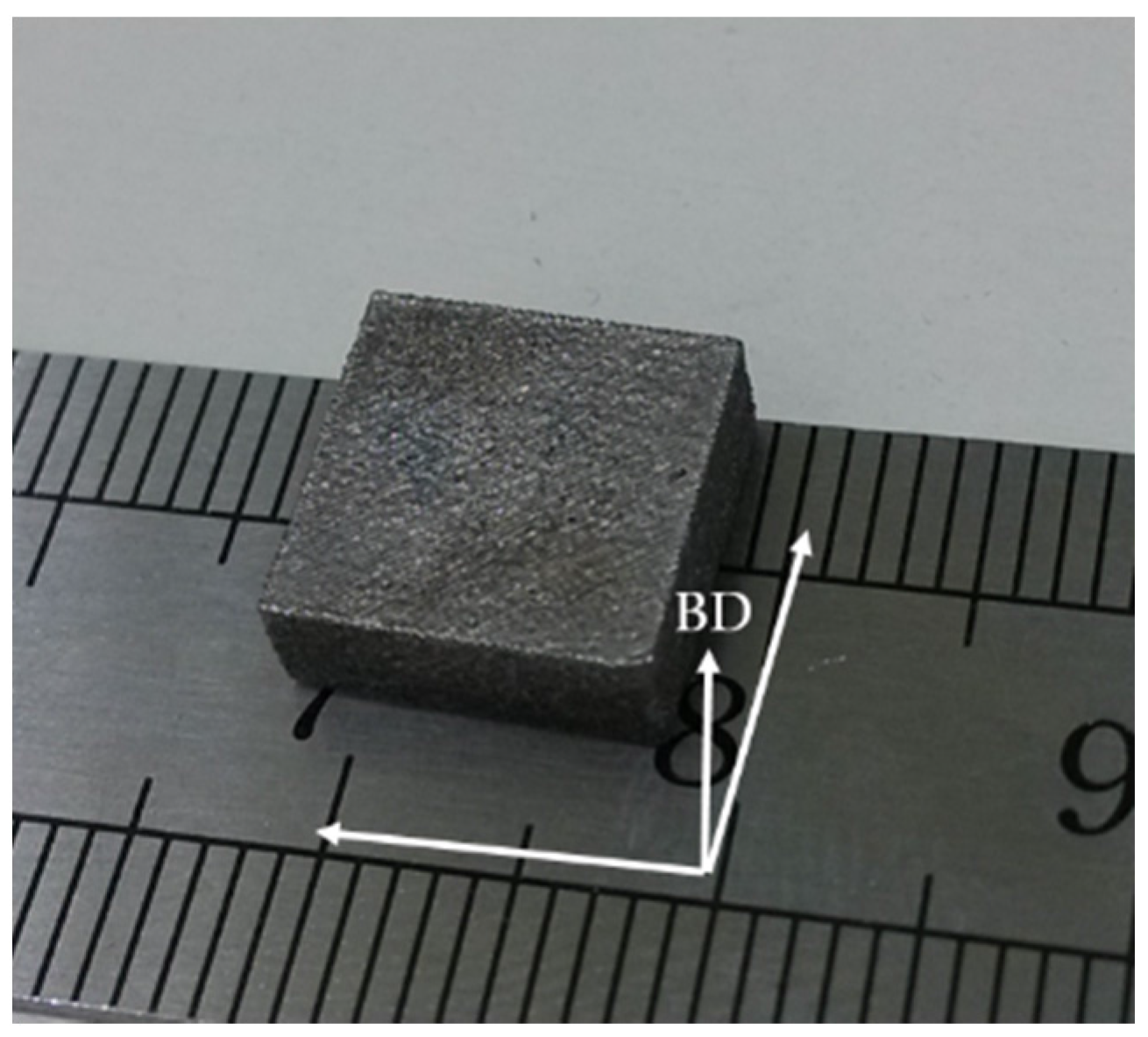
2.1. Powder Spheroidization and the Selective Laser Melting Process
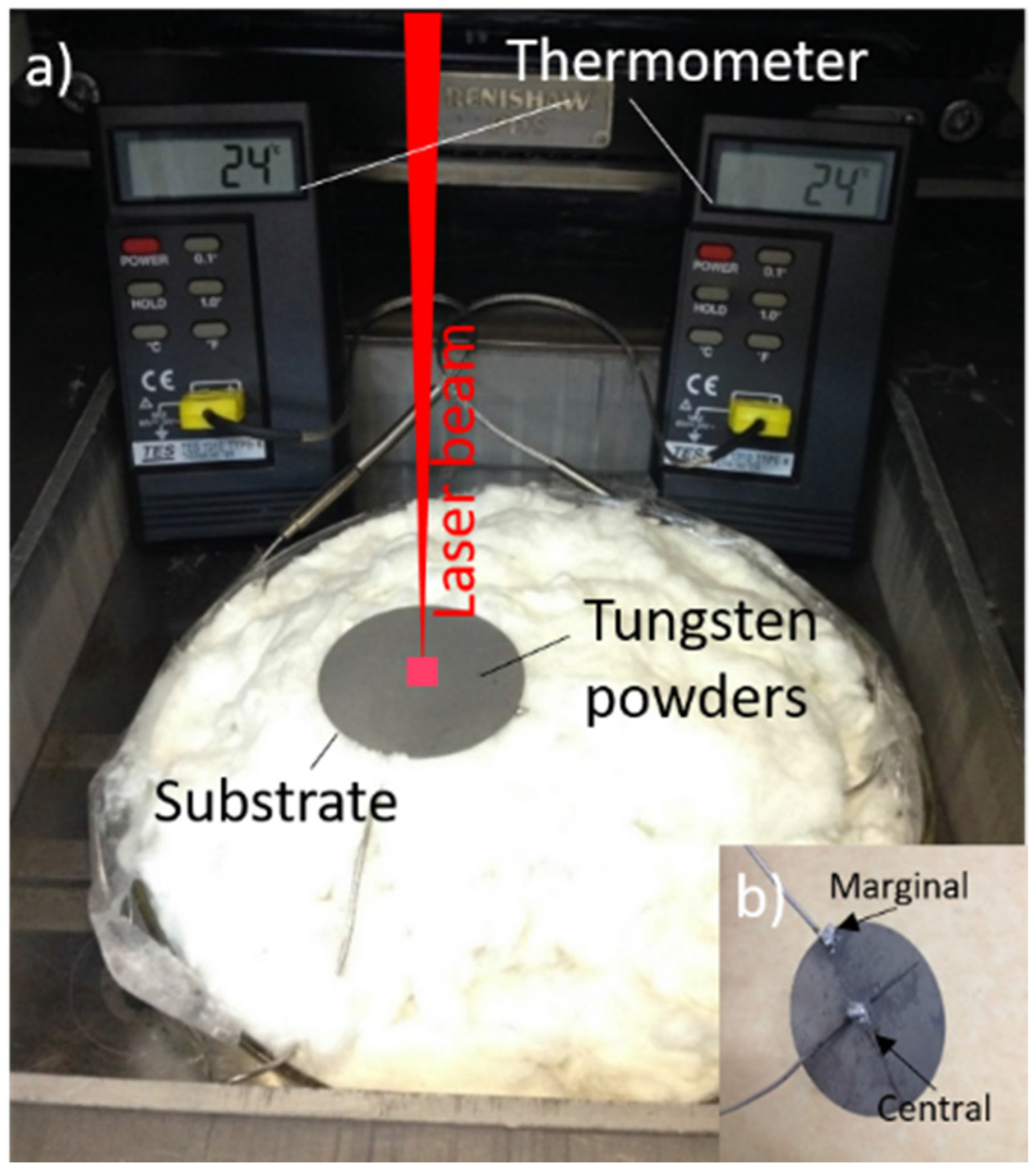
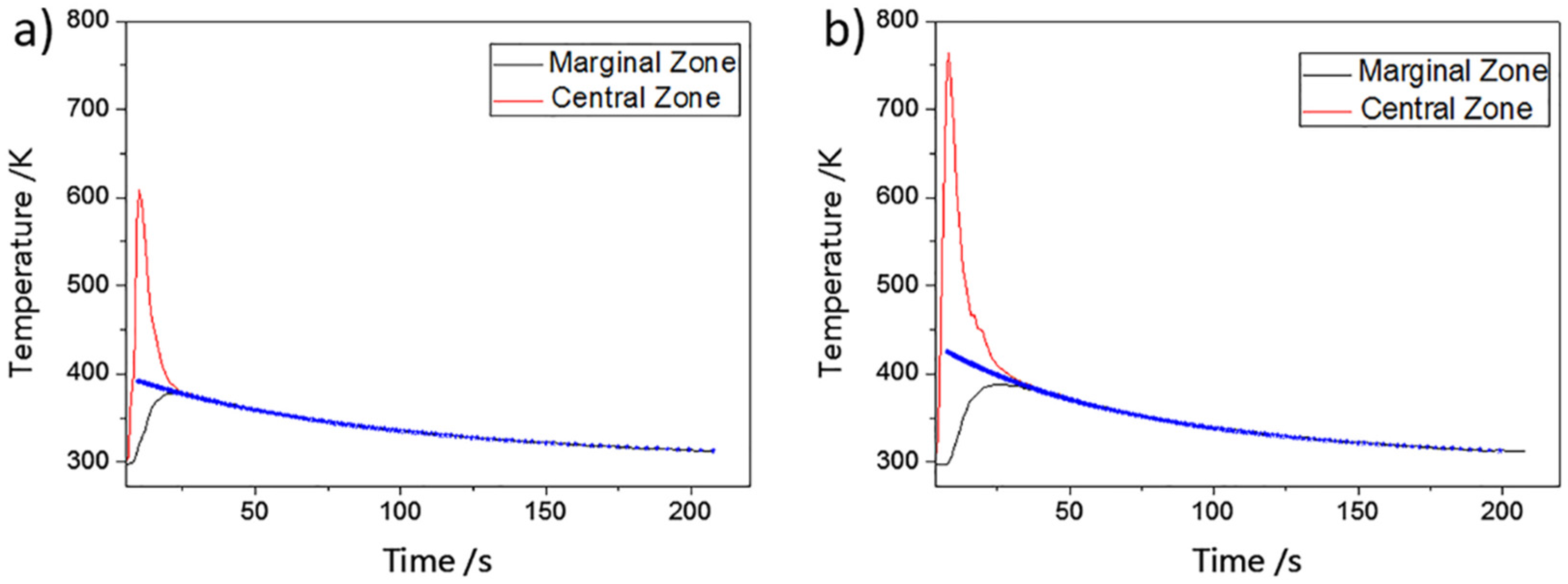
2.2. Mean Laser Absorptivity Measurement
2.3. Characterization of Specimens
3. Results
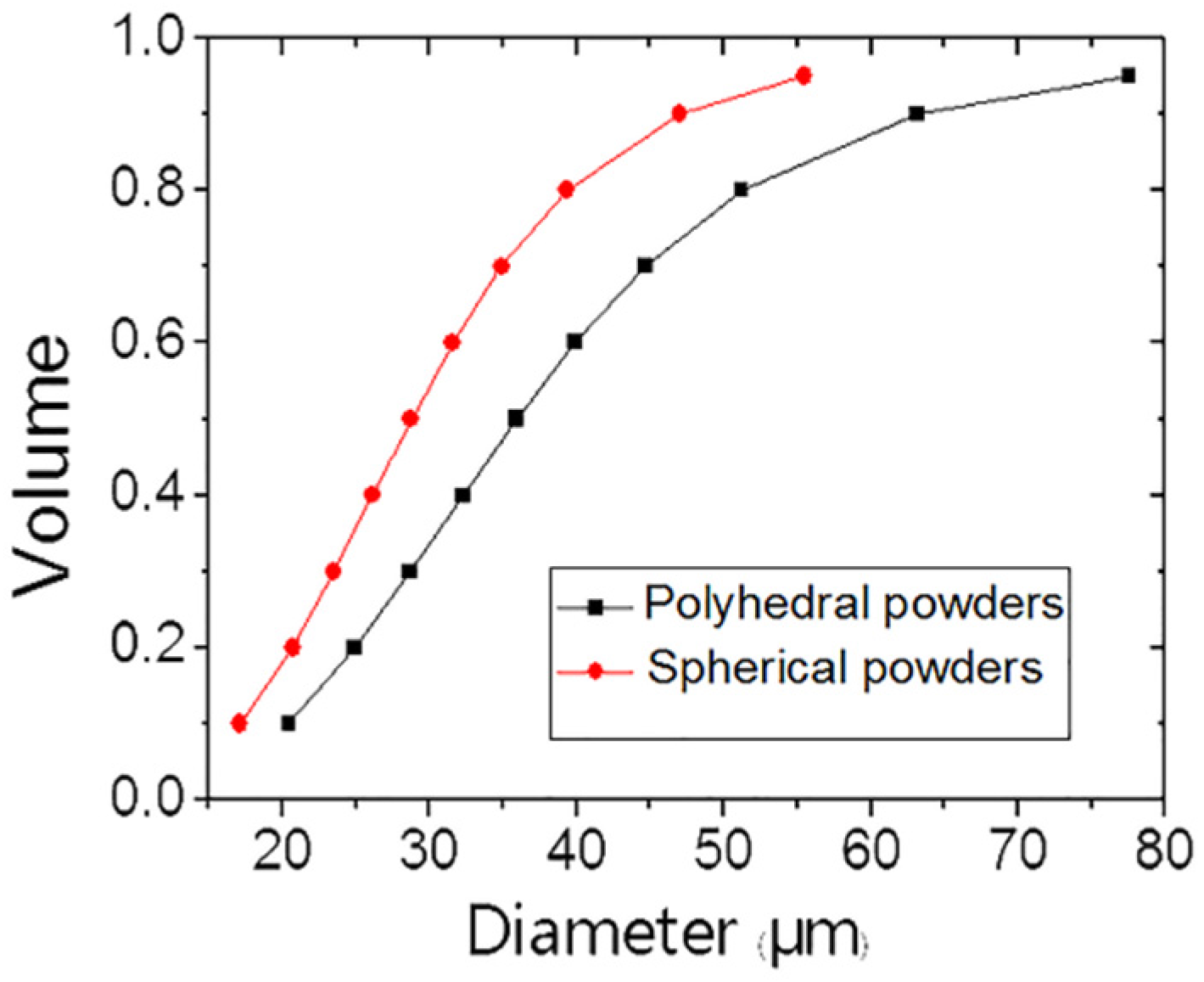
3.1. Polyhedral and Spherical Tungsten Powders
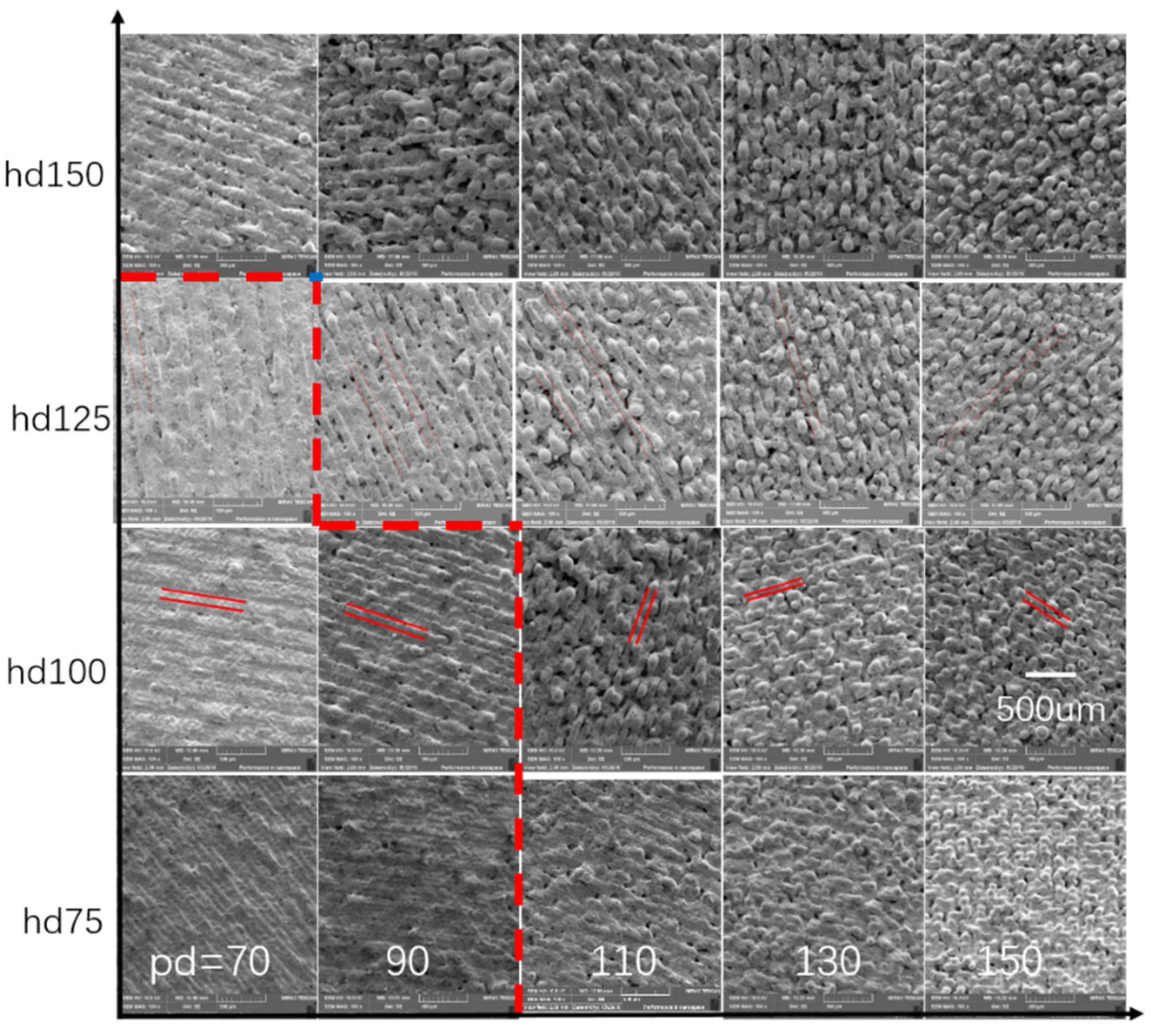
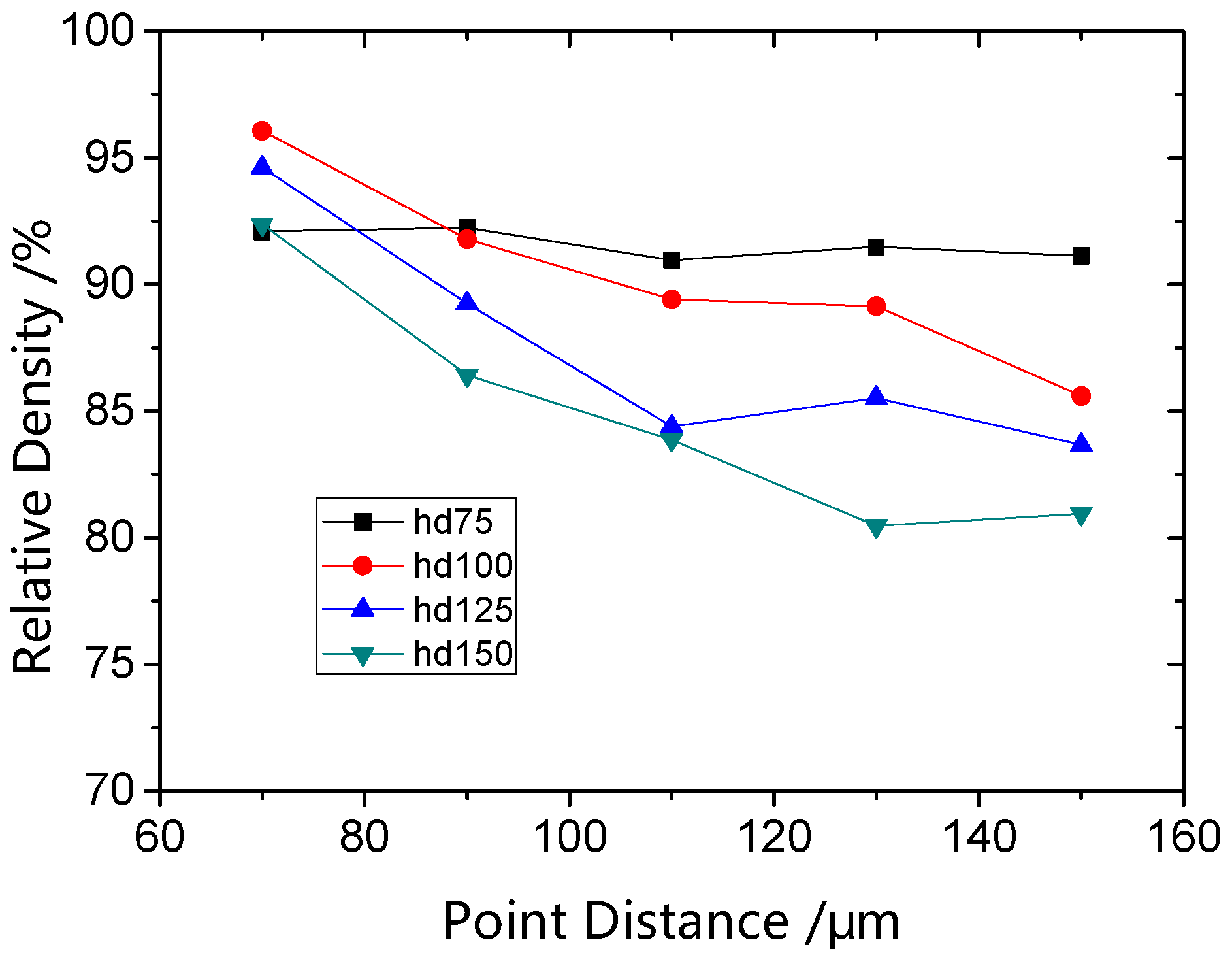
3.2. Parameters Optimization and Surface Morphologies Evolution
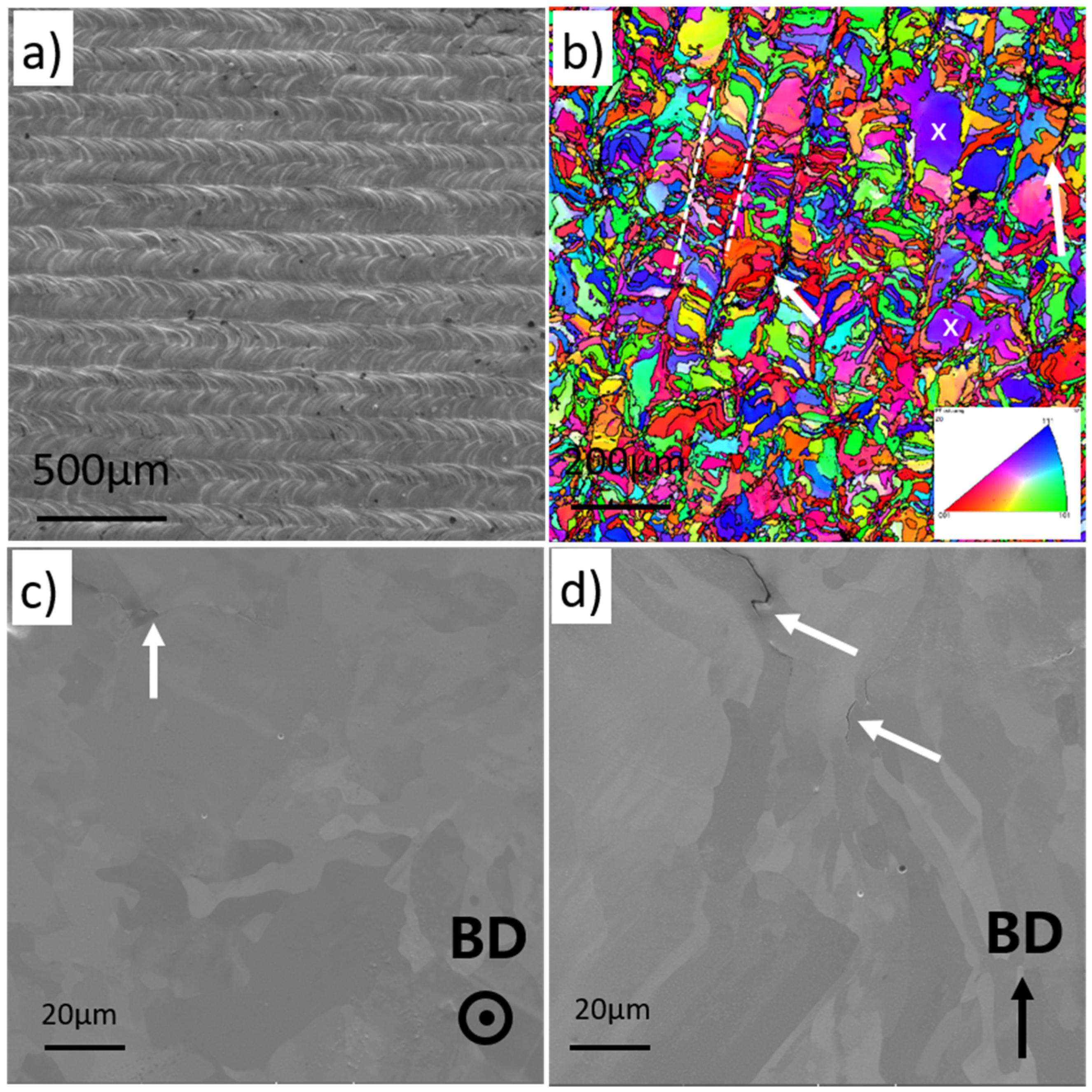
3.3. Microstructure Characterization
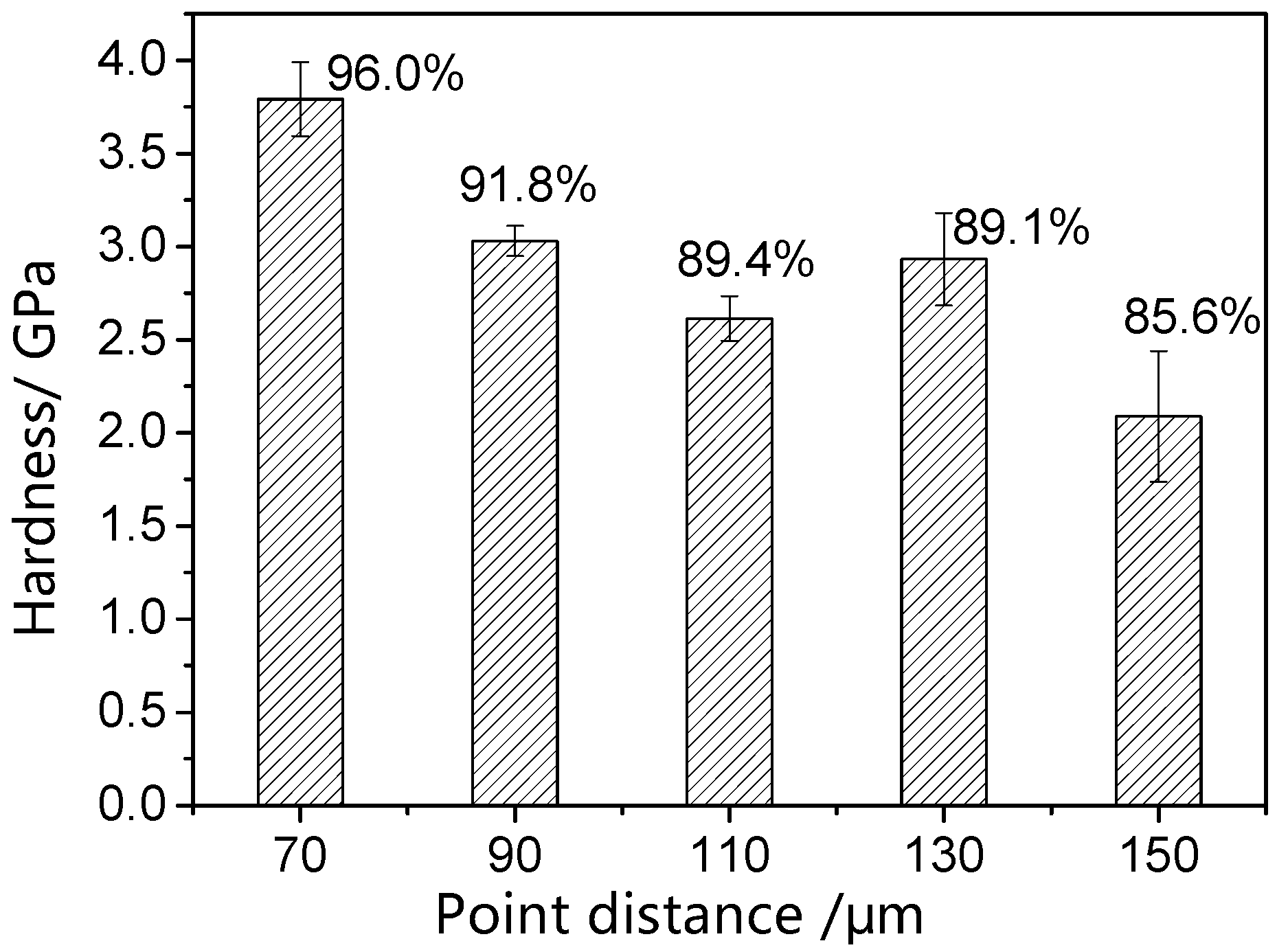
3.4. Surface Hardness
4. Discussion
Effect of Powder Spheroidization on Densification
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, L.; Jiang, L.; Topping, T.D.; Dai, C.; Wang, X.; Carpenter, R.; Haines, C.; Schoenung, J.M. In situ oxide dispersion strengthened tungsten alloys with high compressive strength and high strain-to-failure. Acta Mater. 2017, 122, 19–31. [Google Scholar] [CrossRef]
- Wei, Q.; Jiao, T.; Ramesh, K.T.; Ma, E.; Kecskes, L.J.; Magness, L.; Dowding, R.; Kazykhanov, V.U.; Valiev, R.Z. Mechanical behavior and dynamic failure of high-strength ultrafine grained tungsten under uniaxial compression. Acta Mater. 2006, 54, 77–87. [Google Scholar] [CrossRef]
- Philipps, V. Tungsten as material for plasma-facing components in fusion devices. J. Nucl. Mater. 2011, 415, S2–S9. [Google Scholar] [CrossRef]
- Ezato, K.; Suzuki, S.; Seki, Y.; Yamada, H.; Hirayama, T.; Yokoyama, K.; Escourbiac, F.; Hirai, T. Progress of iter full tungsten divertor technology qualification in Japan: Manufacturing full-scale plasma-facing unit prototypes. Fusion Eng. Des. 2016, 109, 1256–1260. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.Z.; Liu, W.; Shen, Z.J. Suppressing pore-boundary separation during spark plasma sintering of tungsten. J. Nucl. Mater. 2013, 438, 199–203. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Pintsuk, G.; Linke, J.; Hirai, T.; Rodig, M.; Ma, Y.; Ge, C.C. Transient high heat load tests on pure ultra-fine grained tungsten fabricated by resistance sintering under ultra-high pressure. Fusion Eng. Des. 2010, 85, 115–121. [Google Scholar] [CrossRef]
- Antusch, S.; Norajitra, P.; Piotter, V.; Ritzhaupt-Kleissl, H.J.; Spatafora, L. Powder injection molding—An innovative manufacturing method for He-cooled demo divertor components. Fusion Eng. Des. 2011, 86, 1575–1578. [Google Scholar] [CrossRef]
- Antusch, S.; Commin, L.; Heneka, J.; Piotter, V.; Plewa, K.; Walter, H. A new fully automatic pim tool to replicate two component tungsten demo divertor parts. Fusion Eng. Des. 2013, 88, 2461–2465. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.F.; Wikman, S.; Cui, D.Q.; Shen, Z.J. Intragranular cellular segregation network structure strengthening 316l stainless steel prepared by selective laser melting. J. Nucl. Mater. 2016, 470, 170–178. [Google Scholar] [CrossRef]
- Zhou, X.; Li, K.L.; Zhang, D.D.; Liu, X.H.; Ma, J.; Liu, W.; Shen, Z.J. Textures formed in a cocrmo alloy by selective laser melting. J. Alloys Compd. 2015, 631, 153–164. [Google Scholar] [CrossRef]
- Hong, M.H.; Min, B.K.; Kwon, T.Y. The influence of process parameters on the surface roughness of a 3d-printed co-cr dental alloy produced via selective laser melting. Appl. Sci. 2016, 6, 401. [Google Scholar] [CrossRef]
- Popovich, A.; Sufiiarov, V.; Polozov, I.; Borisov, E.; Masaylo, D.; Orlov, A. Microstructure and mechanical properties of additive manufactured copper alloy. Mater. Lett. 2016, 179, 38–41. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Gusarov, A.; Yadroitsava, I.; Smurov, I. Single track formation in selective laser melting of metal powders. J. Mater. Process. Technol. 2010, 210, 1624–1631. [Google Scholar] [CrossRef]
- Ebert, R.; Ullmann, F.; Hildebrandt, D.; Schille, J.; Hartwig, L.; Kloetzer, S.; Streek, A.; Exner, H. Laser processing of tungsten powder with femtosecond laser radiation. J. Laser Micro Nanoeng. 2012, 7, 38–43. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Cai, Q.Z.; Liu, J.H. Formation of nanocrystalline tungsten by selective laser melting of tungsten powder. Mater. Manuf. Process. 2012, 27, 1267–1270. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.H.; Zhang, D.D.; Shen, Z.J.; Liu, W. Balling phenomena in selective laser melted tungsten. J. Mater. Process. Technol. 2015, 222, 33–42. [Google Scholar] [CrossRef]
- Deprez, K.; Vandenberghe, S.; Van Audenhaege, K.; Van Vaerenbergh, J.; Van Holen, R. Rapid additive manufacturing of mr compatible multipinhole collimators with selective laser melting of tungsten powder. Med. Phys. 2013, 40, 012501. [Google Scholar] [CrossRef] [PubMed]
- Yadroitsev, I.; Bertrand, P.; Smurov, I. Parametric analysis of the selective laser melting process. Appl. Surf. Sci. 2007, 253, 8064–8069. [Google Scholar] [CrossRef]
- Wang, L.Z.; Liu, Y.; Chang, S. Fabrication of spherical AlSi10Mg powders by radio frequency plasma spheroidization. Metall. Mater. Trans. A 2016, 47A, 2444–2453. [Google Scholar] [CrossRef]
- Panwisawas, C.; Qiu, C.L.; Sovani, Y.; Brooks, J.W.; Attallah, M.M.; Basoalto, H.C. On the role of thermal fluid dynamics into the evolution of porosity during selective laser melting. Scr. Mater. 2015, 105, 14–17. [Google Scholar] [CrossRef]
- Gusarov, A.V.; Yadroitsev, I.; Bertrand, P.; Smurov, I. Heat transfer modelling and stability analysis of selective laser melting. Appl. Surf. Sci. 2007, 254, 975–979. [Google Scholar] [CrossRef]
- Frenk, A.; Hoadley, A.F.A.; Wagniere, J.D. Insitu technique for measuring the absorption during laser surface remelting. Metall. Trans. B 1991, 22, 139–141. [Google Scholar] [CrossRef]
- Parker, W.J.; Jenkins, R.J.; Abbott, G.L.; Butler, C.P. Flash method of determining thermal diffusivity, heat capacity, and thermal conductivity. J. Appl. Phys. 1961, 32, 1679–1684. [Google Scholar] [CrossRef]
- Paradis, P.F.; Ishikawa, T.; Yoda, S. Viscosity of liquid undercooled tungsten. J. Appl. Phys. 2005, 97, 106101. [Google Scholar] [CrossRef]


| Power Type | C | O | N |
|---|---|---|---|
| Polyhedral powder | 0.0066 | 0.021 | 0.0052 |
| Spherical powder | 0.0083 | 0.010 | 0.0018 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Yu, C.; Zhou, X.; Ma, J.; Liu, W.; Shen, Z. Dense Pure Tungsten Fabricated by Selective Laser Melting. Appl. Sci. 2017, 7, 430. https://doi.org/10.3390/app7040430
Wang D, Yu C, Zhou X, Ma J, Liu W, Shen Z. Dense Pure Tungsten Fabricated by Selective Laser Melting. Applied Sciences. 2017; 7(4):430. https://doi.org/10.3390/app7040430
Chicago/Turabian StyleWang, Dianzheng, Chenfan Yu, Xin Zhou, Jing Ma, Wei Liu, and Zhijian Shen. 2017. "Dense Pure Tungsten Fabricated by Selective Laser Melting" Applied Sciences 7, no. 4: 430. https://doi.org/10.3390/app7040430





